- *Corresponding Author:
- Tong Guan
Department of Rheumatology, The First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine, Guangzhou, Guangdong Province 510000, China
E-mail: lsytgzya@163.com
| This article was originally published in a special issue,“Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “245-253” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Rheumatoid arthritis is a common inflammatory autoimmune disease; tofacitinib and cyclophosphamide have been discovered for the treatment of rheumatoid arthritis. However, single-agent therapy has limitations and combination therapy has become a research hotspot. Nevertheless, studies on the combination therapy of tofacitinib and cyclophosphamide have not been clearly reported. This study aims to investigate the efficacy of their combination therapy at both cellular and animal levels, providing new references for the combination therapy of rheumatoid arthritis. Tofacitinib and/or cyclophosphamide interventions were performed on human fibroblast-like synoviocytes rheumatoid arthritis cells to assess cell proliferation, apoptosis levels, and expression of inflammatory factors. In a rheumatoid arthritis animal model, joint tissue pathology, synovial tissue apoptosis levels, inflammatory factors, bone metabolism, Janus kinase signal transducer and activator of transcription signaling pathway and cluster of differentiation 4+ T-cell balance were evaluated. The combined treatment of tofacitinib and cyclophosphamide in human fibroblast-like synoviocytes rheumatoid arthritis cells resulted in decreased cell survival rate, reduced levels of interleukin-1, interleukin-6, tumor necrosis factor alpha, and B-cell lymphoma 2 messenger ribonucleic acid, increased B-cell lymphoma 2-associated protein X messenger ribonucleic acid levels, and decreased receptor activator of nuclear factor kappa-B ligand, receptor activator of nuclear factor kappa-B, C-terminal telopeptide of type I collagen, and N-terminal propeptide of type I procollagen levels in rheumatoid arthritis animal models. Additionally, there was a decrease in p-Janus kinase 2, and p-signal transducer and activator of transcription 3 protein expression levels, and alterations in the frequencies of T helper type 1, T helper type 2, T helper type 17, and regulatory T cells. Combining tofacitinib and cyclophosphamide can regulate cluster of differentiation 4+ T-cell balance, inhibit the p-Janus kinase 2/p-signal transducer and activator of transcription 3 pathway, reduce human fibroblast-like synoviocytes-rheumatoid arthritis cell proliferation and inflammation progression, and enhance synovial protection, leading to improved therapeutic efficacy compared to individual rheumatoid arthritis treatments.
Keywords
Tofacitinib, cyclophosphamide, combination therapy, rheumatoid arthritis, inflammation
Rheumatoid Arthritis (RA) is a prevalent inflammatory joint disease characterized by joint inflammation, proliferation, swelling, production of autoantibodies, as well as cartilage and bone destruction[1,2]. Epidemiological studies have shown that the global prevalence of RA is approximately 1 %, with relatively higher incidence in women[3]. As a systemic and chronic immune disease, early-stage RA patients often experience varying degrees of joint activity disorders and pain, and in advanced stages, joint stiffness and deformities may occur, resulting in a higher disability rate[4,5]. Currently, the primary treatment strategy for RA focuses on reducing inflammation and alleviating swelling and pain. In clinical practice, traditional disease-modifying drugs, steroids, non-steroidal anti-inflammatory drugs, and other therapies are commonly used for RA treatment. In many cases, patients experience some relief of symptoms after receiving monotherapy. However, there are also patients who do not respond to medication, exhibiting a phenomenon known as drug resistance[6]. Therefore, exploring combination drug therapies for RA has become a hot topic in clinical research.
The pathogenesis of RA involves complex regulation of immune cells, immune factors, and signaling pathways[7,8]. The long-term accumulation of inflammation leads to synovial cell proliferation and synovial thickening, which in turn results in the destruction of cartilage and bone. Ultimately, this leads to the loss of joint function[9]. Human Fibroblast- Like Synoviocytes (HFLS) mediate immune reactions and inflammation in the synovial region, which is also a major pathogenic mechanism of RA. The pathological and physiological mechanisms of RA have not been fully elucidated. However, some studies have indicated that immune reactions occur before the onset of arthritis symptoms[10]. Therefore, exploring the immune reactions of synovial cells and the treatment mechanisms for RA is even more important.
Research has found that adaptive immunity also plays an important role in the pathogenesis of RA, with T cells and B cells both involved to varying degrees in the development of RA[11-13]. By regulating the balance between regulatory T cells (Treg) and T helper 17 (Th17) cells, the symptoms of RA can also be improved[14]. In addition, during the pathogenesis of RA, monocytes in the blood are recruited to the synovial membrane and differentiate into proinflammatory M1 macrophages. These macrophages release various cytokines and chemokines such as Tumor Necrosis Factor-Alpha (TNF-α), Interleukin-6 (IL-6), and IL-1 Beta (β). This leads to the amplification of local inflammation and damage in the synovial tissue[15,16]. The Janus Kinase (JAK)/ Signal Transducer and Activator of Transcription (STAT) signaling pathway, as well as the cyclic GMP-AMP Synthase-Stimulator of Interferon Genes (cGAS-STING) pathway, are both involved in the pathogenesis and progression of RA[17,18].
Tofacitinib (TOF) is a JAK inhibitor that has been shown to inhibit the JAK/STAT signaling pathway. By blocking the synthesis of various cytokines and the occurrence of inflammatory immune responses, TOF can help prevent the progression of RA[19]. Although TOF is a valuable option for the treatment of RA, it is not without its limitations and drawbacks. The incidence of herpes zoster infection may be higher in patients undergoing treatment with TOF compared to the general population of RA patients[20]. Long-term use of TOF may potentially increase the incidence of malignancies[21]. CP is an alkylating agent that is widely used in the treatment of cancer as well as autoimmune diseases such as RA[22]. However, the therapeutic effect of CP in RA is short-lived, and its use is associated with serious toxicity and negative impacts on quality of life. It cannot be used longterm and carries a risk of inducing malignancies[23]. Reports on the combined use of TOF and CP in the treatment of RA are scarce. However, a study found that in RA patients, pulmonary rheumatoid nodules appeared during treatment with TNF inhibitors, and CP treatment was ineffective. Subsequently, treatment with TOF led to a rapid improvement in the patient’s condition[24]. This suggests that the combination of TOF and CP may have distinct effects on immune regulation in the treatment of RA.
Therefore, this study aims to investigate the therapeutic efficacy of the combination of TOF and CP in RA by utilizing cellular biology methods to examine the changes in inflammatory factors and bone metabolism levels in both in vitro and in vivo RA models before and after treatment. Additionally, the study aims to uncover the underlying mechanisms of action of this combination therapy, with the goal of providing new research insights for the combination treatment of RA.
Materials and Methods
Cells culture:
HFLS-RA cells (IL-1β derived) were acquired from the American Type Culture Collection (ATCC), (Manassas, Virginia, United Sates of America (USA)). These cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Life Technologies) supplemented with 10 % Fetal Bovine Serum (FBS), (Hyclone) Laboratories, South Logan, Utah, USA) in a humidified incubator at 37° with 5 % Carbon dioxide (CO2).
Cell model and groups:
The HFLS-RA cells were divided into 4 groups; control group, TOF group, CP group and TOF+CP group. The TOF group cells were treated with TOF (5 μM) for 24 h. The CP group cells were treated with CP (5 μM) for 24 h.
Animal model:
Male DBA/1 mice (6-7 w old, weighing (18±2) g) were randomly divided into 5 groups, with 8 mice per group; control group, model group, TOF group, CP group, and TOF+CP group. A 1:1 mixture of bovine type II collagen (2 mg/ml) acetic acid solution and complete Freund’s adjuvant (5 mg/ml) was prepared to form an emulsion, which was kept for later use. Except for the control group mice, the rest of the mice were injected intradermal at the base of the tail with the aforementioned mixture emulsion (0.1 ml) on the 1st d. After 7 d, a second booster immunization was performed at the same site with the same mixture emulsion (0.1 ml). On the 31st d after the initial immunization, the mice were observed for toe swelling, and the appearance of inflammatory erythema or joint deformity indicated successful replication of the RA model.
Animal model treatment:
After successful replication of the RA model, mice in each group were given daily intraperitoneal injections of the corresponding drugs. In the TOF group and CP group, mice were given intraperitoneal injections of TOF at a dose of 0.6 mg/kg and CP at a dose of 0.6 mg/kg, respectively, twice a week for duration of 30 d. The day after the last administration, mice were sacrificed, and blood was collected for serum separation and storage. The left ankle and tibia joints were collected and fixed with 4 % paraformaldehyde. Decalcification was performed using a 10 % Ethylenediaminetetraacetic Acid (EDTA) solution. After dehydration in a graded ethanol series, the samples were embedded in paraffin, sliced, and stained with Hematoxylin and Eosin (H&E). Pathological changes in the ankle and tibia joint tissues of mice in each group were observed under an optical microscope. The right knee joint was taken separately, and the synovial tissue of the knee joint was bluntly dissected and stored in a -80° ultra-low temperature freezer for future use.
3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) Assay:
Logarithmic-phase HFLS-RA cells were seeded at a density of 5000 cells per well in 96-well plates with complete culture medium. The following day, cell interventions were performed according to the drug treatment. Six parallel wells were set up for each treatment. After the intervention was completed, the culture medium was removed and 20 μl of MTT solution with a concentration of 5 g/l was added to each well for 4 h. After dissolving the MTT crystals with 200 μl of dimethyl sulfoxide, the Optical Density (OD) values of each treatment group were detected at 450 nm using an Enzyme Linked Immunosorbent Assay (ELISA) reader.
ELISA:
The IL-1, IL-6, TNF-α, Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL), RANK, C-Terminal Telopeptide of Type I Collagen (CTX-I) and N-Terminal Propeptide of Type I Procollagen (PINP) contents in cells and serum were measured using an ELISA kit from Abcam. Briefly, we prepared a series of diluted standard solutions and added the samples to separate test tubes. Then, we added the enzyme to each test tube and incubated the mixtures at 37° for 10 min. After the incubation, the reaction was stopped, and the OD was measured at 450 nm using a spectrophotometer.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR):
Total Ribonucleic Acid (RNA) from cells and synovial tissue were isolated using the Trizol reagent (Invitrogen, Carlsbad, California, USA), following the product instructions. Quantitative RT-PCR was then performed with Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) as the control. The relative expression of B-cell lymphoma 2 (Bcl-2) and Bcl- 2-Associated Protein X (BAX) were calculated using the 2-ΔΔCT method. The primer sequences used in this study were as follows; Bcl-2: forward 5’-GGTGGGGTCATGTGTGTGG-3’ and reverse 5’-CGGTTCAGGTACTCAGTCATCC-3’; BAX, forward 5’-GCGCTCGTGTTTCTGGACA-3’ and reverse 5’-AGTATAGACACTCGTCACTGGTG-3’; GAPDH, forward 5’-ACGGATTTGGTCGTATTGGGCG-3’ and reverse 5’-GCTCCTGGAAGATGGTGATGGG-3’.
Western blot assay:
Total proteins were extracted in synovial tissue using Radioimmunoprecipitation assay lysis buffer (Solarbio) and quantified with a bicinchoninic acid assay kit (Solarbio). 20 mg of proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Solarbio) and transferred onto Polyvinylidene fluoride membranes (Millipore, Massachusetts, USA). Following blocking with 5 % skim milk, the membranes were incubated for 1 h at room temperature with primary antibodies against JAK2, pJAK2, STAT3 and pSTAT3. β-tubulin was used as a control. All antibodies were sourced from Abcam.
Immune cell balance assay:
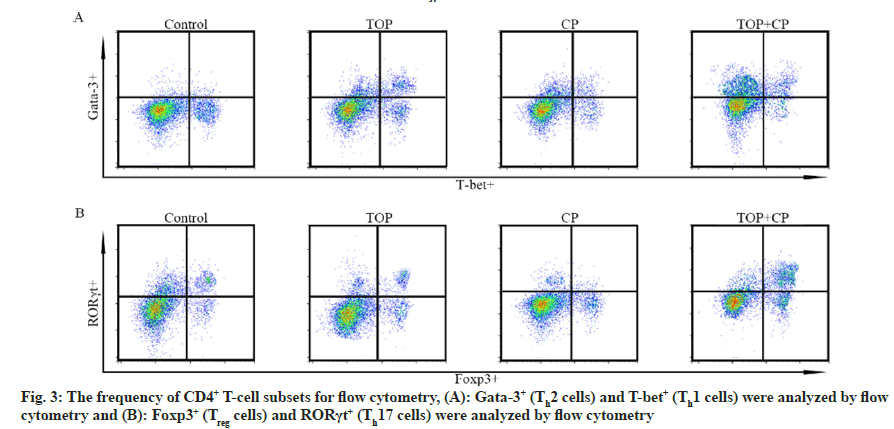
Mononuclear lymphocytes were isolated from collected DBA/1 mice blood and then subjected to a flow cytometry analysis to determine the percentages of Th1, Th2, Th17 and Treg cells in the peripheral blood. The Treg cells were identified using Forkhead box P3 (FOXP3+). Th1 cells were detected with the use of T-bet+, while Th2 cells were detected with Gata-3+. Lastly, Th17 cells were spotted using RORγt+. All sourced from Invitrogen.
Statistical analysis:
All data were presented as mean±Standard Deviation (SD) and analyzed using GraphPad Prism 8.0 Software. One-way Analysis of Variance (ANOVA) followed by Tukey’s multiple comparisons test was performed for intergroup comparisons. p<0.05 was considered statistically significant. All experiments were repeated at least three times to ensure the reliability of the results.
Results and Discussion
To investigate the effects of the combination of TOF and CP, the study will first conduct in vitro experiments to validate the changes in cell survival rate and apoptosis-related genes in HFLS-RA cells after intervention with the combination therapy. Based on the results shown in fig. 1, when compared to the control group using HFLS-RA as the baseline, cell survival rate decreased after 24 h of intervention with either TOF or CP alone. However, when TOF was combined with CP, the cell survival rate further decreased and was significantly lower than that of the TOF or CP alone groups. The excessive proliferation of RA synovial cells is a manifestation of RA. In this case, the level of cell apoptosis was detected using RT-qPCR. Compared to the control group, the expression level of Bcl-2 mRNA significantly decreased in the TOF group, while the expression level of BAX mRNA significantly increased in the TOF group. Compared to the TOF or CP alone groups, the TOF+CP group showed a significant decrease in Bcl-2 mRNA expression level and a significant increase in BAX mRNA expression level. Indeed, these results indicate that the combination of TOF and CP can enhance the inhibition of HFLS-RA cell proliferation as shown in Table 1.
| Group | Cell survival rate (%) | Bcl-2 mRNA | BAX mRNA |
|---|---|---|---|
| Control | 99.8±0.05 | 2.16±0.05 | 0.58±0.21 |
| TOF | 84.36±5.62* | 1.34±0.07* | 1.22±0.06* |
| CP | 97.15±3.75 | 2.11±0.11 | 0.55±0.03 |
| TOF+CP | 65.28±1.06#& | 0.66±0.03#& | 1.97±0.16#& |
Note: *p<0.05 vs. the control group; #p<0.05 vs. the TOF group and &p<0.05 vs. the CP group
Table 1: Effect of TOF and CP Intervention on the Proliferation of HFLS-RA Cells
In the progression of RA, inflammation is inevitably involved. Although the effectiveness of RA in reducing osteoarthritis has been confirmed, it is still unknown whether the combination of TOF and CP can achieve this effect. Therefore, this study compared the levels of inflammatory factors before and after combination therapy (Table 2). Compared to the control group, the levels of IL-1, IL-6, and TNF-α were reduced in both the TOF and CP alone groups, but only the TOF group showed a significant decrease. Compared to the TOF or CP alone groups, the TOF+CP group showed a significant decrease in the levels of IL-1, IL-6, and TNF-α. By detecting the levels of inflammatory factors, it is once again confirmed that the efficacy of TOF combined with CP in reducing inflammatory factors in HFLS-RA cells is stronger than that of using either drug alone, especially for CP.
| Group | IL-1 (pg/ml) | IL-6 (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|
| Control | 130.21±6.12 | 10.84±0.14 | 50.28±3.26 |
| TOF | 97.53±3.74* | 8.25±0.12* | 21.35±1.49* |
| CP | 127.26±5.05 | 9.74±0.09 | 46.51±1.53 |
| TOF+CP | 42.64±3.17#& | 2.43±0.05#& | 12.34±0.98#& |
Note: *p<0.05 vs. the control group; #p<0.05 vs. the TOF group and &p<0.05 vs. the CP group
Table 2: Effect of TOF and CP Intervention on the Inflammation Cytokine of HFLS-RA Cells
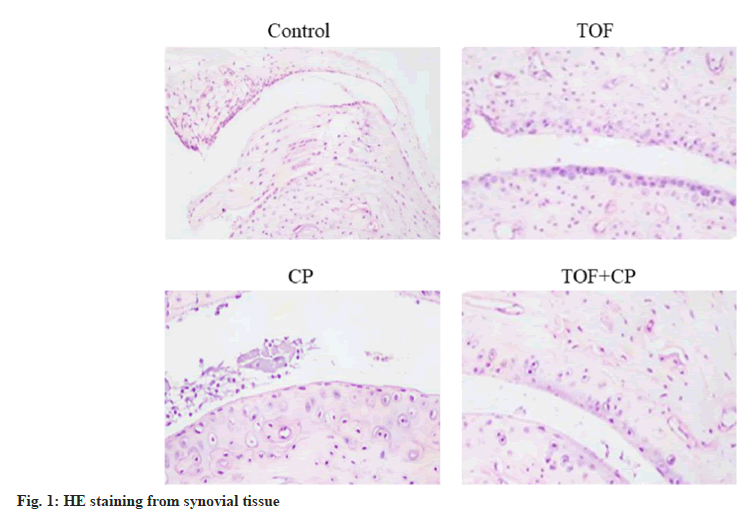
Through preliminary in vitro experiments, we have not only discovered that the combination of TOF and CP can inhibit HFLS-RA cell proliferation and reduce inflammation levels, but we have also identified a unique synergistic effect of TOF and CP in combination. During the treatment of RA, it is not only about inhibiting inflammation, but the ultimate focus lies in improving the synovial tissue of the joints. In the collagen-induced RA animal model using DBA/1 mice, the levels of RANKL, RANK, CTX-I, and PINP were measured. Compared to the control group, these levels were significantly reduced in the TOF group. Compared to the TOF or CP groups, there was a significant reduction in the levels of RANKL, RANK, CTX-I and PINP. In addition, observation through H&E staining (fig. 2) revealed significant inflammatory infiltration in the synovial tissue of the control group mice, including HFLS and osteoclasts. The arrangement of synovial tissue cells was irregular, and there was evident proliferation and swelling. Compared to the TOF or CP groups, the TOF+CP group exhibited significant improvement in synovial tissue proliferation and osteoclast infiltration as shown in Table 3.
| Group | RANKL (ng/l) | RANK (ng/l) | CTX-I (pg/ml) | PINP (ng/l) |
|---|---|---|---|---|
| Control | 17.52±1.84 | 8.72±0.74 | 53.14±5.75 | 0.08±0.013 |
| TOF | 13.26±1.28* | 6.82±0.61* | 39.55±4.93* | 0.06±0.011* |
| CP | 17.07±1.31 | 8.16±0.57 | 51.25±4.86 | 0.08±0.018 |
| TOF+CP | 6.85±1.11#& | 3.18±0.42#& | 17.89±4.74#& | 0.02±0.014#& |
Note: *p<0.05 vs. the control group; #p<0.05 vs. the TOF group and &p<0.05 vs. the CP group
Table 3: Effect of TOF and CP Treatment on Bone Metabolic Factor of Synovial Tissue
To strengthen our hypothesis, we performed another round of assessments on the levels of inflammation and apoptosis in the synovial tissue of RA mice. The results, as shown in Table 4, indicate that compared to the control group, the levels of IL-1, IL-6, and TNF-α were significantly reduced in the TOF group. Compared to the TOF or CP groups, the TOF+CP group exhibited a significant reduction in the levels of IL-1, IL-6, and TNF-α. In Table 5, it was found that the expression level of Bcl-2 mRNA in the TOF group was significantly lower than that in the control group, while the expression level of BAX mRNA was significantly higher than that in the control group. Compared to the TOF or CP groups, the TOF+CP group exhibited a significant decrease in Bcl-2 mRNA expression and a significant increase in BAX mRNA expression.
| Group | IL-1 (pg/ml) | IL-6 (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|
| Control | 388.24±64.32 | 39.44±5.12 | 15.72±1.54 |
| TOF | 321.05±58.91* | 26.91±3.29* | 12.88±1.13* |
| CP | 378.57±47.26 | 34.86±4.08 | 15.01±1.04 |
| TOF+CP | 190.47±20.73#& | 18.95±2.17#& | 7.63±1.03#& |
Note: *p<0.05 vs. the control group; #p<0.05 vs. the TOF group and &p<0.05 vs. the CP group
Table 4: Effect of TOF and CP Treatment on Inflammation of Synovial Tissue
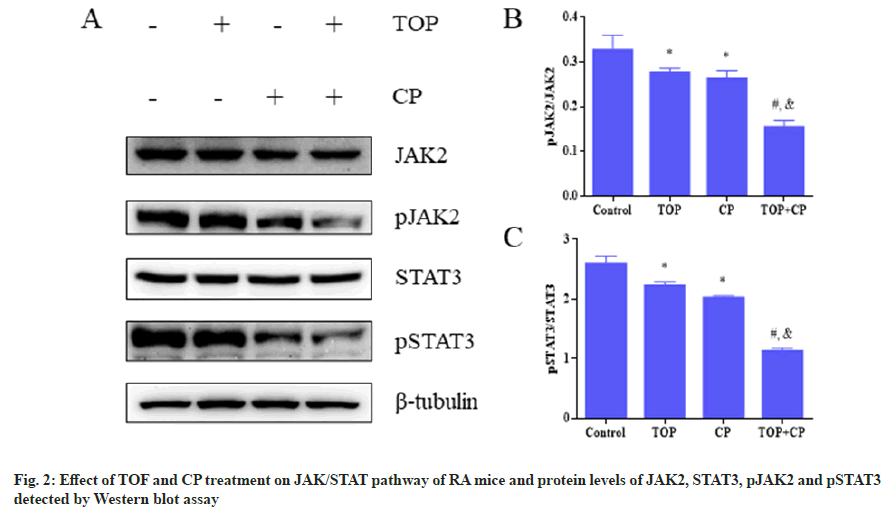
The preceding research has demonstrated that the combination of TOF and CP synergistically improves the progression of RA. In order to investigate the mechanism of action of TOF in combination with CP, we examined the roles of cell signaling and immune regulation in this process separately. By assessing the phosphorylation levels of JAK/STAT pathway proteins, it was observed that the expression levels of pJAK2 and pSTAT3 were the lowest in the TOF+CP group, and significantly lower than in the TOF group or CP group. Furthermore, in comparison to the control group, the expression levels of pJAK2 and pSTAT3 proteins were significantly lower in the TOF group and CP group (fig. 2). Experimental results from fig. 3 and Table 5 revealed that, in comparison to the control group, both the TOF group and CP group exhibited a significant reduction in the cell frequencies of Th1 and Th2, along with a significant increase in the cell frequencies of Treg and Th17. Compared to the TOF group or CP group, the TOF+CP group demonstrated a further significant decrease in the cell frequencies of Th1 and Th2, and a further significant increase in the cell frequencies of Treg and Th17.
| Group | Bcl-2 mRNA | BAX mRNA |
|---|---|---|
| Control | 1.35±0.11 | 0.73±0.33 |
| TOF | 0.84±0.08* | 1.53±0.12* |
| CP | 1.67±0.01 | 0.56±0.09 |
| TOF+CP | 0.26±0.05#& | 3.14±0.08#& |
Note: *p<0.05 vs. the control group; #p<0.05 vs. the TOF group and &p<0.05 vs. the CP group
Table 5: Effect of TOF and CP Treatment on Apoptosis of Synovial Tissue
RA is a chronic autoimmune disease characterized by synovial inflammation, joint damage, and systemic complications[25]. The aim of this study is to investigate the combined therapeutic effect of TOF and CP on the progression of RA. Combined therapy is not a novel concept, as numerous studies have previously proposed combination therapies for RA[26,27]. This study conducted research experiments from both in vitro and in vivo perspectives, focusing on aspects such as cell proliferation, levels of inflammatory factors, bone metabolism, and joint repair. It aimed to uncover the actions and mechanisms of TOF+CP, ultimately revealing the synergistic effects present in this combination therapy. This will provide new insights into addressing the adverse effects of monotherapy and drug resistance, offering potential solutions to these challenges.
In the progression of RA, damage to the synovial tissue of the joints and inflammation often occur, both of which are typically in the later stages of the disease. Guagnano, et al.[28], have suggested that reducing the occurrence of inflammation can serve as an indicator for evaluating RA treatment. Manman, et al. found that upregulating IL-6 and TNF-α exacerbates the inflammatory response in RA[29]. Furthermore, a study suggests that inflammatory factors in RA can enhance drug permeation by overcoming the skin’s stratum corneum barrier, increasing drug bioavailability[30]. It is evident that regulating the levels of inflammatory factors plays a crucial role in the treatment of RA. In both in vivo and in vitro experiments, this study found that the combined therapy of TOF+CP significantly reduced the levels of inflammatory factors IL-1, IL-6, and TNF-α. Furthermore, CP did not exhibit a significant effect in this process, as there was no significant difference observed between the CP group and the control group when compared. A similar trend was observed in the experiments involving the inhibition of synovial cell proliferation and the repair of joint bones in the TOF+CP group.
Additionally, we assessed the protective effect of TOF+CP on joint tissues in a collagen-induced RA mouse model. The combination therapy significantly reduced the levels of bone resorption and degradation markers, including RANKL, RANK, CTX-I, and PINP. Histological analysis also revealed that, in comparison to the TOF group or CP group, the TOF+CP group exhibited improved synovial tissue morphology. These results suggest that the combination therapy not only inhibits inflammation but also provides protection against bone and joint damage. In the research by Ma, et al.[31], the traditional Chinese medicine Hongjingtian was found to downregulate RANKL-related mRNA and protein expression to inhibit bone destruction in the treatment of RA. The study by Liu, et al.[32], also indicates that the combination therapy reduces RA by downregulating the expression of RANKL and RANK proteins. The research demonstrated a reduction in bone turnover associated with bone metabolismrelated genes like CTX-I and serum procollagen type IN peptide[33,34]. In the process of demonstrating the synergistic promotion of RA treatment by TOF+CP, the limitations of monotherapy were overcome. To elucidate its mechanism of action, we investigated immune regulation and the JAK/STAT pathway.
Compared to monotherapy, the combination therapy significantly reduced the phosphorylation levels of JAK2 and STAT3. This indicates that TOF+CP can inhibit the activation of the JAK/STAT pathway, which is involved in the inflammatory response. Furthermore, the combination therapy resulted in a further reduction in the frequency of Th1/Th2 cells and an increase in the frequency of Th17/Treg cells. This suggests that the combination therapy promotes a shift towards an anti-inflammatory immune response. Interestingly, in this study, the monotherapy of CP did not exhibit effects similar to TOF, either in vitro or in vivo. The standalone intervention of CP did not significantly reduce inflammatory factor levels, regulate bone metabolism, or inhibit HFLS-RA proliferation. Guo, et al.[35], research found that after the intervention with active ingredients, HFLS-RA cell proliferation was significantly inhibited. In this study, the combination therapy, when compared to CP alone, demonstrated the ineffective role of CP in inhibiting cell proliferation, reducing inflammatory factor levels, and regulating bone metabolism. However, in the final experimental study, it was found that CP played a significant role in inhibiting the phosphorylation of JAK2 and STAT3 proteins and regulating the balance of CD4+ T-cells. CP has been reported as a treatment standard for inflammation, while JAK inhibitors are a treatment choice for RA[36]. The development of treatments for RA is closely intertwined with this disease. Therefore, even though CP may not directly regulate the levels of inflammatory factors in this study, it serves as a mediator by inhibiting the JAK/STAT pathway and utilizing JAK/STAT to control the progression of inflammation.
Furthermore, the JAK/STAT pathway has been reported to mediate IL-6, TNF-α, IL-17, and RANKL[37]. It’s worth noting that Th17 cells exhibit plasticity in the pathogenesis of RA and may serve as a novel therapeutic target[38]. Research by Abimannan, et al.[39], has found that reducing the frequencies of Th17 and Th1 cells during the treatment of RA patients can inhibit the production of TNF-α and IL-6, providing a new approach for targeted therapy. These studies provide strong evidence of the significant effects of CP in inhibiting the JAK/STAT pathway and regulating the balance of CD4+ T-cells. Furthermore, our research revealed that the combination therapy had distinct effects in terms of its mechanisms of action and performance indicators. In summary, through our research, we have elucidated that the combination of TOF and CP in the treatment of RA plays significant roles individually in inhibiting cell proliferation, reducing inflammatory factor levels, promoting joint repair, inhibiting the JAK/STAT pathway, and regulating CD4+ T-cell balance. Ultimately, they work together to produce a synergistic effect.
The combination of TOF and CP can exert effects in inhibiting RA cell proliferation, reducing inflammatory factors, and protecting against bone and joint damage. This effect is closely related to the synergistic promotion associated with inhibiting JAK/STAT pathway activation and enhancing the Th2/Th1 and Treg/Th17 balance. This study provides new insights into the limitations of monotherapy and the potential benefits of combination therapy in the treatment of RA.
Conflict of interests:
The authors declared no conflict of interests.
References
- McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017;389(10086):2328-37.
[Crossref] [Google Scholar] [PubMed]
- Scherer HU, Haupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun 2020;110:102400.
[Crossref] [Google Scholar] [PubMed]
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388(10055):2023-38.
- Almutairi KB, Nossent JC, Preen DB, Keen HI, Inderjeeth CA. The prevalence of rheumatoid arthritis: A systematic review of population-based studies. J Rheumatol 2021;48(5):669-76.
[Crossref] [Google Scholar] [PubMed]
- Kerschbaumer A, Sepriano A, Smolen JS, van der Heijde D, Dougados M, van Vollenhoven R, et al. Efficacy of pharmacological treatment in rheumatoid arthritis: A systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2020;79(6):744-59.
[Crossref] [Google Scholar] [PubMed]
- Turk MA, Hayworth JL, Nevskaya T, Pope JE. Ocular manifestations in rheumatoid arthritis, connective tissue disease and vasculitis: A systematic review and metaanalysis. J Rheumatol 2021;48(1):25-34.
[Crossref] [Google Scholar] [PubMed]
- Weyand CM, Goronzy JJ. The immunology of rheumatoid arthritis. Nat Immunol 2021;22(1):10-8.
[Crossref] [Google Scholar] [PubMed]
- Mueller AL, Payandeh Z, Mohammadkhani N, Mubarak SM, Zakeri A, Alagheband Bahrami A, et al. Recent advances in understanding the pathogenesis of rheumatoid arthritis: New treatment strategies. Cells 2021;10(11):3017.
[Crossref] [Google Scholar] [PubMed]
- Schonenberger KA, Schupfer AC, Gloy VL, Hasler P, Stanga Z, Kaegi-Braun N, et al. Effect of anti-inflammatory diets on pain in rheumatoid arthritis: A systematic review and meta-analysis. Nutrients 2021;13(12):4221.
[Crossref] [Google Scholar] [PubMed]
- Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity 2017;46(2):183-96.
[Crossref] [Google Scholar] [PubMed]
- Huang Y, Wang H, Ba X, Chen Z, Wang Y, Qin K, et al. Decipher manifestations and Treg/Th17 imbalance in multi-staging rheumatoid arthritis and correlation with TSDR/RORC methylation. Mol Immunol 2020;127:1.
[Crossref] [Google Scholar] [PubMed]
- Sun W, Meednu N, Rosenberg A, Rangel-Moreno J, Wang V, Glanzman J, et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat Commun 2018;9(1):5127.
[Crossref] [Google Scholar] [PubMed]
- Dahdah A, Habir K, Nandakumar KS, Saxena A, Xu B, Holmdahl R, et al. Germinal center B cells are essential for collagen-induced arthritis. Arthritis Rheumatol 2018;70(2):193-203.
[Crossref] [Google Scholar] [PubMed]
- Silva JC, Mariz HA, Rocha LF, Oliveira PS, Dantas AT, Duarte AL, et al. Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics 2013;68(6):766-71.
[Crossref] [Google Scholar] [PubMed]
- Fukui S, Iwamoto N, Takatani A, Igawa T, Shimizu T, Umeda M, et al. M1 and M2 monocytes in rheumatoid arthritis: A contribution of imbalance of M1/M2 monocytes to osteoclastogenesis. Front Immunol 2018;8:1958.
[Crossref] [Google Scholar] [PubMed]
- Croia C, Bursi R, Sutera D, Petrelli F, Alunno A, Puxeddu I. One year in review 2019: Pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 2019;37(3):347-57.
[Google Scholar] [PubMed]
- Shen H, Jin L, Zheng Q, Ye Z, Cheng L, Wu Y, et al. Synergistically targeting synovium STING pathway for rheumatoid arthritis treatment. Bioactive Mater 2023;24:37-53.
[Crossref] [Google Scholar] [PubMed]
- Lin JJ, Tao K, Gao N, Zeng H, Wang DL, Yang J, et al. Triptolide inhibits expression of inflammatory cytokines and proliferation of fibroblast-like synoviocytes induced by IL-6/sIL-6R-mediated JAK2/STAT3 signaling pathway. Curr Med Sci 2021;41(1):133-9.
[Crossref] [Google Scholar] [PubMed]
- Itoh I, Kasuno K, Yamamoto C, Takahashi N, Shimizu H, Ojima T, et al. IgA vasculitis developed as an adverse effect of tofacitinib taken for rheumatoid arthritis. Inter Med 2020;59(6):817-21.
[Crossref] [Google Scholar] [PubMed]
- Strand V, Ahadieh S, French J, Geier J, Krishnaswami S, Menon S, et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res Ther 2015;17:362.
[Crossref] [Google Scholar] [PubMed]
- Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): A phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017;390(10093):457-68.
[Crossref] [Google Scholar] [PubMed]
- Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother Pharmacol 2016;78:661-71.
[Crossref] [Google Scholar] [PubMed]
- Suarez-Almazor ME, Belseck E, Shea B, Tugwell P, Wells GA, Cochrane musculoskeletal group. Cyclophosphamide for treating rheumatoid arthritis. Cochrane Database Syst Rev 2000;2010(7):Cd001157.
- Kondo M, Murakawa Y, Honda M, Yanagawa T, Nagasaki M, Moriyama M, et al. A case of rheumatoid arthritis with multiple lung rheumatoid nodules successfully treated with tofacitinib. Mod Rheumatol Case Rep 2021;5(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Venetsanopoulou AI, Alamanos Y, Voulgari PV, Drosos AA. Epidemiology of rheumatoid arthritis: Genetic and environmental influences. Exp Rev Clin Immunol 2022;18(9):923-31.
[Crossref] [Google Scholar] [PubMed]
- Gao Y, Gao YN, Wang MJ, Zhang Y, Zhang FQ, He ZX, et al. Efficacy and safety of tofacitinib combined with methotrexate in the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Heliyon 2023;9(5):e15839.
[Crossref] [Google Scholar] [PubMed]
- Jones G, Nash P, Hall S. Advances in rheumatoid arthritis. Med J Australia 2017;206(5):221-4.
- Guagnano MT, D’Angelo C, Caniglia D, di Giovanni P, Celletti E, Sabatini E, et al. Improvement of inflammation and pain after three months’ exclusion diet in rheumatoid arthritis patients. Nutrients 2021;13(10):3535.
[Crossref] [Google Scholar] [PubMed]
- Geng M, Xu K, Meng L, Xu J, Jiang C, Guo Y, et al. Up-regulated DERL3 in fibroblast-like synoviocytes exacerbates inflammation of rheumatoid arthritis. Clin Immunol 2020;220:108579.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Gao Z, Chao S, Lu W, Zhang P. Transdermal delivery of inflammatory factors regulated drugs for rheumatoid arthritis. Drug Deliv 2022;29(1):1934-50.
[Crossref] [Google Scholar] [PubMed]
- Ma Y, Zhang J, Yu H, Zhang Y, Zhang H, Hao C, et al. Traditional Chinese medicine Rhodiola sachalinensis Borissova from Baekdu Mountain (RsBBM) for rheumatoid arthritis: Therapeutic effect and underlying molecular mechanisms. Molecules 2022;27(18):6058.
[Crossref] [Google Scholar] [PubMed]
- Liu H, Wang G, Wang J, Wang T, Tian J, Wang L, et al. Bushentongluo recipe (BSTL) attenuates bone destruction by inhibiting NF-κB/RANK/RANKL pathway in Collagen-Induced Arthritis (CIA) rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2021;37(3):205-11.
[Google Scholar] [PubMed]
- Huang Y, Lin S, Zhan F, Xiao L, Zhan Y, Wang R. OX40-Fc fusion protein alleviates PD-1-Fc-aggravated rheumatoid arthritis by inhibiting inflammatory response. Comput Math Methods Med 2022;2022.
[Crossref] [Google Scholar] [PubMed]
- Wei L, Sun Y, Kong XF, Zhang C, Yue T, Zhu Q, et al. The effects of dopamine receptor 2 expression on B cells on bone metabolism and TNF-α levels in rheumatoid arthritis. BMC Musculoskeletal Disord 2016;17:352.
[Crossref] [Google Scholar] [PubMed]
- Guo C, He L, Hu N, Zhao X, Gong L, Wang C, et al. Aconiti lateralis Radix Praeparata lipid-soluble alkaloids alleviates IL-1β-induced inflammation of human fibroblast-like synoviocytes in rheumatoid arthritis by inhibiting NF-κB and MAPKs signaling pathways and inducing apoptosis. Cytokine 2022;151:155809.
[Crossref] [Google Scholar] [PubMed]
- Watanabe R, Hashimoto M. Perspectives of JAK inhibitors for large vessel vasculitis. Front Immunol 2022;13:881705.
[Crossref] [Google Scholar] [PubMed]
- Hu L, Liu R, Zhang L. Advance in bone destruction participated by JAK/STAT in rheumatoid arthritis and therapeutic effect of JAK/STAT inhibitors. Int Immunopharmacol 2022;111:109095.
[Crossref] [Google Scholar] [PubMed]
- Kotake S, Yago T, Kobashigawa T, Nanke Y. The plasticity of Th17 cells in the pathogenesis of rheumatoid arthritis. J Clin Med 2017;6(7):67.
[Crossref] [Google Scholar] [PubMed]
- Abimannan T, Peroumal D, Parida JR, Barik PK, Padhan P, Devadas S. Oxidative stress modulates the cytokine response of differentiated Th17 and Th1 cells. Free Radical Biol Med 2016;99:352-63.
[Crossref] [Google Scholar] [PubMed]