- *Corresponding Author:
- Wei Zhao
Department of Respiratory Medicine, Qiqihar Medical University, Second Affiliated Hospital, Qiqihar, Heilongjiang 161000, China
E-mail: zhao7667g@sina.com
| This article was originally published in a special issue, “Clinical Advancements in Life Sciences and Pharmaceutical Research” |
| Indian J Pharm Sci 2024:86(5) Spl Issue “261-272” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Ordinary acetylcysteine nebulization therapy requires doctors to manually control the nebulizer, which requires professional operation and is unsuitable for large-scale pneumonia prevention and control work. Therefore, this article aimed to explore the application of intelligent medicine combined with acetylcysteine nebulization inhalation in treating neonatal pneumonia, providing a theoretical basis for its clinical application. This article selected 50 newborns with pneumonia from Qiqihar Medical University, Second Affiliated Hospital, to ensure the representativeness and consistency of the sample. Fifty patients with neonatal pneumonia can be randomly divided into groups, with 25 cases in each group. It can ensure the randomness of grouping and reduce the bias of experimental results. In this study, the control group was treated with a single use of acetylcysteine nebulization inhalation. In contrast, the experimental group was treated with intelligent medical technology and acetylcysteine nebulization inhalation. Symptoms, fever, and degree of pulmonary inflammation before and after treatment can be observed. After treatment, the proportion of children with non-inflammation in the experimental and control groups was 46.66 % and 17.9 %, respectively. The proportion of children in the control group with non-inflammatory was 28.76 % lower than that in the experimental group with non-inflammatory. A more comprehensive and scientific evaluation of the application of intelligent medical technology in treating neonatal pneumonia can provide more personalized and efficient treatment plans for similar cases in the future and promote the application of medical technology in neonatal medicine.
Keywords
Intelligent healthcare, nebulization inhalation, neonatal pneumonia, acetylcysteine treatment, intelligent monitoring
Neonatal pneumonia is a lung infection that occurs within 28 d of birth. The traditional method of treating this infection involves antibiotics, which can have significant side effects on liver and kidney function. However, nebulized acetylcysteine inhalation has emerged as a promising alternative treatment in recent years. Acetylcysteine is an amino acid that contains sulfur and is useful in clearing toxins from the body and reducing lung infection symptoms. This amino acid is delivered to the lungs through nebulized inhalation, which has proven effective and safe treatment. Unlike traditional drugs, nebulized acetylcysteine has minimal side effects and can mitigate the toxic side effects of conventional treatments.
Liu et al.[1] conducted a research study to determine the safety and effectiveness of Bronchoalveolar Lavage (BAL) as a treatment for severe pneumonia in newborns. The study included 100 patients suffering from severe pneumonia, and 50 were randomly selected for the BAL and control groups. The study results showed that 70 % of patients in the BAL group met the invasive mechanical ventilation criteria upon admission, while only 30 % still needed invasive mechanical ventilation after BAL treatment. All patients had stable vital signs and experienced no significant adverse reactions during the gastric lavage. Respiratory Syncytial Virus (RSV) pneumonia is the primary cause of hospitalization and death in newborns globally and there is currently no specific clinical treatment for RSV infection. He et al.[2] aimed to evaluate the effectiveness and safety of interferon treatment for neonatal pneumonia. The study divided newborns with RSV pneumonia into a treatment group (126 cases) and a control group (160 cases). The treatment group received interferon treatment, while the control group received the same routine treatment. The study results showed that the treatment group experienced significant relief from symptoms such as coughing, shortness of breath, asphyxia during breastfeeding, extra peritoneal cyanosis, wet rales, and oxygen inhalation compared to the control group (p<0.05). In summary, interferon treatment effectively improved symptoms associated with neonatal RSV pneumonia and had minimal adverse reactions[2]. Healthcare professionals have recently administered colistin via intravenous injection and nebulization to treat Ventilator-Associated Pneumonia (VAP) caused by colistin-sensitive multidrug-resistant gram-negative bacteria. However, the ability of colistin to penetrate the lungs is limited. In a study by Hussain et al.[3] on treating neonatal pneumonia, the efficacy and safety of intravenous injection and colistin were compared. The results revealed that intravenous injection and nebulized colistin significantly improved clinical cure and microbial eradication rates.
VAP caused by more than one microorganism is not rare and could present some challenges. However, there is no relevant data on multi-microbial VAP in ventilated newborns. Wang et al.[4] study aims to investigate the clinical characteristics and outcomes of multi-microbial VAP in neonatal intensive care units. He compared all clinical features, treatment interventions, and outcomes of multi-microbial VAP and single microbial VAP episodes. He used multiple regression analysis to identify independent risk factors for treatment failure. The study found that multi-microbial VAP is more common in newborns with prolonged intubation time, late life, and potential bronchopulmonary dysplasia. Most clinical features of multi-microbial VAP are similar to those of singlemicrobial VAP. With the advancements in science, technology, and medical practices, the treatment for neonatal pneumonia has been consistently improving. However, there are still many challenges that need to be addressed at present.
Intelligent healthcare has become a crucial development trend in medicine. Acetylcysteine nebulization inhalation is a new treatment method gradually gaining recognition and becoming popular in the medical community. Bronchial asthma is a chronic inflammatory disease of the airways that has a heterogeneous nature. The type of inflammation, such as eosinophils and neutrophils, plays a crucial role in the disease. The study conducted by Ignatieva et al.[5] aimed to evaluate the effectiveness of respiratory inhalation of the standard therapeutic dose of ultrafine particle glucocorticoid thiamethoxam bromide along with inhaling 10 % acetylcysteine solution once a day for 10 consecutive days as the baseline treatment. The results showed a significant improvement in respiratory function and increased physical activity tolerance, with an effective rate of 93.3 %.
Qian et al.[6] conducted a study to analyze the effectiveness of budesonide nebulization inhalation in treating neonatal pneumonia. The study included 36 hospitalized children with severe neonatal pneumonia, who were divided into two groups; a reference group and an experimental group. Each group had 18 cases. The control group received routine symptomatic treatment, while the experimental group received budesonide nebulized inhalation and routine symptomatic treatment. The results showed that the experimental group’s treatment was significantly better than the control group’s (p<0.05). Nebulized budesonide inhalation reduces inflammation in children and maximizes the therapeutic effects of treatment for newborn pneumonia. This finding is significant for clinical advancement. Fiberoptic bronchoscopy can be used to assess refractory Mycoplasma pneumoniae in its early stages, which can help reduce airway obstruction and prevent the development of complications.
Zhang et al.[7] developed an intelligent system that analyzes clinical data on risk factors to detect and assess Mycoplasma pneumoniae early. He created a model of the risk variables and performed statistical analysis on the risk elements that emerged. Cui et al.[8] conducted a study to examine the difference in expression levels between 58 children with pneumonia and 58 healthy children. Using this data, he created a model to monitor the impact of induction on cell viability and death. Additionally, he identified the virus present in the serum of newborns with pneumonia, which is crucial for monitoring and controlling infant pneumonia. This study will help determine pneumonia’s relative expression in newborns and young children, and develop effective treatments. The administration of acetylcysteine has become more streamlined and convenient with the advancements in modern medical technology.
Materials and Methods
Selection of subjects:
This study selected 50 newborns with pneumonia from Qiqihar Medical University, the Second Affiliated Hospital.
Inclusion criteria: Newborns born within 28 d; patients who display clinical symptoms such as shortness of breath and fever, along with imaging features of pneumonia, meet the criteria for diagnosis and a positive blood culture indicates the existence of a bacterial infection.
Exclusion criteria: Premature birth; chronic diseases combined with other chronic diseases, such as congenital heart disease, congenital immunodeficiency, etc., there may be obvious congenital abnormalities that are related to the immune or respiratory system; interpretation of pneumonia research results may be compromised by other infections and unable to provide informed consent.
It is important to follow certain standards in research to ensure consistency and prevent factors that may affect the accuracy of results. However, the patient or guardian failed to provide an informed consent form, an important requirement for conducting research involving human subjects. In this study, 50 newborns with pneumonia were randomly divided into two groups. One group was the control group and received a single acetylcysteine nebulization inhalation treatment, while the other group was the experimental group. The experimental group received intelligent medical treatment in addition to acetylcysteine nebulization inhalation therapy for 8 d. The aim was to observe the symptoms before and after treatment to analyze its effectiveness.
Characteristics of acetylcysteine:
Acetylcysteine is a non-essential amino acid that is derived from cysteine. It contains one sulfurcontaining double bond, which is cysteine and the other is pyruvate. Acetylcysteine is an important metabolite that plays a role in protein synthesis and various life processes. In these processes, glutathione is a critical enzyme that maintains the body’s oxidation-reduction balance. Fig. 1, shows the general structure of acetylcysteine.
Acetylcysteine nebulized inhalation is a targeted therapy with high bioavailability and convenient personalized administration. It has been widely used to treat diseases such as neonatal pneumonia.
One of the main advantages of acetylcysteine nebulization inhalation is its ability to provide local effects and accomplish focused therapy. The nebulized liquid made with acetylcysteine can be inhaled directly through the respiratory system to treat lung tissue directly[9,10]. Through local delivery, medication concentrations in the lungs can be increased while systemic side effects can be decreased. This helps to reduce oxidative damage and pulmonary inflammation. The effective concentration of medications in target tissues is known as bioavailability. Nebulized inhalation of acetylcysteine can increase this concentration. Unlike oral administration, this drug bypasses the absorption period of the gastrointestinal tract and directly enters the lungs through the respiratory tract. This prevents the breakdown of gastric acid, enzymes, and other factors that could lead to drug breakdown failure[11,12].
Mechanism of action:
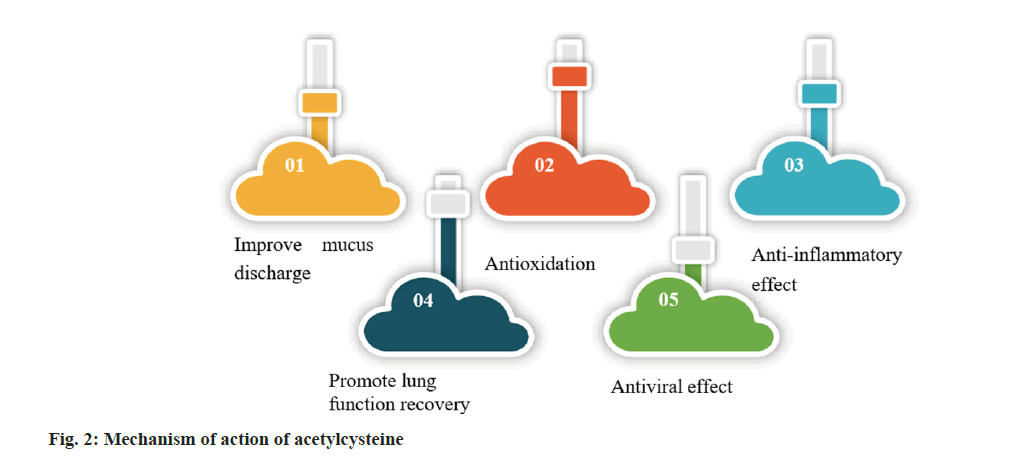
Acetylcysteine has shown promising results in treating neonatal pneumonia. Fig. 2, shows its mechanism of action.
Improve mucus discharge: Acetylcysteine is known to increase the concentration of reducing power in the body, which can help break down mucus. In cases of neonatal pneumonia, excessive accumulation of mucus can lead to respiratory problems, such as increased resistance to breathing. Acetylcysteine can help by reducing the viscosity of mucus, making it easier to clear out of the respiratory system. This, in turn, can reduce airway resistance and improve overall respiratory efficiency.
Antioxidant effect: Acetylcysteine has strong antioxidant effects. Neonatal pneumonia is a common infectious disease, and its pathogenesis may be closely related to inflammatory reactions[13,14]. Acetylcysteine is an antioxidant that can eliminate harmful molecules, safeguard the integrity of cell membranes, reduce oxidative damage and prevent pathogen damage to lung tissue. It plays a crucial role in maintaining redox homeostasis in lung tissue and contributes to glutathione synthesis. Glutathione is a significant antioxidant that helps sustain a reducing environment within cells and delays oxidative damage.
Anti-inflammatory effects: Acetylcysteine helps synthesize glutathione and maintains redox equilibrium in lung tissue, delaying oxidative damage and preserving a reducing environment in cells.
Promote lung function recovery: Acetylcysteine can enhance alveolar surface tension and improve lung function, leading to better patient recovery by preventing oxidative stress and inflammation associated with pneumonia[15,16].
Antiviral effect: Acetylcysteine has the potential to be an antiviral medication. It can prevent viruses from invading, spreading, and replicating. Moreover, acetylcysteine can strengthen the immune system and prevent viral infections. This study establishes a theoretical foundation for using acetylcysteine in treating pneumonia and other infectious diseases. It is expected to provide patients with more comprehensive treatment options and better outcomes.
Intelligent healthcare combined with acetylcysteine nebulization inhalation:
Assessment and monitoring of pediatric patients: Neonatal pneumonia is an infection or inflammation in newborns occurring within 28 d after birth. It is caused by various factors such as bacteria, viruses and fungi[17,18]. The main pathogens causing neonatal pneumonia are bacteria, such as Streptococcus pneumoniae, Staphylococcus aureus, Escherichia coli and Klebsiella pneumoniae. Viral infections are mostly caused by RSV, adenoviruses and influenza viruses[19,20].
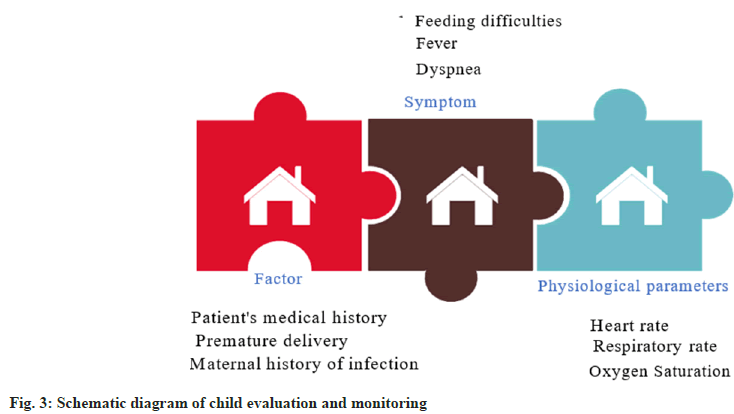
Intelligent healthcare is a field that leverages advanced computer technology and artificial intelligence to collect, process, analyze, and diagnose large volumes of medical data. This results in personalized medical services that are more accurate and effective for patients. Our paper focuses on using intelligent healthcare and acetylcysteine nebulization inhalation therapy for neonatal pneumonia. It evaluates and monitors the effectiveness and safety of pediatric patients, which is crucial to ensure patient safety and efficacy. A schematic diagram of the pediatric evaluation and monitoring process is illustrated in fig. 3.
Fig. 3, illustrates that evaluating and monitoring pediatric patients involves collecting medical history, including factors such as premature birth and maternal infection history, and observing symptoms such as breathing difficulties, fever, and feeding issues. Moreover, medical teams monitor physiological parameters, including heart rate, respiratory rate, and oxygen saturation. This helps them to make accurate and personalized treatment plans based on realtime and comprehensive data. Intelligent medical systems can record various physiological indicators of patients in real-time and analyze them, providing more information for patients[21,22]. Medical staff can adjust nebulization dosage, concentration and duration to achieve the best therapeutic effect for pediatric patients. The intelligent monitoring system provides real-time feedback, reminders, and other functions to help medical personnel detect abnormal conditions promptly, take timely response measures, and ensure the safety of children’s lives. Evaluating and monitoring pediatric patients requires close cooperation among medical staff to ensure the accuracy and timeliness of each stage of the treatment process.
By integrating intelligent medical technology, medical teams can access more data support, leading to safer and more personalized diagnosis and treatment plans for children. This improves the success rate of treatment for children and enhances the quality of rehabilitation for children [23,24].
Individualized adjustment of nebulization therapy plan:
Healthcare professionals can use a smart monitoring system to keep track of a child’s condition. This system monitors physiological parameters such as heart rate, oxygen saturation, and breathing rate. Based on the patient’s specific situation, medical staff can adjust the spray dose, concentration, and course of treatment. Acetylcysteine is necessary for mild patients, but for severe patients, a higher dosage is required[25,26]. The spray length can also be chosen based on the patient’s condition and tolerance. Smart medicines can ensure that children receive the best treatment outcomes during the diagnostic and treatment phase by providing healthcare professionals with real-time, precise diagnosis and treatment plans, and quick feedback.
Applications:
Drug management system: Artificial intelligence and a drug knowledge base form the foundation of an intelligent drug management system. In order to provide patients with the best possible therapeutic outcome, it can assist doctors in giving medication more precisely and individually, as well as in keeping an eye on the side effects and medication process.
Intelligently adjust the dosage of nebulized acetylcysteine based on the patient’s physiological characteristics and treatment response.
Fnew=Fold+k1×HZ+ k2×LY (1)
Where, Fnew represents the adjusted nebulized dose of acetylcysteine; Fold is the baseline dose; Hz represents the patient’s physiological characteristics and LY represents the treatment response. This information generates visual reports for medical staff, which can help them analyze and guide personalized patient treatment. The system can immediately alarm if the patient experiences abnormal conditions. This allows medical staff to respond quickly and adjust the treatment plan. The system also monitors the patient’s medication and efficacy, intelligently calculating abnormal condition indicators and generating corresponding warning levels.
Levelj+k3×abnormal (2)
Where, Levelj is alarm level indicates abnormal situations and is used to alert medical professionals. With the help of intelligent medication management systems, acetylcysteine nebulization inhalation and intelligent healthcare have become possible. This system allows for faster and more accurate modifications to treatment plans, enhancing the level of personalization in patient care. This technology improves patient safety, convenience, and scientific accuracy by combining real-time monitoring features with medical data. Intelligent drug delivery systems can potentially improve patient medication compliance and rehabilitation outcomes. They also provide medical professionals with additional tools for decision-making, which can further develop intelligent medical services.
Respiratory treatment system:
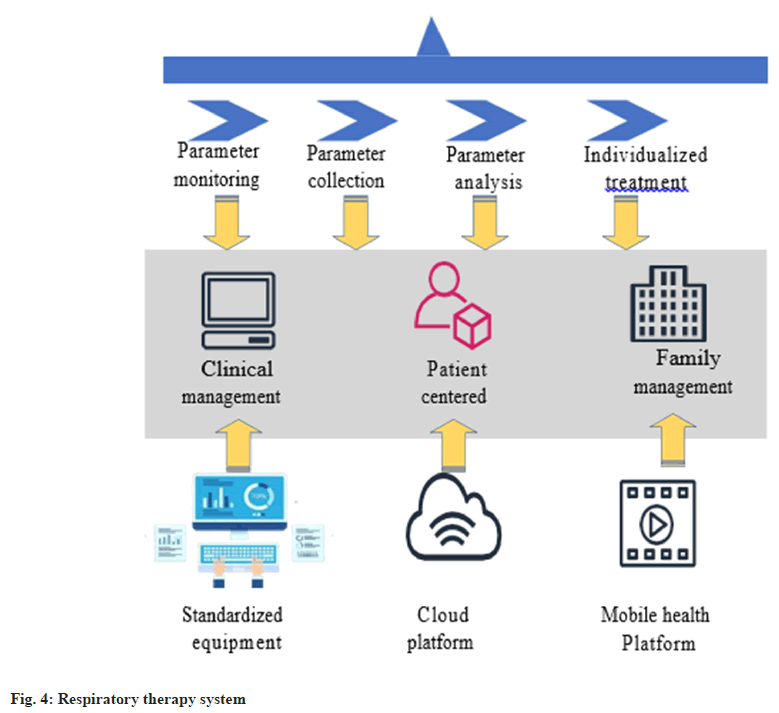
The intelligent respiratory therapy system utilizes intelligent ventilator and sensor technology to adjust breathing parameters to the treatment plan, enhancing efficacy, safety and cost-effectiveness while reducing treatment times[27,28]. The intelligent respiratory therapy system is a vital technological advancement in medicine that provides patients with more precise and personalized respiratory care. The system’s advanced sensing technology monitors the patient’s respiratory functions. The device has sensors that accurately measure physiological markers like breathing rate, tidal volume, and respiratory volume. The real-time monitoring feature of the system allows medical personnel to comprehensively understand the patient’s respiratory condition and promptly detect any abnormal situations. The intelligent respiratory treatment system is shown in fig. 4.
As shown in fig. 4, the intelligent respiratory treatment system provides parameter monitoring, collection, analysis and personalized treatment. The equipment is standardized and patient-centered, which ensures clinical and home management with dual protection. It connects to the mobile health platform through a cloud platform, enabling real-time monitoring. The system gathers patient’s real-time physiological information to modify the treatment plan for acetylcysteine nebulization automatically. For example, the dosage and concentration of acetylcysteine nebulization can be automatically adjusted based on the patient’s tidal volume and gas exchange status, ensuring customized treatment[29,30]. This modification improves patient’s adherence to therapy, reduces discomfort, and enhances treatment effectiveness.
The intelligent respiratory treatment system utilizes AI algorithms to adjust the respiratory treatment plan based on the patient’s physiological parameters and treatment response. This technology can intelligently generate customized breathing techniques for each patient. It also dynamically adjusts drug concentration in real-time based on monitoring data to better adapt to individual differences and patient conditions
Cnew=Cold+k1×Ds (3)
Where, Cnew is the adjusted nebulized concentration of acetylcysteine, Cold is the original concentration. By monitoring data in real-time, the drug concentration can better match the patient’s situation and individual differences. This allows for an adjusted nebulized concentration of acetylcysteine to be used based on the original concentration.
Dnew=Dold+ K2×Ds (4)
Among them, Dnew is the adjusted nebulized dose of acetylcysteine; Dold is the original dose and K2 is the dose adjustment coefficient learned by the system.
The system can adjust the nebulized dose of acetylcysteine and provide medical personnel with necessary information about the patient, such as medical history and development trends, through a visual interface. This helps medical teams to develop personalized diagnosis and treatment plans. Patients can achieve the best absorption effect during treatment by monitoring data in real time and intelligently adjusting the atomization time. The system also learns the original dose and dose adjustment coefficient, which are important factors in the treatment process:
Tnew=Told+ k3×Ds (5)
Where, Tnew is the adjusted atomization time; Told is the original atomization time and K3 is the atomization time adjustment coefficient learned by the system. The system uses an atomization time adjustment coefficient learned by dynamically learning patient’s physiological characteristics and treatment responses. This helps generate intelligent treatment plans that ensure personalized control of acetylcysteine concentration, dosage, and nebulization time. As a result, patients receive more accurate and efficient respiratory treatment.
When combined with artificial intelligence algorithms and sensing technology, intelligent respiratory therapy systems can monitor a patient’s respiratory status in real-time and make personalized adjustments. This leads to more targeted and effective treatment, which improves the overall treatment experience of patients and provides better decisionsupport tools for medical staff. This technological innovation promotes the widespread application of intelligent medical technology in respiratory therapy, which benefits patients and medical professionals.
Remote medical consultation:
Doctors can use telemedicine to advise and monitor their patients from their homes. Telemedicine involves using the internet to connect intelligent medical devices with patients. Through video consultations and online chats, doctors can quickly understand the effectiveness of aerosol inhalation of acetylcysteine and any changes in their symptoms and medication use. This information is then used to revise the patient’s medical plan and provide personalized advice tailored to their needs.
Patients can easily report their physical condition and make corresponding responses according to their situation, which allows doctors to adjust their treatment plans accordingly. Additionally, doctors can obtain the patient’s physiological parameters and spray status through remote monitoring to better understand their health status.
Results and Discussion
This article examines the clinical symptoms observed in children, including cough, shortness of breath, cyanosis around the abdominal wall, choking on milk and moist rales. The article evaluates whether there is a significant improvement in these symptoms after treatment. Table 1, shows the symptoms of the two groups of children before treatment.
Table 1, shows no significant difference in the symptom situation between the two groups of children before treatment. Most children had different symptoms, with shortness of breath being the most common. In both groups, 24 children in the control group and 23 children in the experimental group exhibited shortness of breath, respectively.
| Symptom | Control group | Experimental group |
|---|---|---|
| Cough | 20 | 22 |
| Shortness of breath | 24 | 23 |
| Cyanosis around the abdominal wall | 13 | 12 |
| Choking milk | 15 | 17 |
| Moist rale | 13 | 12 |
Table 1: Symptoms of two groups of children before treatment
Table 2, shows that both groups of children experienced varying degrees of symptom improvement after treatment, with the experimental group showing a higher degree of improvement. The number of children with each symptom is similar in both groups, indicating that the treatment effect of the experimental group is more significant.
| Symptom | Control group | Experimental group |
|---|---|---|
| Cough | 15 | 5 |
| Shortness of breath | 12 | 6 |
| Cyanosis around the abdominal wall | 5 | 0 |
| Choking milk | 8 | 2 |
| Moist rale | 9 | 3 |
Table 2: Symptoms of two groups of children after treatment
By monitoring a patient’s respiratory rate in realtime, medical staff can detect abnormal conditions such as shortness of breath and promptly adjust treatment plans. Acetylcysteine nebulized inhalation can promote mucus excretion, have antioxidant and anti-inflammatory properties, and help in lung function recovery. This can significantly improve a patient’s respiratory distress and patency, and stabilize their respiratory condition after treatment.
In response to symptoms such as choking on milk in pediatric patients, an intelligent medical system can personalize adjustments based on their physiological needs. The system can continuously monitor children and dynamically adjust parameters such as drug concentration, dosage and nebulization time. This ensures that children receive more personalized treatment, reducing discomfort and fatigue. Acetylcysteine nebulization inhalation is a comprehensive treatment method that, when combined with intelligent medical systems, is expected to achieve better therapeutic effects and improve the quality of life of infants in the comprehensive treatment of neonatal pneumonia.
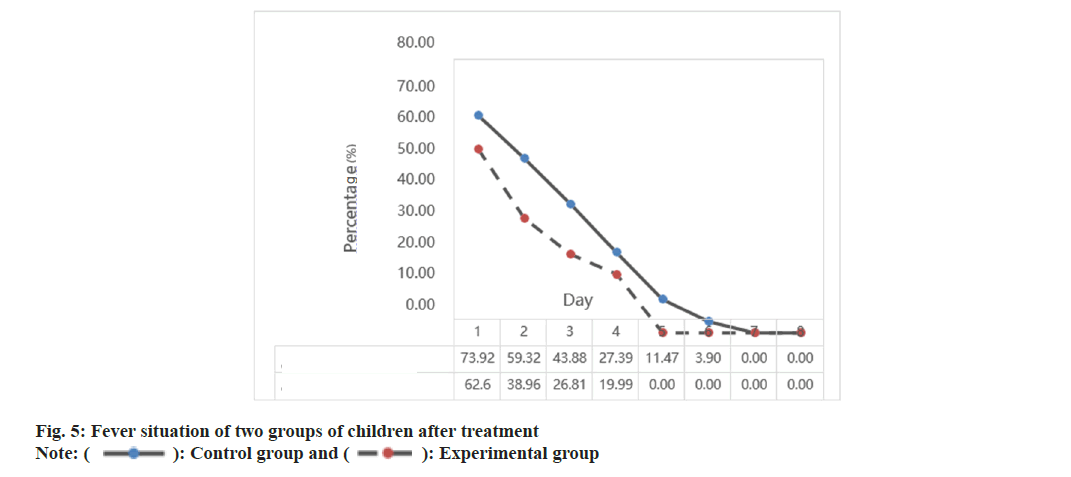
The fever situation of the two groups of children after treatment is shown in fig. 5. In fig. 5, it is shown that none of the children in the experimental group had a fever on the 5th d after treatment. This indicates that their fever was gone by the 5th d. In contrast, the control group still had a 0 % fever rate on the 7th d after treatment, which means that the control group’s treatment was slower in effect. An intelligent medical system has been developed to monitor children’s temperature and physiological indicators in real-time. This system can detect and analyze fever situations quickly using intelligent algorithms. Adjusting parameters such as acetylcysteine nebulization concentration and duration can help inhibit infection and alleviate fever.
Although single acetylcysteine nebulization inhalation can improve fever, its effect is limited. Neonatal pneumonia has complex etiology and pathogenesis, and a single drug cannot fully address the complexity of the disease. The intelligent medical system combines the acetylcysteine nebulization inhalation scheme with a personalized treatment strategy to provide a more comprehensive and accurate treatment for neonatal pneumonia. This approach responds more effectively to individual differences and patient disease changes, enhancing targeted treatment. As a result, the combination of acetylcysteine nebulization inhalation and the intelligent medical system significantly improves fever status.
This article explains how inflammatory markers in the blood can be detected and used to evaluate the level of inflammatory response. The degree of inflammation can be categorized into five groups; A (very severe), B (relatively severe), C (generally severe), D (not severe but symptomatic) and E (no inflammation).
| Level | Control group | Experimental group |
|---|---|---|
| A | 42.38 % | 49.5 % |
| B | 36.76 % | 39.39 % |
| C | 20.86 % | 11.11 % |
| D | 0 % | 0 % |
| E | 0 % | 0 % |
Table 3: Inflammation levels in the two groups of children before treatment
Table 3, presents the level of inflammation in both groups of children prior to treatment, ranging from grade A to C. None of the children fell under grades D and E. The level of inflammation in the two groups after treatment is illustrated in fig. 6.
After treatment, the experimental group showed 0 % of A-grade (very serious) children, as shown in fig. 6. In comparison, the control group had 12.93 % of A-grade children. The experimental group’s treatment method significantly improved inflammation, with 46.66 % of children with grade E (non-inflammation) compared to the control group’s 17.9 %. Pneumonia’s etiology is complex, with multiple pathogenic microorganisms and inflammatory pathways involved. It is difficult for a single drug to cover all pathogens. However, a comprehensive treatment strategy based on intelligent medical systems can more accurately control lung inflammation. When combined with acetylcysteine nebulization inhalation, it can effectively reduce lung inflammation response and promote lung function recovery. This synergistic effect can provide more comprehensive and personalized treatment for neonatal pneumonia patients, improving the treatment success rate. The intelligent medical system uses advanced sensing technology and real-time monitoring to accurately detect physiological indicators, such as pulmonary inflammation in pediatric patients. The system can intelligently adjust the acetylcysteine nebulization treatment plan based on monitoring data to ensure patients receive personalized treatment plans most suitable for their pulmonary inflammation status.
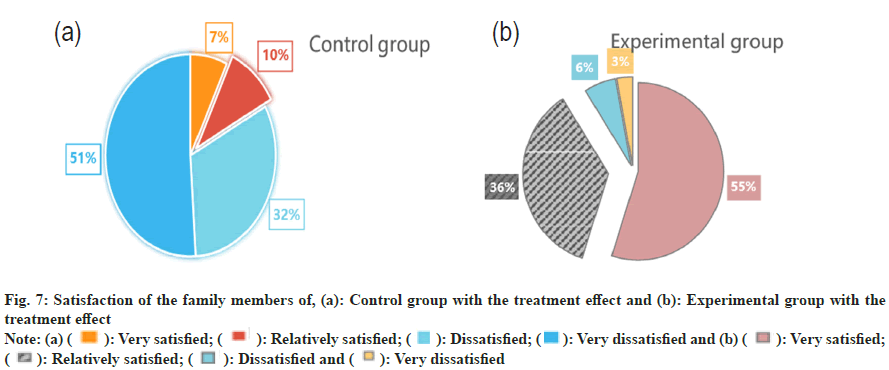
It is important to check if the child experiences any allergic reactions to acetylcysteine, such as vomiting, itching, redness, anaphylaxis, difficulty in feeding and other allergic symptoms. According to Table 4, the control group had a higher complication incidence than the experimental group. Specifically, the control group had the highest incidence of vomiting at 11 %. Meanwhile, the experimental group had a lower incidence of vomiting at 2 %, 9 % less than the control group. Allergic reactions involve complex immune cell and biochemical processes. This article proposes a comprehensive treatment strategy based on an intelligent medical system that provides a more individualized response to allergic reactions. The strategy includes acetylcysteine nebulization inhalation, which can effectively inhibit allergic inflammation and delay the onset of allergic reactions. This article examines the satisfaction of two groups of relatives of children with the treatment’s effectiveness as shown in fig. 7.
| Complication | Control group | Experimental group |
|---|---|---|
| Vomit | 11 % | 2 % |
| Pruritus | 5 % | 1 % |
| Erythema | 8 % | 0 % |
| Anaphylactic shock | 3 % | 0 % |
| Feeding difficulties | 7 % | 1 % |
Table 4: Incidence of complications
According to fig. 7a, most family members in the control group are extremely dissatisfied with the treatment effect. Only 7 % are very satisfied, which is 44 % less than the proportion of those who are very dissatisfied (51 %). Fig. 7b, demonstrates that the experimental group had a high proportion of satisfied family members, with a rate of 55 %. This is significantly higher than the proportion of dissatisfied family members with pneumonia, only 3 %. The intelligent medical system provides accurate and real-time monitoring. Through intelligent monitoring devices, doctors can obtain patient’s realtime vital signs, such as respiratory and heart rates. This helps detect changes in the patient’s condition on time and take necessary treatment measures in advance. The timeliness and accuracy of the system make family members feel more at ease and satisfied with the treatment effect. By comprehensively and systematically evaluating the aspects mentioned above, a more comprehensive understanding of the safety of acetylcysteine nebulized inhalation can be gained in the treatment of neonatal pneumonia. This helps identify potential problems promptly and take appropriate measures to ensure the patient’s safety and good treatment effect.
Fig. 7: Satisfaction of the family members of, (a): Control group with the treatment effect and (b): Experimental group with the treatment effect
Note: (a) (  ): Very satisfied; (
): Very satisfied; ( ): Relatively satisfied; (
): Relatively satisfied; (  ): Dissatisfied (
): Dissatisfied ( ): Very dissatisfied and (b) (
): Very dissatisfied and (b) ( ): Very satisfied; (
): Very satisfied; ( ): Relatively satisfied; (
): Relatively satisfied; (  ): Dissatisfied and (
): Dissatisfied and (  ): Very dissatisfied
): Very dissatisfied
The combination of intelligent medical technology and nebulized acetylcysteine inhalation can treat neonatal pneumonia, providing strong support for improving the efficacy and satisfaction of patients and their families. This article utilized intelligent medical technology to efficiently and timely monitor the health status of patients, adjust treatment plans and achieve personalized treatment. Meanwhile, as an anti-inflammatory drug, nebulized acetylcysteine inhalation can help reduce inflammation levels and promote the recovery of lung function. With the development of intelligent medical technology, it can undoubtedly be widely applied, bringing more convenient, safer, and more effective diagnostic and treatment experiences to medical staff and patients. In the future, this article can continue to conduct in-depth research and continuously innovate technologies to improve the treatment mode and treatment effects for patients with neonatal pneumonia.
Funding:
This study was supported by, The Clinical Study on the Treatment of Neonatal Pneumonia with Acetylcysteine Nebulized Inhalation (No: SFZD 2019156).
Conflict of interests:
The authors declared no conflict of interests.
References
- Liu J, Zhao HR, Wei HL, Chen C, Qiu RX, Ren XL, et al. Efficacy of bronchoalveolar lavage as adjunct therapy in the treatment of neonatal severe pneumonia: A prospective case-control study. J Trop Pediatr 2020;66(5):528-33.
[Crossref] [Google Scholar] [PubMed]
- He L, Yang L, Zhang H, Luo Q. Efficacy and safety of interferon on neonates with respiratory syncytial virus pneumonia. Exp Ther Med 2020;20(6):220.
[Google Scholar] [PubMed]
- Hussain K, Salat MS, Ambreen G, Mughal A, Idrees S, Sohail M, et al. Intravenous vs. intravenous plus aerosolized colistin for treatment of ventilator-associated pneumonia-A matched case-control study in neonates. Exp Opin Drug Safety 2020;19(12):1641-9.
[Crossref] [Google Scholar] [PubMed]
- Wang HC, Tsai MH, Chu SM, Liao CC, Lai MY, Huang HR, et al. Clinical characteristics and outcomes of neonates with polymicrobial ventilator-associated pneumonia in the intensive care unit. BMC Infect Dis 2021;21(1):965.
[Crossref] [Google Scholar] [PubMed]
- Ignatieva VI, Opimakh SG, Dobrianskyi DV, Gumeniuk GL, Ilnytskyi RI, Kuzmenko NM. Application of acetylcysteine in inhalation form in complex treatment of patients with bronchial asthma with neutrophilic type of inflammation. Infusion Chemother 2020;3(1):34-6.
- Qian W, Wei Z. Clinical effect analysis of budesonide atomization inhalation in the treatment of neonatal pneumonia. Med Clin Med 2023;4(7):88-93.
- Zhang H, Yang J, Zhao W, Zhou J, He S, Shang Y, et al. Clinical features and risk factors of plastic bronchitis caused by refractory Mycoplasma pneumoniae pneumonia in children: A practical nomogram prediction model. Eur J Pediatr 2023;182(3):1239-49.
[Crossref] [Google Scholar] [PubMed]
- Cui J, Wang J, Lv Y, Xu D. LncRNA NEAT1 regulates infantile pneumonia by sponging miR-146b. Mol Biotechnol 2021;63(8):694-701.
[Crossref] [Google Scholar] [PubMed]
- Zheng Q, Lu Y, Lure F, Jaeger S, Lu P. Clinical and radiological features of novel coronavirus pneumonia. J X-Ray Sci Technol 2020;28(3):391-404.
[Crossref] [Google Scholar] [PubMed]
- Wu C, Zhang Y, Yang L, Shen F, Ma C, Shen M. Effect of capsaicin atomization-induced cough on sputum excretion in tracheotomized patients after hemorrhagic stroke: A randomized controlled trial. J Speech Lang Hear Res 2021;64(11):4085-95.
[Crossref] [Google Scholar] [PubMed]
- Schloss J, Leach M, Brown D, Hannan N, Kendall-Reed P, Steel A. The effects of N-acetyl cysteine on acute viral respiratory infections in humans: A rapid review. Adv Integr Med 2020;7(4):232-9.
[Crossref] [Google Scholar] [PubMed]
- Song J, Yao L, Shi J, Li J, Xu C. Protective effects of N-acetylcysteine on a chemical-induced murine model of asthma. J Asthma 2021;58(9):1208-15.
[Crossref] [Google Scholar] [PubMed]
- Singh G, Pandey A, Shandilya G, Gupta A, Rawat JD, Wakhlu A, et al. Evaluation of nebulized N-acetyl cysteine in outcome of esophageal atresia with tracheoesophegeal fistula. J Pediatr Surg 2020;55(12):2635-9.
[Crossref] [Google Scholar] [PubMed]
- Liu H, Wang W, Gao X. Comparison of the efficacy of ambroxol hydrochloride and N-acetylcysteine in the treatment of children with bronchopneumonia and their influence on prognosis. Exp Ther Med 2020;20(6):130.
[Google Scholar] [PubMed]
- Brodier EA, Raithatha M, Kannan S, Karunasekara N. Use of nebulised N-acetylcysteine as a life-saving mucolytic in intensive care: A case report. J Intensive Care Soc 2020;21(4):296-8.
[Crossref] [Google Scholar] [PubMed]
- Phelps MK, Olson LM, Patel MA, Thompson MJ, Murphy CV. Nebulized heparin for adult patients with smoke inhalation injury: A review of the literature. J Pharm Technol 2020;36(4):130-40.
[Crossref] [Google Scholar] [PubMed]
- Jo YS, Choi IS, So YK. The use of inhaled N-acetylcysteine for laryngopharyngeal reflux disease: A randomized controlled trial. J Voice 2021;35(4):618-24.
[Crossref] [Google Scholar ] [PubMed]
- Xiong J, Zhang L, Bao L. Complications and mortality of venovenous extracorporeal membrane oxygenation in the treatment of neonatal respiratory failure: A systematic review and meta-analysis. BMC Pulm Med 2020;20(1):124.
[Crossref] [Google Scholar] [PubMed]
- Korppi M. Antibiotic therapy in children with community-acquired pneumonia. Acta Paediatr 2021;110(12):3246-50.
[Crossref] [Google Scholar] [PubMed]
- Gao YQ, Qiu RX, Liu J, Zhang L, Ren XL, Qin SJ. Lung ultrasound completely replaced chest X-ray for diagnosing neonatal lung diseases: A 3-year clinical practice report from a neonatal intensive care unit in China. J Maternal Fetal Neonatal Med 2022;35(18):3565-72.
[Crossref] [Google Scholar] [PubMed]
- Garwood TJ, Lehman CP, Walsh DP, Cassirer EF, Besser TE, Jenks JA. Removal of chronic Mycoplasma pneumoniae carrier ewes eliminates pneumonia in a bighorn sheep population. Ecol Evol 2020;10(7):3491-502.
[Crossref] [Google Scholar] [PubMed]
- Ng EH, Shah V. Guidelines for surfactant replacement therapy in neonates. Paediatr Child Health 2021;26(1):35-41.
- Zhang L, Yan H, Wang H, Wang L, Bai B, Ma Y, et al. MicroRNA (miR)-429 promotes inflammatory injury by targeting Kruppel-Like Factor 4 (KLF4) in neonatal pneumonia. Curr Neurovasc Res 2020;17(1):102-9.
[Crossref] [Google Scholar] [PubMed]
- Lowery AS, Gelbard A, Wootten C. The incidence of laryngotracheal stenosis in neonates with a history of ventilator-associated pneumonia. Laryngoscope 2020;130(9):2252-5.
[Crossref] [Google Scholar] [PubMed]
- Kargar S, Roshan M, Ghoreishi SM, Akhlaghi A, Kanani M, Shams-Abadi AA, et al. Extended colostrum feeding for 2 weeks improves growth performance and reduces the susceptibility to diarrhea and pneumonia in neonatal Holstein dairy calves. J Dairy Sci 2020;103(9):8130-42.
[Crossref] [Google Scholar] [PubMed]
- Bringhenti L, Pallu M, Silva JC, Tomazi T, Tomazi AC, Rodrigues MX, et al. Effect of treatment of pneumonia and otitis media with tildipirosin or florfenicol+flunixin meglumine on health and upper respiratory tract microbiota of preweaned Holstein dairy heifers. J Dairy Sci 2021;104(9):10291-309.
[Crossref] [Google Scholar] [PubMed]
- Xiaobin WU, Jialin YU, Xuemei LI. The role of fucosylated human milk oligosaccharide in treating neonatal Streptococcus agalactiae pneumonia. Chin J Microecol 2020;32(3):264-8.
- Darlow CA, Hope W. Flomoxef for neonates: Extending options for treatment of neonatal sepsis caused by ESBL-producing Enterobacterales. J Antimicrobial Chemother 2022;77(3):711-8.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Xia RM, Ren XL, Li JJ. The new application of point-of-care lung ultrasound in guiding or assisting neonatal severe lung disease treatment based on a case series. J Matern Fetal Neonatal Med 2020;33(23):3907-15.
[Crossref] [Google Scholar] [PubMed]
- Alanezi G, Almulhem A, Aldriwesh M, Bawazeer M. A triple antimicrobial regimen for multidrug-resistant Klebsiella pneumoniae in a neonatal intensive care unit outbreak: A case series. J Infect Public Health. 2022;15(1):138-41.
[Crossref] [Google Scholar] [PubMed]
 ): Control group and (
): Control group and (  ): Experimental group
): Experimental group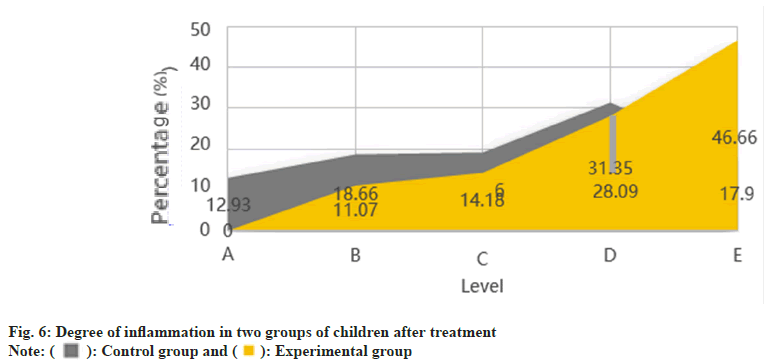
 ): Control group and (
): Control group and ( ): Experimental group
): Experimental group