- *Corresponding Author:
- Hong Li
Department of Hematology, The First Hospital of Shanxi Medical University, Taiyuan, Shanxi Province 030001, China
E-mail: 19511428208@163.com
| This article was originally published in a special issue,“Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “372-383” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
There is controversy about the changes of intestinal and respiratory microbiota characteristics in patients with bronchopulmonary dysplasia and some studies have reported inconsistent or even contrary results. We performed a meta-analysis to explore the characteristics of the respiratory tract and gut microbiota in preterm infants with bronchopulmonary dysplasia. A complete search of the database (last updated on September 1, 2023) was conducted based on the preferred reporting project statement for systematic review and meta-analysis to identify eligible case-control studies that tested the gut and respiratory tract microbiota of patients with bronchopulmonary dysplasia. The results of meta-analysis were expressed as effect size and 95 % confidence interval. The bias control was assessed using Newcastle-Ottawa scale and funnel plot analysis. Seven studies (Newcastle-Ottawa scale score range from 6-8) were included, three studies (n=76, observation group n=36/control group n=40) collected intestinal microbial samples, and four studies (n=98, observation group n=46/control group n=52) collected respiratory tract microbial samples. The results showed that the richness and diversity of respiratory tract and intestinal microbial communities were decreased in bronchopulmonary dysplasia patients. In this meta-analysis, we analyzed the characteristics of respiratory tract and intestinal microbiota in premature infants with bronchopulmonary dysplasia, and found that the abundance and diversity of respiratory tract and intestinal microbiota in bronchopulmonary dysplasia patients were reduced. The intestinal microflora of Firmicutes, Actinobacteria, Veillonella and Escherichia/ Shigella in premature infants with bronchopulmonary dysplasia increased. Proteobacteria, Bacteroidetes, Cyanobacteria, Enterococcus, Enterobacteriaceae and Streptococcus were reduced. Firmicutes increased in the respiratory tract of premature infants with bronchopulmonary dysplasia, while Proteobacteria, Bacteroidetes and Cyanobacteria decreased. In addition, the characteristics of respiratory microbiome in premature infants with bronchopulmonary dysplasia may be affected by region or race.
Keywords
Bronchopulmonary dysplasia, respiratory distress syndrome, trauma, hypertension, inflammation
Bronchopulmonary Dysplasia (BPD) is the most common chronic lung disease of preterm infants[1], and preterm infants with BPD may experience various long-term lung complications, such as asthma, respiratory infections, and low exercise capacity[2-7]. It is most common in premature infants who have received mechanical ventilation and supplemental oxygen for a long time[8,9]. Premature infants may develop respiratory failure due to different reasons, such as Respiratory Distress Syndrome (RDS) and Acute Lung Injury (ALI), and people have to resort to mechanical ventilation and other means to meet their strong ventilation and oxygen requirements[10]. BPD is characterized by inflammation, alveolar simplification, pulmonary microvascular malformation and pulmonary hypertension[11]. Studies have pointed out that longterm assisted ventilation may lead to chronic tissue damage of the respiratory tract[12]. Long-term high oxygen in the lung due to mechanical ventilation is believed to lead to inflammation, destruction of blood vessels and alveoli, and is one of the important causes of BPD[13]. The pathogenesis of BPD is thought to be multifactorial, and it is associated with several prenatal and postnatal factors, including preterm birth, mechanical trauma, oxygen poisoning, infection or inflammation, growth restriction and genetic predisposition[14].
Microorganisms play a crucial role in maintaining the balance of the human immune response, building barriers, and influencing the development of neonatal diseases[15]. There is growing evidence that dysregulation of the microbiota may be involved in lung inflammation and contribute to the development of BPD. The study of Ryan et al.[16] analyzed the influence of the composition of intestinal flora on the expression of systemic immune genes and found that the fecal samples of Escherichia coli and Shigella in children with BPD significantly increased compared with normal infants. In addition, studies have pointed out that the imbalance of respiratory microflora may participate in the pathogenesis of BPD and may be related to the severity of BPD[17,18]. Therefore, it is necessary to explore the intestinal and respiratory microbial characteristics of BPD patients as a new entry point for future diagnosis and treatment.
At present, there is controversy about the characteristics of intestinal and respiratory microbiota changes in BPD patients, and some studies have reported inconsistent or even contrary results. Therefore, we conducted a meta-analysis to explore the characteristics of respiratory and gut microbiota in preterm infants with BPD.
Materials and Methods
This article has been reported in accordance with the Preferred Reporting Project and the PRISMA2009 checklist as stated in the Systematic Review and Meta-Analysis (PRISMA) and has been registered with the PROSPERO Registry (CRD42023471852) [19]. We used computers to search all relevant English literature in PubMed, Web of Science and Embase databases from their establishment to September 1, 2023 (regardless of region or race). The search terms are as follows, BPD (Medical Subject Headings (MeSH) terms), lung dysplasia, BPD, dysplasia, BPD, 16 s. The search mode is formed by combining MeSH subject words with free words. Table 1 shows the search process on PubMed. We also searched the literature manually with the help of related articles functions and reference lists.
| Search | PubMed |
|---|---|
| #1 | Bronchopulmonary dysplasia (MeSH Terms) |
| #2 | (Bronchopulmonary dysplasia) or (lung dysplasia) or (BPD) or (dysplasia, bronchopulmonary) or (bronchopulmonary dysplasia) |
| #3 | (#1) or (#2) |
| #4 | 16S |
| #5 | (#3) and (#4) |
Table 1: Article Retrieval Process Table.
Research selection:
All studies that explored differences in gut/respiratory microbiota between preterm BPD and control preterm infants were included in this systematic review and meta-analysis.
Inclusion criteria: Original full-text publications; confirmed BPD; the gestational age and sex ratio of the children in the control group and the BPD group were basically similar; available and sufficient data (sample size, mean and standard deviation or any data that can be converted) to calculate the Effect Size (ES) of the two groups; the study included any of the following measures of intestinal/respiratory microbiome comparison between BPD infants and control infants (1 d postnatal and sampling time ≤7 d) and Alpha (α) diversity index (Shannon and Observed OTUs), phylum level population difference and genus level population difference were included.
Exclusion criteria: Case reports, hypotheses, book chapters, conference abstracts, animal or cell experiments, reviews; there are other non-BPD complications; studies of additional interventions for participating infants and extraction of microbiota outside the gut/respiratory tract were excluded from this study.
Data extraction:
Researchers are responsible for selecting studies and extracting data according to pre-set exclusion criteria, and any differences are discussed and ultimately resolved by both parties. When additional data or data transformation is needed, contact the study authors via email for assistance. When the authors did not reply, the standard statistical formula was used for data conversion. If the required data cannot be obtained from the authors, the study is excluded. We also collected the following characteristic information about the included studies. The first author; nationality; year of publication; gestational age; maternal antibiotic treatment; antenatal steroids; cesarean section; duration of parenteral nutrition; duration of mechanical ventilation; apgar 1 min; apgar 5 min and the above indicators to be studied
Quality assessment:
To assess the risk of bias in each study, the Newcastle- Ottawa Scale (NOS) was used by two researchers. Any differences that arise are discussed and finally resolved by the two researchers. NOS is widely used to assess the quality of non-randomized studies in systematic reviews/meta-analyses. Using the tool, study quality was assessed based on eight items divided into three groups; study group selection, group comparability (comparability can be given up to two stars), and exposure or outcome determination for case control or cohort studies. A star rating system was used to rate the quality of included studies on a scale from 0 (low quality) to 9 (high quality).
Statistical analysis:
We used Engauge Digitizer to extract data, representing all bacterial abundance data as mean (x̄ ) and Standard Deviation (SD), and performed a metaanalysis using STATA/SE 15.0 (StataCorp, Texas, USA) with a Meta module for Windows. ES was used to evaluate the α diversity of the BPD group and the control group and the abundance of different strains, with a confidence interval of 95 %. Chi-square (χ2) and I2 tests were used for included references. When I2<50 % and p>0.1, there was no significant heterogeneity in the study and fixed effect model could be used for analysis. Otherwise, it indicates that there is significant heterogeneity in the study and the random effects model is used for analysis. To assess the risk of publication bias, a funnel plot was performed for intestinal/respiratory microbiota of different genera, where publication bias would result in an asymmetric funnel plot.
Results and Discussion
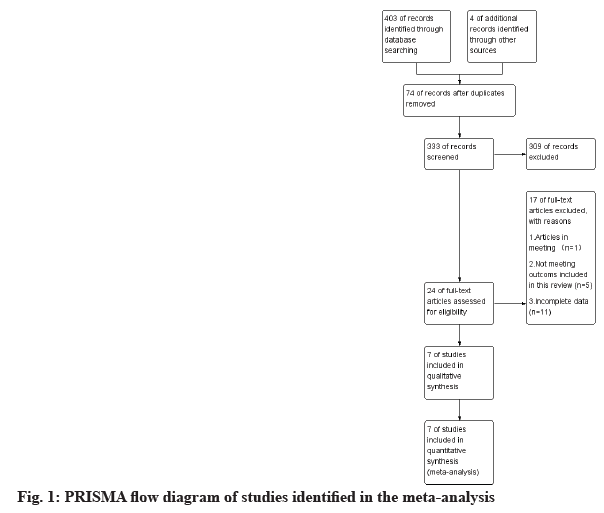
A total of 403 literatures were retrieved according to the pre-determined search strategy, as shown in fig. 1. Four of additional records identified through other sources, 74 duplicates were deleted, and the remaining 333 were screened by title and abstract review. A total of 309 articles were excluded because the type of study did not meet the requirements, animal or cell experiments, or were not relevant to this study. Among the remaining 24 articles, 17 articles were excluded for the following reasons; articles in meeting (n=1); not meeting outcomes included in this review (n=5) and incomplete data (n=11). Finally, a total of 7 literatures were included[20-26]. Additional examination of the references listed in these studies did not provide any additional eligible studies.
The characteristics of the included studies are shown in Table 2 and Table 3. There were 7 literatures (n=174, n=82 in the observation group/92 in the control group)[20-26], 3 literatures (n=76, n=36 in the observation group/40 in the control group) collected intestinal microbial samples[20,21,26], and 4 studies (n=98, observation group n=46/control group n=52) respiratory microbial samples[22-25], 16S ribosomal Ribonucleic Acid (16S rRNA) sequencing was used in all studies. Four articles were conducted in China (including one in Taiwan, China)[20,21,25,26], and the other three were from the United States[22], Australia[23] and Italy[24].
| Author | Country | Year | Gestational age (week) | Materials and methods | BPD (male) | Control (male) | BPD (n) | Control (n) | Richness and diversity |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al.[20] | China | 2021 | 27.5 (27.0-28.75)/27.5 (27.0-28.75) | Faecal, 16S rRNA sequencing | 6 | 7 | 8 | 10 | observed OTUs/ACE/Shannon index |
| Li et al.[21] | China | 2022 | (26-35) w | Faecal, 16S rDNA sequencing | - | - | 10 | 10 | Observe/ACE/Shannon index/Chao1/Simpson |
| Lohmann et al.[22] | USA | 2014 | 26.2±1.9/28.9±1.4 | Tracheal aspirates, 16S rDNA sequencing | 3 | 7 | 10 | 12 | observed OTUs/Shannon index |
| Selway et al.[23] | Australia | 2023 | 26.4 (±1.7) | Buccal swabs and tracheal aspirates, 16S rDNA sequencing | - | - | 20 | 20 | observed/Faith |
| Tirone et al.[24] | Italy | 2022 | 26.5±1.5/26.5±1.5 | Bronchoalveolar Lavage Fluid (BALF), 16S rDNA sequencing | 6 | 7 | 8 | 15 | Shannon index |
| Xu et al.[25] | China | 2022 | 30.28±2.22/31.57±0.61 | Tracheal aspirates, 16S rDNA sequencing | 4 | 4 | 8 | 5 | Shannon index |
| Zhang et al.[26] | China | 2022 | 27.4±1.5/29.5±0.9 | Faecal,16S rDNA sequencing | 11 | 10 | 18 | 20 | Shannon index |
Table 2: Characteristics of the Included Studies in the Meta-Analysis
| Author | Maternal antibiotic treatment | Antenatal steroids BPD/control | Cesarean section BPD/control | Duration of mechanical ventilation (days) |
|---|---|---|---|---|
| Chen et al.[20] | 0 | 3/5 | 7/7 | - |
| Li et al.[21] | - | - | - | - |
| Lohmann et al.[22] | - | - | 8/11 | - |
| Selway et al.[23] | - | - | - | - |
| Tirone et al.[24] | 3/6 | 8/13 | 6/14 | 20.07±26.91/1.02±1.98 |
| Xu et al.[25] | 1/1 | 6/3 | 5/5 | 25.83±8.96/6.17±1.88 |
| Zhang et al.[26] | 6/5 | 12/13 | 14/17 | 32.5±17.0/12.9±7.6 |
Table 3: Characteristics of the Included Studies in the Meta-Analysis
OS was used for quality evaluation of the included studies, as shown in Table 4. The overall quality was high (7 marks in 3 studies, 6 marks in 2 studies, 8 marks in 2 studies, mean±SD: 7±0.82). No studies were excluded due to low NOS scores.
| First author | Selectiona | Comparabilityb | Exposurec | Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | A | B | C | ||||
| Chen et al.[20] | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | △ | 8 |
| Li et al.[21] | ▲ | ▲ | ▲ | ▲ | △ | △ | ▲ | ▲ | △ | 6 |
| Lohmann, et al.[22] | ▲ | ▲ | ▲ | ▲ | ▲ | △ | ▲ | ▲ | △ | 7 |
| Selway, et al.[23] | ▲ | ▲ | ▲ | ▲ | ▲ | △ | ▲ | ▲ | △ | 7 |
| Tirone, et al.[24] | ▲ | ▲ | △ | ▲ | ▲ | △ | ▲ | ▲ | △ | 6 |
| Xu et al.[25] | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | △ | 8 |
| Zhang et al.[26] | ▲ | ▲ | ▲ | ▲ | ▲ | △ | ▲ | ▲ | △ | 7 |
Note: (a): Study population selection; (b): Comparability of cases and controls; (c): Measurement of exposure factors; (1): Is the case definition adequate; (2): Representativeness of the cases; (3): Selection of controls; (4): Definition of controls; (A): Ascertainment of exposure; (B): Same method of ascertainment for cases and controls and (C): Nonresponse rate
Table 4: Quality Assessment of Included Studies Using the Nos (Case-Control Studies)
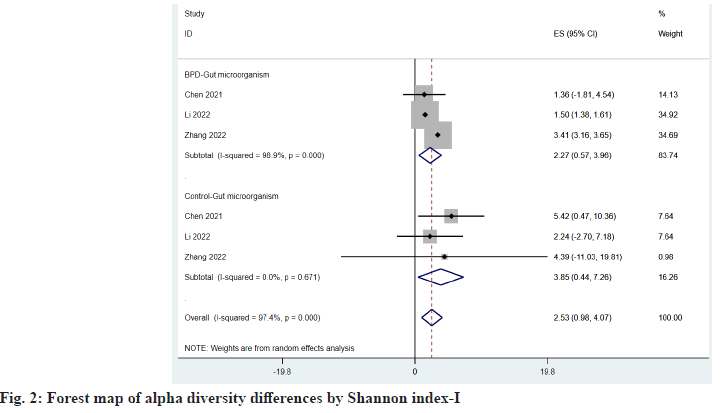
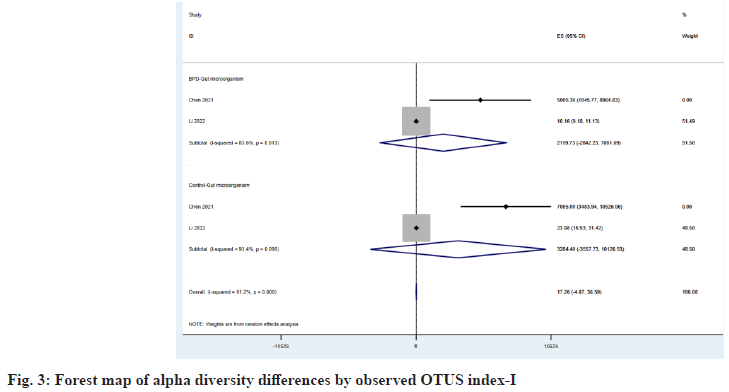
The α diversity index reflects the abundance and diversity of microbial communities in a specimen. A total of three observed studies provided the Shannon index[20,21,26], and two observed Operational Taxonomic Units (OTUs) index provided the Observed OTUs index[20,21], which compared the ES of the two indexes between the BPD group and the control group as shown in fig. 2 and fig. 3, respectively being 2.27 (95 % Confidence Interval (CI): 0.57, 3.96) vs. 3.85 (95 % CI: 0.44, 7.26) and 2119.73 (95 % CI: -2842.23, 7081.69) vs. 3284.40 (95 % CI: -3557.73, 10126.53), we found that microbial abundance and diversity were reduced in children with BPD.
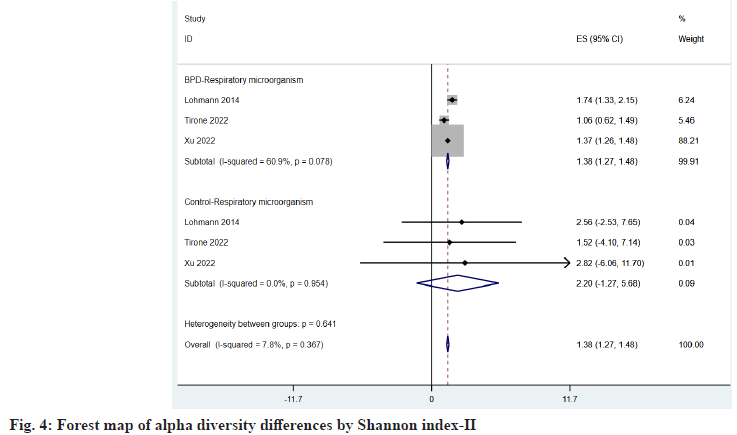
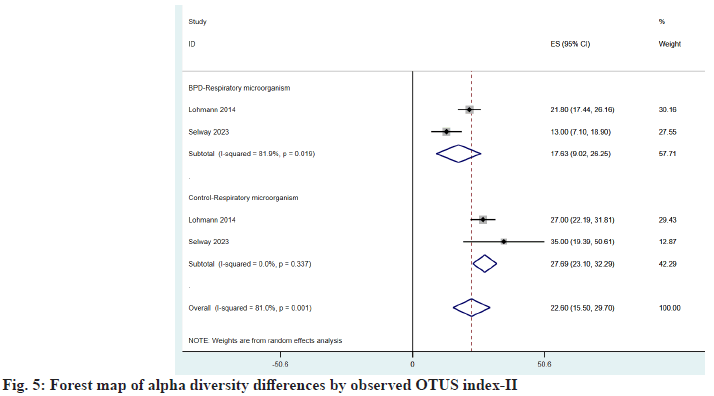
Respiratory microorganisms three of the articles included in the study provided the Shannon index[22,24,25], and two observed OTUs index[22,23], and the same conclusion was drawn after comparison, as shown in fig. 4 and fig. 5, which were 1.38 (95 % CI: 1.27, 1.48) vs. 2.20 (95 % CI: -1.27, 5.68) and 17.63 (95 % CI: 9.02, 26.25) vs. 22.60 (95 % CI: 15.50, 29.70), found that microbial abundance and diversity were reduced in children with BPD.
Among the included articles, two studies each analyzed the level changes of intestinal microflora[20,26] and respiratory microflora of BPD patients[22,25], respectively. These include Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria and Cyanobacteria.
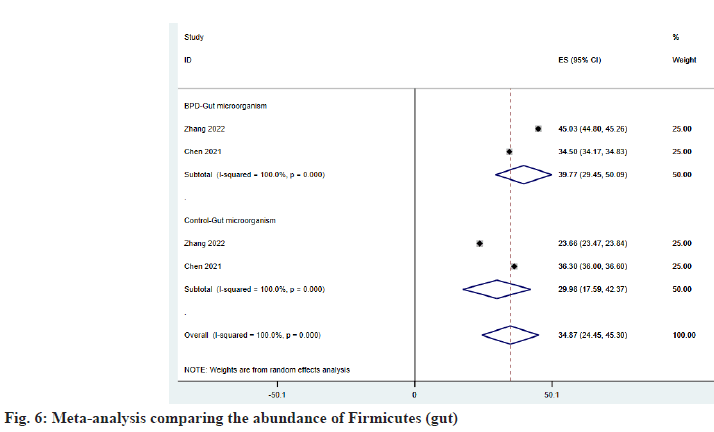
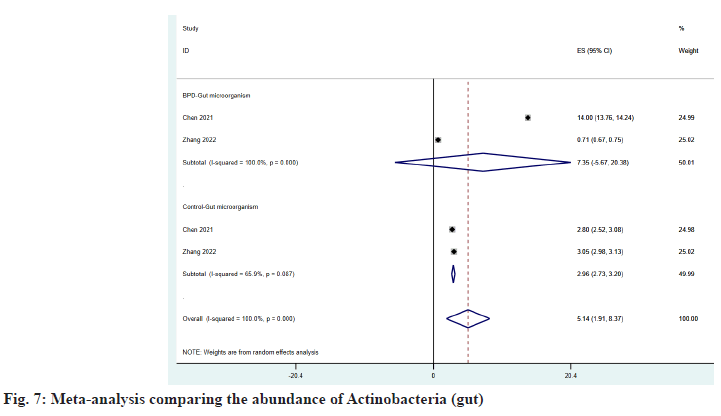
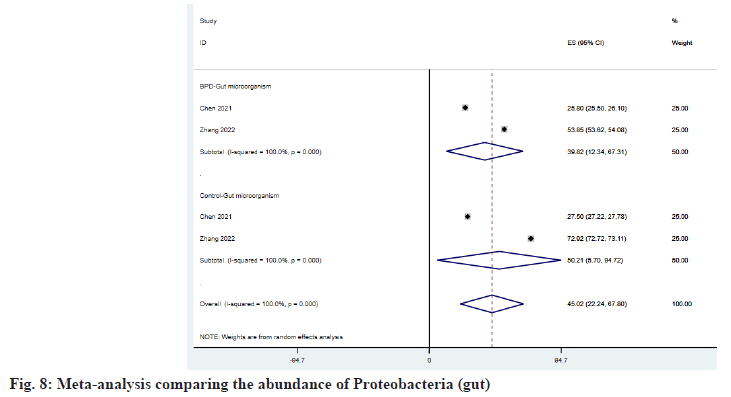
As shown in fig. 6 and fig. 7, Firmicutes and Actinobacteria have increased in the intestine of patients with BPD, and ESBPD vs. ES control is 39.77 (95 % CI: 29.45, 50.09) vs. 34.87 (95 % CI: 24.45, 45.30) and 7.35 (95 % CI: -5.67, 20.38) vs. 2.96 (95 % CI: 2.73, 3.20), while Proteobacteria was reduced in the intestine of patients with BPD, as shown in fig. 8. ESBPD vs. ES control was 39.82 (95 % CI: 12.34, 67.31) vs. 50.21 (95 % CI: 5.70, 94.72). In addition, Chen et al.[20] also found in his study that the average abundance of Bacteroidetes and Cyanobacteria in the gut of BPD patients was lower than that of the control group, 16.3 % vs. 21 % and 1.9 % . 2.6 % respectively.
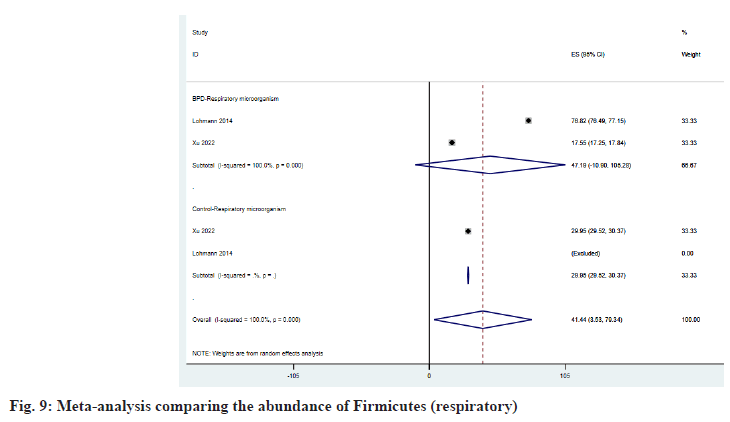
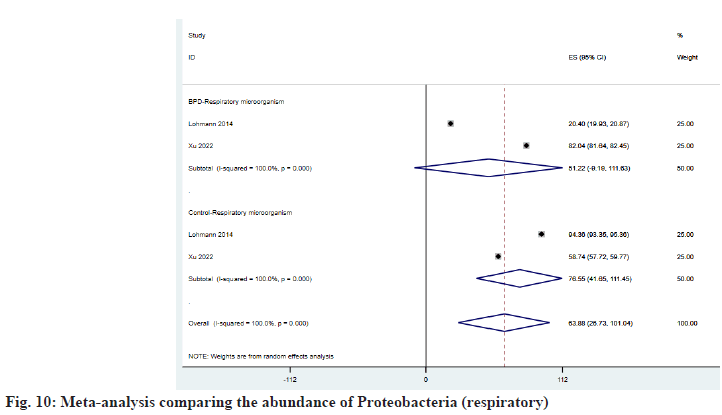
As shown in fig. 9, Firmicutes increased in the respiratory tract of BPD patients and ESBPD vs. . ES control was 47.19 (95 % CI: -10.90, 105.28) vs. 29.95 (95 % CI: 29.52, 30.37). According to fig. 10, we can learn that Proteobacteria has decreased in the respiratory tract of patients with BPD, and the ESBPD vs. ES control is 51.22 (95 % CI: -9.19, 111.63) vs. 76.55 (95 % CI: 41.65, 111.45). Xu et al.[25] found in her study that Cyanobacteria was not detected in the respiratory tract of BPD patients, while the average abundance of Cyanobacteria in the control group was about 5.95 %. In addition, Xu et al.[25] also found that the proportion of Bacteroidetes in the control group (about 1.854 %) was higher than that of BPD group (0.206 %).
Among the included articles, two studies each analyzed the changes of intestinal microbiota and respiratory microbiota in BPD patients[21,26], respectively[22,25] including Streptococcus, Enterococcus, Veillonella, Escherichia/Shigella, Staphylococcus, Enterobacteriaceae and Stenotrophomonas.
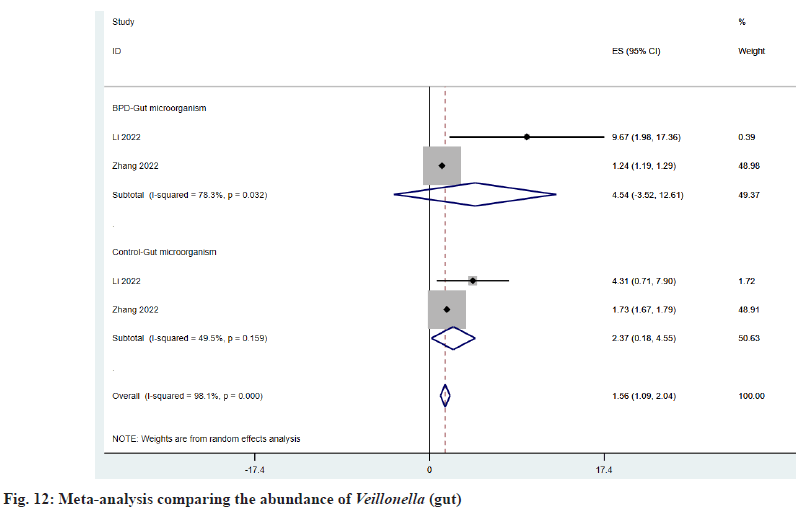
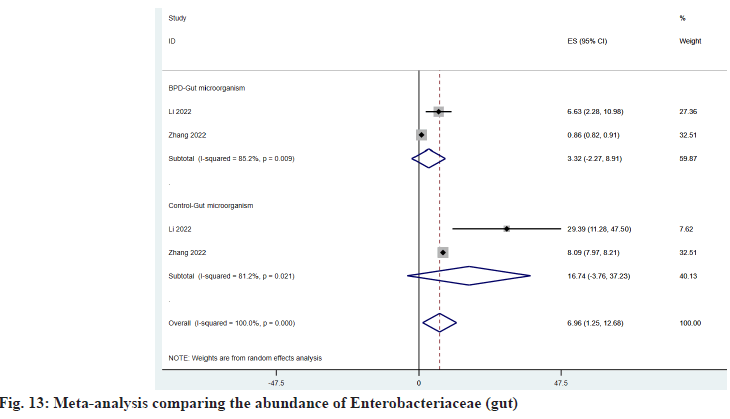
As shown in fig. 11, Enterococcus is decreased in the intestine of BPD patients, and ESBPD vs. ES control is 26.49 (95 % CI: -23.85, 76.82) vs. 34.74 (95 % CI: -9.73, 79.20). As shown in fig. 12, Veillonella was increased in the intestine of BPD patients, and ESBPD vs. ES control was 4.54 (95 % CI: -3.52, 12.61) vs. 2.37 (95 % CI: 0.18, 4.55). According to fig. 13, it can be seen that Enterobacteriaceae was reduced in the intestine of patients with BPD, and the ESBPD vs. ES control was 3.32 (95 % CI: -2.27, 8.91) vs. 16.74 (95 % CI: -3.76, 37.23). In addition, we also found that in the study of Li et al.[21], the average intestinal Streptococcus abundance in BPD patients decreased (0.33 % vs. 2.97066 %), while Escherichia/Shigella increased (49.841 % vs. 11.32311 %).
Regarding Staphylococcus, Lohmann et al.[22] and Xu et al.[25] showed opposite conclusions. Lohmann et al.[22] found that no Staphylococcus was detected in the control group. The average abundance of Staphylococcus in respiratory tract microbe samples of BPD group was 68.879 %. In the study of Xu et al.[25], the average abundance of Staphylococcus in the samples of children in the control group was about 19.1 %, while no Staphylococcus was detected in the BPD group. For Stenotrophomonas, Lohmann et al.[22] and Xu et al.[25] also proposed the opposite view. In Lohmann et al.[22] study, Stenotrophomonas was only detected in the samples of children in the control group (the average abundance was about 4.58 %). However, in the study of Xu et al.[25], Stenotrophomonas was only detected in the samples of children in the BPD group (the average abundance was about 20.02 %). At the same time, we found that in the study of Xu et al.[25], the average abundance of Streptococcus in BPD samples (about 0.1775 %) was higher than that of the control group (about 0.09 %). Enterobacteriaceae has not been detected in the control group, while the average abundance of Enterobacteriaceae in the BPD group is about 0.6815 %. In the study of Lohmann et al.[25], we also found that the average abundance of Escherichia/Shigella in BPD group (about 1.8 %) was lower than that in control group (about 9.435 %).
Due to the small number of studies involving microbial phyla level and genus level, we only used funnel plot to analyze publication bias for studies involving microbial α diversity. According to the funnel plot, none of the analyses showed publication bias.
To our knowledge, this is the first meta-analysis to look at changes in gut and respiratory microbiome in patients with BPD. This meta-analysis included seven studies that analyzed gut and respiratory microbiome changes in 82 BPD patients and 92 non-BPD control infants from four countries. Despite differences in gut and respiratory microbiota between individuals, this study provides evidence of overall changes in gut and respiratory microbiota occurring in patients with BPD.
More and more studies on the pneumo-intestinal axis theory have shown that intestinal microbes are related to lung homeostasis[27], while other studies have pointed out that respiratory microorganisms may be related to lung inflammation, especially BPD[28]. Shannon index and OTUS index can reflect the number of species detected in samples (i.e. richness) and their relative abundance distribution (i.e. evenness). In this study, we observed that the Shannon index and OTUS index of children in the BPD group were lower than those in the control group, indicating that the richness and diversity of microbial communities in the respiratory tract and intestinal tract were reduced in BPD patients, and such differences in alpha diversity may play a predictive role in the development of BPD.
Existing studies have pointed out that Actinobacteria, Proteobacteria, Bacteroidetes and Firmicutes are the four main microbial phyla in the intestinal tract of preterm infants[29]. According to the research we included, the microbial flora in the respiratory tract and intestinal tract of BPD patients is as follows; Bacteroidetes and Cyanobacteria decreased, and Actinobacteria increased in the intestinal tract of BPD patients. At the same time, our study also found that in BPD patients after birth (≤7 d), Firmicutes increased in both respiratory tract and intestinal tract (95 % CI, respectively), and Proteobacteria decreased. Many studies have suggested that the instability of intestinal microbial community structure is related to the increase in the abundance of Proteobacteria[30], and the increase in the abundance of Proteobacteria may also lead to the activation of inflammatory substances and cause respiratory tract injury[31], which is contrary to our findings. It is worth noting that, in Chen and Zhang study, Firmicutes decreased and Proteobacteria increased in the stool samples of BPD children from 14 d to 28 d after birth. We believe that when the sampling time was at the early stage of BPD development, the change of Proteobacteria in the community might not have started yet. Changes in the relative abundance of Proteobacteria in the intestinal and respiratory flora of BPD patients may predict the development of BPD.
At the genus level, we found that Enterococcus, Enterobacteriaceae and Streptococcus decreased in the intestinal tract of preterm infants with BPD, while in the respiratory tract of preterm infants with BPD, we found that Streptococcus and Enterobacteriaceae increased while Escherichia/Shigella decreased, and the differences in intestinal and respiratory microorganisms of premature infants with BPD still need to be further studied. We also found that Veillonella, Escherichia/Shigella were increased in the intestines of children with BPD. Rojas et al.[32] study pointed out that Veillonella ability to compete in the intestines would be increased when there was inflammation in the body. The inflammatory environment created during BPD helps Veillonella multiply in the gut. In addition, previous studies have found that Escherichia/Shigella overgrowth as a potential antigenic bacteria[33-35], which is consistent with our findings. The overgrowth of Escherichia/ Shigella is believed to trigger inflammasome activation and participate in pulmonary inflammation. The contradiction is concentrated in the study of Lohmann et al.[22] and Xu et al.[25] on the changes of respiratory tract microorganisms in children with BPD. Xu et al.[25] found that Staphylococcus decreased and Stenotrophomonas increased in the respiratory tract of children with BPD, while Lohmann et al.[22] experimental results were contrary. We think this may be due to ethnic or regional differences between the two studies that may have contributed to the differences in the structure of the two studies. Our study is the first to explore the microbiota characteristics of preterm infants with BPD through a meta-analysis and systematic review. We also divided them into intestinal and respiratory microbiota groups according to different sampling sites, trying to compare the changes of respiratory microbiota and intestinal microbiota in premature infants with BPD. There are some limitations to our study. First of all, the research on the microbiota characteristics of premature infants with BPD is still in its infancy, resulting in fewer studies to be included. Secondly, most studies have significant heterogeneity, which cannot be eliminated by using random effects model. In addition, this study did not take into account the impact of ethnic and regional differences on the research structure.
In this meta-analysis, we analyzed the characteristics of respiratory and intestinal microbiota in premature infants with BPD and found that the abundance and diversity of respiratory and intestinal microbiota in BPD patients were reduced. The intestinal microflora of Firmicutes, Actinobacteria, Veillonella and Escherichia/Shigella in premature infants with BPD increased. Proteobacteria, Bacteroidetes, Cyanobacteria, Enterococcus, Enterobacteriaceae and Streptococcus were reduced. Firmicutes increased in the respiratory tract of premature infants with BPD, while Proteobacteria, Bacteroidetes and Cyanobacteria decreased. In addition, the characteristics of respiratory microbiome in premature infants with BPD may be affected by region or race. Our research helps to define the characteristics of the respiratory and intestinal microbiota of BPD patients and provides a basis for the future treatment of BPD by regulating the microbiota balance.
Conflict of interests:
The authors declared no conflict of interests.
References
- Thébaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers 2019;5(1):78.
- Smith LJ, van Asperen PP, McKay KO, Selvadurai H, Fitzgerald DA. Reduced exercise capacity in children born very preterm. Pediatrics 2008;122(2):e287-93.
[Crossref] [Google Scholar] [PubMed]
- Ralser E, Mueller W, Haberland C, Fink FM, Gutenberger KH, Strobl R, et al. Rehospitalization in the first 2 years of life in children born preterm. Acta Paediatr 2012;101(1):e1-5.
[Crossref] [Google Scholar] [PubMed]
- Narang I, Rosenthal M, Cremonesini D, Silverman M, Bush A. Longitudinal evaluation of airway function 21 years after preterm birth. Am J Respir Crit Care Med 2008;178(1):74-80.
[Crossref] [Google Scholar] [PubMed]
- Filippone M, Bonetto G, Corradi M, Frigo AC, Baraldi E. Evidence of unexpected oxidative stress in airways of adolescents born very pre-term. Eur Respir J 2012;40(5):1253-9.
[Crossref] [Google Scholar] [PubMed]
- Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics 2006;118(1):108-13.
[Crossref] [Google Scholar] [PubMed]
- Broström EB, Thunqvist P, Adenfelt G, Borling E, Katz-Salamon M. Obstructive lung disease in children with mild to severe BPD. Respir Med 2010;104(3):362-70.
[Crossref] [Google Scholar] [PubMed]
- Northway Jr WH, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: Bronchopulmonary dysplasia. New Engl J Med 1967;276(7):357-68.
[Crossref] [Google Scholar] [PubMed]
- Poets CF, Lorenz L. Prevention of bronchopulmonary dysplasia in extremely low gestational age neonates: Current evidence. Arch Dis Child Fetal Neonatal Ed 2018;103(3):F285-91.
[Crossref] [Google Scholar] [PubMed]
- Omar SA, Abdul-Hafez A, Ibrahim S, Pillai N, Abdulmageed M, Thiruvenkataramani RP, et al. Stem-cell therapy for Bronchopulmonary Dysplasia (BPD) in newborns. Cells 2022;11(8):1275.
[Crossref] [Google Scholar] [PubMed]
- Pammi M, Lal CV, Wagner BD, Mourani PM, Lohmann P, Luna RA, et al. Airway microbiome and development of bronchopulmonary dysplasia in preterm infants: A systematic review. J Pediatr 2019;204:126-33.
[Crossref] [Google Scholar] [PubMed]
- Mohamed T, Abdul-Hafez A, Gewolb IH, Uhal BD. Oxygen injury in neonates: Which is worse? Hyperoxia, hypoxia or alternating hyperoxia/hypoxia. J Lung Pulm Respir Res 2020;7(1):4-13.
[Crossref] [Google Scholar] [PubMed]
- Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: Implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2007;292(5):L1073-84.
[Crossref] [Google Scholar] [PubMed]
- Davidson LM, Berkelhamer SK. Bronchopulmonary dysplasia: Chronic lung disease of infancy and long-term pulmonary outcomes. J Clin Med 2017;6(1):4.
[Crossref] [Google Scholar] [PubMed]
- Collado MC, Cernada M, Neu J, Pérez-Martínez G, Gormaz M, Vento M. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr Res 2015;77(6):726-31.
[Crossref] [Google Scholar] [PubMed]
- Ryan FJ, Drew DP, Douglas C, Leong LE, Moldovan M, Lynn M, et al. Changes in the composition of the gut microbiota and the blood transcriptome in preterm infants at less than 29 weeks gestation diagnosed with bronchopulmonary dysplasia. mSystems 2019;4(5):10-128.
[Crossref] [Google Scholar] [PubMed]
- Lal CV, Travers C, Aghai ZH, Eipers P, Jilling T, Halloran B, et al. The airway microbiome at birth. Sci Rep 2016;6(1):31023.
[Crossref] [Google Scholar] [PubMed]
- Wagner BD, Sontag MK, Harris JK, Miller JI, Morrow L, Robertson CE, et al. Airway microbial community turnover differs by BPD severity in ventilated preterm infants. PLoS One 2017;12(1):e0170120.
[Crossref] [Google Scholar] [PubMed]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg 2010;8(5):336-41.
[Crossref] [Google Scholar] [PubMed]
- Chen SM, Lin CP, Jan MS. Early gut microbiota changes in preterm infants with bronchopulmonary dysplasia: A pilot case-control study. Am J Perinatol 2021;38(11):1142-9.
[Crossref] [Google Scholar] [PubMed]
- Li Y, He L, Zhao Q, Bo T. Microbial and metabolic profiles of bronchopulmonary dysplasia and therapeutic effects of potential probiotics Limosilactobacillus reuteri and Bifidobacterium bifidum. J Appl Microbiol 2022;133(2):908-21.
[Crossref] [Google Scholar] [PubMed]
- Lohmann P, Luna RA, Hollister EB, Devaraj S, Mistretta TA, Welty SE, et al. The airway microbiome of intubated premature infants: Characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr Res 2014;76(3):294-301.
[Crossref] [Google Scholar] [PubMed]
- Selway CA, Collins CT, Makrides M, Sullivan TR. Variable preterm oral microbiome stabilizes and reflects a full-term infant profile within three months. Pediatr Res 2023:1-9.
[Crossref] [Google Scholar] [PubMed]
- Tirone C, Paladini A, de Maio F, Tersigni C, D’Ippolito S, di Simone N, et al. The relationship between maternal and neonatal microbiota in spontaneous preterm birth: A pilot study. Front Pediatr 2022;10:909962.
[Crossref] [Google Scholar] [PubMed]
- Xu Q, Yu J, Liu D, Tan Q, He Y. The airway microbiome and metabolome in preterm infants: Potential biomarkers of bronchopulmonary dysplasia. Front Pediatr 2022;10:862157.
- Zhang Z, Jiang J, Li Z, Wan W. The change of cytokines and gut microbiome in preterm infants for bronchopulmonary dysplasia. Front Microbiol 2022;13:804887.
[Crossref] [Google Scholar] [PubMed]
- Warner BB, Hamvas A. Lungs, microbes and the developing neonate. Neonatology 2015;107(4):337-43.
[Crossref] [Google Scholar] [PubMed]
- Thekkeveedu RK, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir Med 2017;132:170-7.
[Crossref] [Google Scholar] [PubMed]
- Gritz EC, Bhandari V. The human neonatal gut microbiome: A brief review. Front Pediatr 2015;3:17.
[Crossref] [Google Scholar] [PubMed]
- Shin NR, Whon TW, Bae JW. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 2015;33(9):496-503.
[Crossref] [Google Scholar] [PubMed]
- Li F, Ye J, Shao C, Zhong B. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: A systematic review and meta-analysis. Lipids Health Dis 2021;20(1):122.
[Crossref] [Google Scholar] [PubMed]
- Rojas-Tapias DF, Brown EM, Temple ER, Onyekaba MA, Mohamed AM, Duncan K, et al. Inflammation-associated nitrate facilitates ectopic colonization of oral bacterium Veillonella parvula in the intestine. Nat Microbiol 2022;7(10):1673-85.
[Crossref] [Google Scholar] [PubMed]
- Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in Nonalcoholic Steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013;57(2):601-9.
[Crossref] [Google Scholar] [PubMed]
- Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep 2015;5(1):8096.
[Crossref] [Google Scholar] [PubMed]
- Ozkul C, Yalinay M, Karakan T, Yilmaz G. Determination of certain bacterial groups in gut microbiota and endotoxin levels in patients with nonalcoholic steatohepatitis. Turk J Gastroenterol 2017;28(5):361-9.
[Crossref] [Google Scholar] [PubMed]