- *Corresponding Author:
- Amit Mittal
Department of Pharmacy, I.K. Gujral Punjab Technical University, Jalandhar, Punjab 144601, India
E-mail: amitmittal77@yahoo.com
| Date of Received | 22 December 2023 |
| Date of Revision | 26 June 2024 |
| Date of Acceptance | 01 October 2024 |
| Indian J Pharm Sci 2024;86(5):1542-1553 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Juglans regia L. is a wonder nut widely used as functional food owing to its multi-nutritional qualities. It is generally found in Southeast Europe extending towards the Himalayas and Southwest China. This historical plant is extensively used ethno-medicinally in the treatment of digestive, respiratory, cardiovascular and skin diseases. Its seeds are highly proteinaceous and polyphenolic containing triacylglycerol-rich oil especially mono and polyunsaturated fatty acids. The diverse lipid profile of the walnut has attracted the researchers of the decade towards scientific validation of its traditional aspects and isolation of important chemical compounds for improving human health. The present review comprehends the ameliorative effect of Juglans regia on various chronic diseases including cancer, gut dysbiosis, cardiovascular and neurodegeneration concerning its specific chemical profile.
Keywords
Juglans regia, walnut, α-linolenic acid, juglone, sankrutin, polyphenolic, cardiovascular
Nuts, the enveloped kernels and seeds, are wonderful gifts from mother earth to mankind. They are considered complete meals as they come packed with energy, proteins, antioxidants, vitamins, and minerals. They contain heart-friendly Monounsaturated Fatty Acids (MUFA), omega-3 essential fatty acids, and polyphenols such as carotenes, resveratrol, and lutein. There are various types of nuts available in markets worldwide, including almonds, cashews, hazelnuts, peanuts, chestnuts, walnuts, and pine nuts, each with its unique importance.
Various types of nuts are available in markets worldwide, including almonds, cashews, hazelnuts, peanuts, chestnuts, walnuts, and pine nuts, each possessing its unique importance. Several varieties of walnuts are available in the market, including black walnut (Juglans nigra), English walnut (Juglans regia (J. regia) L.), and butter or white walnut (Juglans cinerea). J. regia, of the family Juglandaceae, is most commonly referred to as walnut. It is native to Kyrgyzstan but is widely distributed across parts of Southern Europe, the United States of America (USA), Turkey, Tajikistan, Australia, New Zealand, China, and India[1]. China leads in walnut production, followed by the USA, Iran, Turkey, Ukraine, Romania, France, and India. In recent years, countries like Chile and Argentina have also seen increased production rates[2]. The Juglandaceae family comprises 60 species in the Northern Hemisphere. Juglans is a plant genus whose seeds are known as walnuts[3]. Walnuts are an integral part of Mediterranean diets and come from deciduous trees found in Europe, Asia, and the eastern and southern United States[4].
Walnuts, whether Persian or black varieties, possess a thicker shell with an intense flavor. As deciduous trees, they are easy to cultivate, with harvesting beginning from early September to November. When 85 % of the hull is removed, the shells are ready for harvest, and proper irrigation is crucial during cultivation and harvesting. Delayed harvesting can result in shell blackening and give the kernels a bitter, rancid flavor[5]. The nut is the powerhouse of vitamin E, MUFA, omega-3 fatty acids, and arachidonic acids[6]. Due to its nutrient-rich oil and high protein content, the walnut is listed on the FAO's priority plants list and is classified as a strategic species[7]. Beyond the nut, other parts of the plant such as the leaves, bark, husk, shell, and fruit also hold pharmacological importance[8]. Walnut protein has been reported to contain minimal allergenic levels, with an Eliciting Dose 01 (ED01) among 14 food allergens, according to a study using the stacked model averaging statistical method. Walnuts are versatile, being used across the cosmetic, food, and pharmaceutical industries[9,10]. They contain a variety of flavonoids, phenolic acids, and related polyphenols, making them powerful antioxidants with anti-atherogenic, anti-inflammatory, and antimutagenic properties[11,12]. Several studies have revealed that regular consumption of walnuts decreases cardiovascular risk by lowering Low-Density Lipoprotein (LDL) cholesterol, reducing inflammation, and improving arterial function[13-15]. The remarkable properties of the walnut plant have been discussed in various complementary and folk medicines. The leaves have been used ethnomedicinally in Turkey to relieve fever and swelling, while the kernels have been used in Iran to treat bowel inflammation. In Palestine, walnuts are used to treat diabetes and asthma, and in Calabria's folk medicine, the shells are used as an anti-malarial remedy[16-18]. The plant is commonly used in traditional medicine for treating topical or dermal inflammations, particularly with its leaves and bark[19,20].
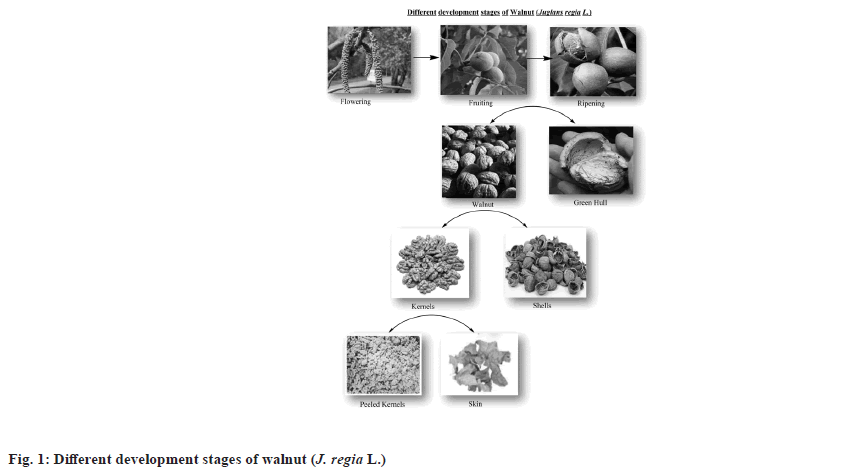
Numerous scientific reports suggest that walnuts, rich in fibers, Polyunsaturated Fatty Acids (PUFAs), polyphenols (such as tannins and ellagic acid), vitamins (riboflavin, tocopherols), proteins (arginine, lysine, tryptophan), non-sodium minerals (calcium, magnesium, potassium, phosphorus), and trace elements (copper, zinc, selenium), possess anti-inflammatory, antioxidant, anti-aging, and cognitive improvement properties. Walnuts, particularly rich in omega-3 and omega-6 fatty acids, have also been found to enhance memory[21]. J. regia L. is a crucial tree with leaves, branches, trunk, roots, flowers, and fruits that are extensively used for nutritional, medicinal, and industrial purposes. The unripened walnut consists of an epicarp, mesocarp, and endocarp (the hard shell of the nut), which encases the edible kernels. The different stages of fruit development are illustrated in fig. 1. The green husk of the walnut is traditionally used in Chinese medicine for its anti-cancer and antioxidant properties, as well as for combating inflammation and dermal infections[22]. The phenolic-rich walnuts of J. regia are oxidized by Polyphenol Oxidase (PPO), while Gallate 1-Β-Glucosyltransferase (GGT) acts as a precursor molecule in the synthesis of hydrolysable tannins. The genome of J. regia contains 130 GGT genes and 2 PPO genes; however, the locations of PPO1 and PPO2 remain unidentified, which is vital for basic research and molecular breeding[23]. This review provides insights into the remarkable nut, J. regia, focusing on its phytochemistry, ethnobotanical importance, and reported pharmacology.
Bioactive Constituents
The kernels of J. regia without shells consist of 4 % water, 15 % protein, 64 % lipids, and 14 % carbohydrates, with 7 % dietary fiber. A 100 g serving of walnuts provides 654 kcal of energy, along with minerals, including 163 % of the daily value for manganese, and vitamins A, C, and folate[24]. Walnut oil is rich in unsaturated fatty acids (both mono and poly), such as Alpha (α)-linolenic acid, linoleic acid, palmitic acid, stearic acid, and oleic acid, which make up the total fat content[25]. Walnut residue after oil extraction is commonly used as fodder, fertilizer, or low-value feed. However, through the technique of enzymolysis, bioactive peptides can be generated from this residue, which possess xanthine oxidase inhibitory properties. These peptides could serve as nutraceutical supplements for populations suffering from malnutrition and excessive junk food consumption[26]. Juglone (5-hydroxy-1,4-naphthoquinone) and pyrogallol are major phenolic compounds that exhibit anti-cancer activity by inhibiting key enzymes required for metabolism[27,28]. Sakuranetin, an aglycone derived from sakuranin found in Juglan species, is a 7-O-methylated flavonoid with various pharmacological activities, including antioxidant, anti-inflammatory, antimycobacterial, antiprotozoal, hypoglycemic, neuroprotective, and antineoplastic effects. Although the pharmacokinetics and toxicity of this compound have not yet been thoroughly examined, it may play a role in promoting health[29]. The structures of bioactive constituents from different categories are presented in Table 1. Walnut shells, which are rich in lignocellulosic content, are used as abrasives in industrial applications. Additionally, due to their biodegradable properties, walnut shells can be utilized for packaging and, in pharmaceutical applications, as adsorbents for heavy metals, synthetic dyes, and hazardous chemicals[29-36].
| S. No. | Secondary metabolites | Structures |
|---|---|---|
| 1 | Steroid derivatives[31] | 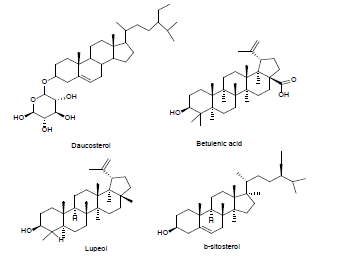 |
| 2 | Flavonoid C-glycoside derivative[32] | 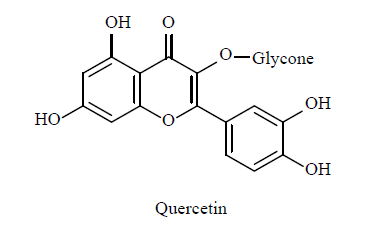 |
| 3 | Flavones derivatives[33] | 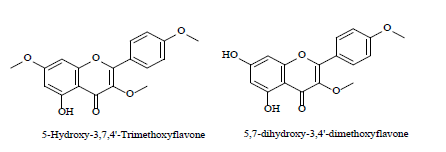 |
| 4 | Essential oil compounds[34] | 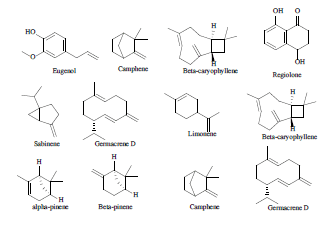 |
| 5 | Tannins derivative | 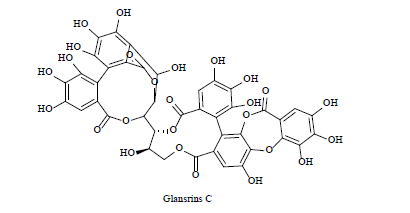 |
| 6 | Organics acid derivatives[35] | 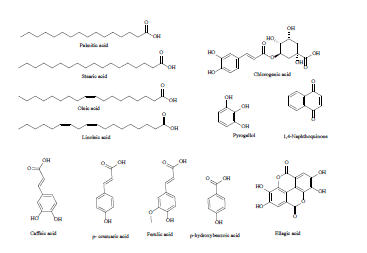 |
| 7 | Phenolic aldehyde derivatives[36] | 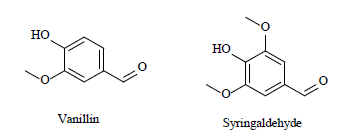 |
| 8 | Monoglyceride derivative |  |
| 9 | Sesquiterpene derivative | 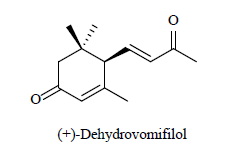 |
| 10 | Diarylheptanoid derivatives[36] | 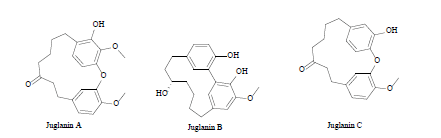 |
Table 1: The list of different bioactive moieties reported to be present in J. regia L.
Pharmacological Activity OF J. regia L.
Neuroprotective activity:
Tan et al.[37] concluded that a walnut-rich diet can combat chronic inflammation and the progression of neurodegenerative diseases. Diets high in J. regia significantly reduce the expression of phosphorylated Nuclear Factor kappa B (NF-κB) and oxidative stress in rodents induced by Lipopolysaccharides (LPS). Furthermore, a balanced gut microbiome contributes to a reduction in microbial-derived pro-inflammatory mediators such as Interleukin-6 (IL-6), IL-1 Beta (β), and TNF-α, aiding in the maintenance of neural health. In Persian medicine, walnuts are believed to promote brain health and alleviate fatigue, as walnut extract increases dopamine levels and LPS in treated rat hippocampal cells. The ellagic acid present in walnut leaves and fruits contributes to their anti-inflammatory activity[38].
Bleomycin-induced pulmonary toxicity protection:
A methanolic extract of J. regia (150 mg/kg) was administered 14 d prior to bleomycin exposure to evaluate its effects on bleomycin-induced pulmonary toxicity. Following the administration of bleomycin (10 u/kg), results indicated that the J. regia-treated rats showed increased expression of glutathione and catalase, along with decreased expression of lactate dehydrogenase and alkaline phosphatase, thus regulating apoptosis via the NF-κB pathway[39]. Therefore, the J. regia extract was reported to protect against the harmful effects caused by bleomycin in cancer individuals[40].
Improvement in male fertility:
J. regia L. has been found to exhibit the highest antioxidant activity after hazelnuts and pistachios. It contains tocopherols, essential fatty acids such as oleic, linoleic, palmitic, and stearic acids, as well as tannins, flavonoids, melatonin, fibers, and sterols[41]. The whole walnut kernel has been reported to improve semen quality, sperm morphology, and motility[42]. In a clinical study, the intake of 75 g/d of walnut kernels for 12 w as part of the routine diet of young males resulted in improved sperm count, vitality, and motility compared to the control group. The increase in omega-3 and omega-6 fatty acids was identified as a key factor responsible for enhancing sperm and hormonal balance. Another study concluded that the intake of 42 g/d of walnut kernels offered potential benefits for men with impotence and sperm quality issues[43,44]. The omega-3/omega-6 ratio in walnut kernels was found to be 4.25, the highest among superfoods such as hempseed, chia seeds, and flax seeds. This ratio, along with α-linolenic acid, is considered essential for long-term health maintenance[45].
Gut microbiome:
Walnuts, rich in polymerized polyphenols, starch, NSP, and n-3 fatty acids, possess a prebiotic effect[46]. After cereals, walnuts have the second-highest fiber content per 100 g. The fiber bypasses digestion in the small intestine and is transported directly to the colon, where it is used as a substrate by microbes. This stimulates the growth of microbiota, which, in turn, leads to the production of metabolites such as Short-Chain Fatty Acids (SCFAs) like butyrate, providing health benefits to the host[47,48].
Gastroprotection via dual Cyclooxygenase (COX)-2/5-Lipoxygenase (LOX) inhibition:
The phenolic extract from walnuts was found to have a preventative effect on indomethacin-induced gastric ulcers. Chronic use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) can induce gastrointestinal ulcers, perforations, and bleeding[49]. In walnut extract-treated groups, there was significant upregulation of 5-Hydroxyprostaglandin Dehydrogenase (15PGDH), which subsequently increased Heme Oxygenase-1 (HO-1) expression and nuclear translocation of Nuclear factor erythroid 2-Related Factor 2 (Nrf2) via Keap 1 inactivation. The downstream regulation of NF-κB and AP-1 led to the modulation of COX-1 enzymes, providing mucoprotective effects. Additionally, the eradication of Helicobacter pylori by walnut extract was attributed either to the repression of Signal Transducer and Activator of Transcription 3 (STAT 3) or the activation of Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ) receptors[50-61].
Anti-aging activity:
Walnut septum extract, rich in ellagic acid and other compounds, demonstrated significant tyrosinase inhibitory activity and has been proposed for use in cosmetic formulations to alleviate dermal hyperpigmentation and reduce wrinkles[61-69]. Table 2 summarizes the major pharmacological activities reported for various bioactive constituents, along with their mechanisms of action.
| S. no. | Pharmacological activity | Bioactive compound reported | Description | Reference |
|---|---|---|---|---|
| 1 | Anti-neoplastic activity | Hydroalcoholic extract | 30 % reduction of prostate and breast cancer | [52-54] |
| Juglone | Cellular apoptosis and mitotic arrest | [55] | ||
| 2 | Anti-oxidant | Ellagitannin derivatives | Metabolic process regulation | [56] |
| Hydroalcoholic extract | Free radical scavenging activity | [57-59] | ||
| 3 | Neuroprotective | Omega-3 | Inflamo-protective | [60] |
| EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) | Anti-inflammatory | [61] | ||
| Walnut aqueous extracts | brain function and connectivity | [62] | ||
| Ellagic acid, melatonin, and γ-tocopherol | Anti-oxidant in β-amyloid reign | [63] | ||
| 4 | Cardio-protective | Walnut kernels | Delayed onset of cardiovascular | [64] |
| Walnut kernels | Boost defense system by improving good cholesterol | [65] | ||
| Ellagic acid | antiatherosclerotic effect. | [66] | ||
| Ellagitannin | Combat oxidative stress and inflammation | [67] | ||
| 5 | Diabetes mellitus | Dietary fats | Glycaemic index and insulin levels improvement | [68,69] |
| 6 | Gut microbiome | Omega-3 and omega-6 fatty acid | Regulate intestinal microbes | [70] |
| Walnut polyphenol extracts | Improve LDL levels, Good Bacteria | [71,72] |
Table 2: List of major pharmacological activity reported by different bioactive constituent and their mechanism of action.
Interpretive Patents
J. regia L. (Persian walnut) is a highly valued medicinal plant. Its various parts, extracts, and decoctions have been extensively used for therapeutic purposes in treating conditions such as hypertension, thrombophlebitis, hypercholesterolemia, and mood disorders[70-78]. According to patent IN201921025805, the hydroalcoholic extract of the green hull of walnut fruit, rich in caffeic acid and ferulic acid, was found to be effective in the treatment of cerebral ischemia. The US20190111098 patent disclosed that a powdered formulation of the fifth part of J. regia was found to be effective in improving penile erection and sperm motility. Another patent, CN107913302, revealed that green dragon paste, containing juglone, oleanolic acid, vanillin, vanillic acid, quercetin, and naphthoquinone, when taken in a 2 g dose on an empty stomach, exhibited anti-inflammatory, anti-bacterial, anti-helminthic, analgesic, antioxidant, and anti-tumorigenic activities. An herbal formulation containing J. regia (fruit shell), Acacia arabica (bark), Mimusops elengi (bark), and Mangifera indica (leaves) has been indicated for periodontal care, specifically for preventing dental plaque and gingivitis[79-81]. A list of patents along with their associated pharmacological activities is presented in Table 3.
| S. No. | Patent Number | Description |
|---|---|---|
| 1 | US20210093688 | 10 parts by weight of 70 % ethanolic extract of J. regia root along with other plant extracts were used to prepare a natural plant preparation indicated for the regulation of cardiovascular disease including hypertension, thrombophlebitis, hypercholesterolemia, and mood disorders[73]. |
| 2 | IN201921025805 | Hydroalcoholic extract of the green coat of J. regia fruit was used in the treatment of cerebral ischemia. Phenolic compounds comprising caffeic acid and ferulic acid are enriched in the hydroalcoholic extract[74]. |
| 3 | US20190111098 | A powder formulation including J. regia, Dimocarpus longan, Ziziphus jujube, Lycium barbarum, Rehmannia glutinosa, and others was prepared for the enhancement of eroticism, libido, and sperm motility. The formulation increases the stability and ease of oral administration. The 400 g of J. regia in 2000 g of powder treated group was found to be effective penile erection value and a sperm motility percentage increase[75]. |
| 4 | EP3441076 | An extract of Julans regia L, Blumea aromatica, and Eugenia caryophyllata, were formulated in a weight ratio of 77:54:98 and indicated to possess anti-neoplatic activity and tumor recurrence by arresting the cellular growth in prophase stage[76]. |
| 5 | CN107913302 | The green dragon paste containing juglone, oleanolic acid, vanillin, vanillic acid, quercetin, and naphthoquinone was taken 2 g empty stomach and found to be inflamo-protective, bactericidal, anti-helminthic, analgesic, combating oxidative stress and anti-tumorogenic activity[77]. |
| 6 | EP3441078 | A formulation capsule containing J. regia (1 part), Artemisia absinthium (0.5-2 parts), Panax quinquefolium Linn. (1-5 parts), and Dendrobium nobile (1-10 parts) was used to treat lupus erythematosus. Additionally, the formulation is also indicated for analgesic, anorectic, hepatoprotective, normoglycaemic, and blood purifier[78]. |
| 7 | WO2015174808 | Nutraceutical drink containing Panax ginseng, Allium sativum, Mel, Laurus nobilis, Syzygium aromaticum, J. regia, and Vitis vinifera and claimed to robustness and enhance sexual functioning. This drink is organic, capable, and apposite for both males and females[79]. |
| 8 | IN1102/MUM/2012 | Herbal formulation containing J. regia (fruit shell), Acacia arabica (bark), Mimusops elengi (bark), and Mangifera indica (leaves) was indicated for the periodontal care i.e., preventing dental plaque and gingivitis. The formulation could be a mouthwash, toothpaste, and mouth rinse or applying the powder directly on the gums and teeth for two weeks daily[80]. |
| 9 | WO2012033422 | A mixture of herbs including J. regia fruit was mixed in 1:6 of 96 % ethanol/ glycerine and 40 % of volcanic mineral water, prepared for the treatment of skin disorders. The Juglans regia possess folic acid and a green coat of unripe fruit rich in ascorbic acid and iodine[81]. |
Table 3: The list of patents disclosing various pharmacological benefitted formulations.
Clinical Trials
A meta-analysis of anthropometric parameters in obesity revealed that an intake of 35 g/d of walnuts for 50 w resulted in significant reductions in body weight, waist circumference, and body mass index; however, it did not lead to a reduction in fat mass[82].
The analysis of randomized controlled trials involving 195 subjects with type 2 diabetes mellitus and lipid profiles was conducted over duration of 8 to 12 w. Participants received a hydroalcoholic extract of J. regia leaves at doses ranging from 100 to 750 mg/d, with an initial dose of 100 mg/d for the 1st week followed by 200 mg/d for the subsequent 7 w. No significant results were found in lipid-lowering effects; however, glycosylated hemoglobin (HbA1c) levels were shown to decline after 8 w of treatment[83]. Another study examining walnut consumption in individuals with type 2 diabetes mellitus assessed Fasting Blood Glucose (FBG) levels, HbA1c, and insulin resistance. The researchers concluded that there was no significant reduction in blood glucose or insulin levels, and no association was found between walnut intake and alleviation of type 2 diabetes. However, including walnuts in the daily diet may help delay the progression of chronic disease[84].
The effect of walnut consumption in the routine diet has been evaluated for metabolic syndrome in adults. In this analysis, 549 individuals from eight randomized controlled trials were studied concerning blood glucose levels, lipid profiles, C-reactive protein concentrations, and anthropometric indices. The results showed a significant decline in triglyceride levels, while other cardiovascular parameters and glucose levels remained unchanged[85]. Furthermore, another study investigated the association between cognitive improvement and walnut intake, concluding that there was no significant cognitive improvement in individuals who consumed walnuts[86].
A clinical trial involving 99 obese women was conducted to study the effects of a combined marine and plant omega-3-rich diet over 12 w. The participants were divided into 3 groups: One received fish alone (300 g/w), another received walnuts alone (18 walnuts/w), and the 3rd group consumed both fish and walnuts (150 g of fish plus 9 walnuts/w). The results revealed that the combined group, which included both marine and plant sources, showed a significant reduction in anthropometric measurements, blood pressure, glycemic indices, and fibrinogen levels[87].
In a clinical trial, 236 adults with borderline blood pressure were supplemented with walnut kernels, comprising approximately 15 % of their daily energy intake, over a duration of 2 y. The study concluded that walnut supplementation resulted in a reduction of systolic blood pressure compared to controls on a normal diet. Walnuts contain n-3 PUFAs, particularly α-linolenic acid, which has potent vasodilatory and anti-inflammatory properties. They are also rich in γ-tocopherols, powerful antioxidants, and non-sodium minerals (potassium, calcium, magnesium) that help lower blood pressure. Additionally, the amino acid arginine, a precursor to nitric oxide release, suggests that walnut intake positively affects endothelial function in blood vessels[88]. In another study, the authors concluded that a walnut-supplemented diet did not significantly contribute to the reduction of hypertension. Although walnuts are rich in zinc, magnesium, and fiber, tocopherols may serve as a complementary component in a healthy dietary plan[89].
A meta-analysis of 14 clinical trials involving 883 individuals was conducted to evaluate the effects of walnut consumption (dosing of 15-57 g/d) on glycemic biomarkers, including FBG levels, insulin, HbA1c, adiponectin, and leptin, over intervention periods ranging from 5 w to 1 y. The results suggested that regular dietary intake of walnuts did not significantly alter FBG, insulin, or HbA1c levels; however, adiponectin and leptin levels increased significantly. Pharmacologically, leptin transmits signals from hypothalamic neurons and mediates the activity of Neuropeptide Y (NPY), Agouti-Related Protein (AgRP), and Pro-Opiomelanocortin (POMC). NPY and AgRP levels stimulate orexigenic (appetite-stimulating) effects, while POMC signals induce anorexic (appetite-suppressing) behavior. Furthermore, leptin-modulated immune cell proliferation may be associated with an individual's nutritional status and immune function. Thus, the studies concluded that adiponectin and leptin levels contribute to immune function and nutritional status, while showing no significant association with improvements in blood glucose level biomarkers in acute or chronic metabolic diseases[90].
A clinical trial involving the intake of various nuts (almonds, pistachios, walnuts, peanuts, and others) examined their influence on gut microflora and gut function in adults. The microbiota, including Dialister, Lachnospira, and Parabacteroides, showed no significant changes in excretion in both walnut intake and control groups. In contrast, Roseburia and Clostridium levels in feces were found to significantly increase in individuals consuming walnuts. The dose and duration of nut intake played a crucial role in these outcomes. Walnuts, rich in omega-3 fatty acids, were identified as the bioactive compounds responsible for maintaining gut microbiota[91].
An extract of walnut tree leaves, combined with extracts from other medicinal plants, was administered to a pediatric population (aged 6-18 y) and found to be effective in treating acute non-bacterial tonsillitis. In Romanian ethnomedicine, J. regia has been recognized since prehistoric times for its activity against dermal infections such as eczema, scrofulosis, and atopic dermatitis. It demonstrates ethnopediatric therapeutic versatility, with a reported effectiveness of 71.42 %[92].
Regular dietary intake of walnuts, consumed every 6 m, has been shown to delay the progression of chronic diseases and downregulate the expression of cancer metabolic biomarkers due to their richness in juglanin, juglone, and ellagitannin metabolites (urolithins)[93]. A study involving 56 g of walnuts in the daily diet for 8 w indicated a significant increase in endothelial function, characterized by enhanced membrane fluidity, increased nitric oxide release, and decreased levels of Vascular Cell Adhesion Molecule-1 (VCAM-1), along with a reduction in endothelin-1. Walnuts are abundant in polyphenolic compounds, dietary fiber, healthy fatty acids, selenium, and magnesium, all of which have beneficial effects on cardiovascular risk, particularly regarding atherosclerosis and arrhythmia[94]. Additionally, walnuts, rich in the bioactive fatty Acid Α-Linolenic Acid (ALA) and non-sodium minerals, help lower the risk of myocardial infarction[95].
Discussion
Nuts are of prime importance worldwide due to their multifactorial health benefits, attributed to their highly nutritious profiles. The genus Juglans encompasses 7 subgenera and 59 species distributed globally. The kernels of J. regia L. (Persian walnut) contain 4 % water, 15 % protein, 64 % lipids, and 14 % carbohydrates, along with 7 % dietary fiber. A 100 g serving of walnuts provides 654 kcal of energy, along with minerals (163 % of the daily value for manganese) and vitamins A, C, and folate. Numerous scientific reports indicate that nuts, rich in fiber, PUFAs, polyphenols (such as tannins and ellagic acid), vitamins (including riboflavin and tocopherols), proteins (such as arginine, lysine, and tryptophan), minerals (like calcium, magnesium, potassium, and phosphorus), and trace elements (such as copper, zinc, and selenium), exhibit anti-inflammatory, antioxidant, anti-aging, and cognitive enhancement properties.
J. regia L. (Persian walnut) is a highly valued medicinal plant. Various parts of the plant, including extracts and decoctions, have been extensively used for their therapeutic properties in treating conditions such as hypertension, thrombophlebitis, hypercholesterolemia, and mood disorders. An herbal formulation containing J. regia (fruit shell), Acacia arabica (bark), Mimusops elengi (bark), and Mangifera indica (leaves) has been indicated for periodontal care, specifically in preventing dental plaque and gingivitis.
Walnuts contain omega-3 PUFAs, specifically α-linolenic acid, which serves as a potent vasodilator and exhibits anti-inflammatory activity. They are also rich in γ-tocopherols, powerful antioxidants, and non-sodium minerals (such as potassium, calcium, and magnesium) that help lower blood pressure. Additionally, walnuts provide the amino acid arginine, a precursor for nitric oxide release, thus enhancing endothelial function in blood vessels. Rich in polyphenolic compounds, dietary fiber, healthy fatty acids, selenium, and magnesium, walnuts have beneficial effects on cardiovascular risk, particularly regarding atherosclerosis and arrhythmia[94]. Moreover, walnuts, rich in the bioactive fatty ALA and non-sodium minerals, can lower the risk of myocardial infarction[95].
The de-oiled cakes from nuts are typically protein rich, but their current utilization is largely limited to cattle feed. These mechanically pressed cakes contain 45 %-55 % protein and 30 %-35 % carbohydrates and fiber. Enzymatic hydrolysis derived de-oiled nut cakes could be evaluated for their potential anti-hypertensive, antioxidant, and neuroprotective activities[96]. Regular inclusion of walnuts in the diet can aid in delaying the progression of chronic diseases, including those related to anthropometric and endocrine dysfunctions, as well as neurodegenerative disorders[97].
Conclusion
J. regia L. (Juglandaceae) is a medicinal plant that contains fibers, PUFAs, polyphenols (including tannins and ellagic acid), vitamins (such as riboflavin and tocopherols), proteins (including arginine, lysine, and tryptophan), non-sodium minerals (such as calcium, magnesium, potassium, and phosphorus), and trace elements (including copper, zinc, and selenium). These components contribute to its ability to combat inflammation, oxidative stress, aging, cancer, and diabetes mellitus, as well as its antimicrobial properties and cognitive and memory enhancement effects. This review highlights that walnuts are nutritionally rich, pharmacologically active, and therapeutically used in various formulations. It is recommended that future studies focus on the identification of active molecules, pharmacokinetic and pharmacodynamic profiling, gene profiling, and elucidating their mechanisms of action.
Acknowledgement:
The authors express their gratitude to Amar Shaheed Baba Ajit Singh Jujhar Singh Memorial (ASBASJSM) College of Pharmacy, Bela, Ropar; School of Pharmaceutical Sciences, Lovely Professional University, Phagwara (Punjab); and RKSD College of Pharmacy, Kaithal (Haryana) for their support.
Conflict of interest:
The authors declared no conflict of interests.
References
- Girzu M, Carnat A, Privat AM, Fialip J, Carnat AP, Lamaison JL. Sedative effect of walnut leaf extract and juglone, an isolated constituent. Pharm Biol 1998;36(4):280-6.
- Martínez ML, Labuckas DO, Lamarque AL, Maestri DM. Walnut (Juglans regia L.): genetic resources, chemistry, by‐products. J Sci Food Agric 2010;90(12):1959-67.
[Crossref] [Google Scholar] [PubMed]
- Du H, Li C, Wen Y, Tu Y, Zhong Y, Yuan Z, et al. Secondary metabolites from pericarp of Juglans regia. Biochem Syst Ecol 2014;54:88-91.
- Moore JN. Genetic resources of temperate fruit and nut crops. Acta Hortic 1990;290:1-974.
- Grant A. Walnut Tree Harvesting: When are walnuts ready to pick. Gardening know how 2021.
- Sen SM, Karadeniz T. The nutritional value of walnut. J Hyg Eng Des 2015;11(18):68-71.
- Gandev S. Budding and grafting of the walnut (Juglans regia L.) and their effectiveness in Bulgaria. Bulg J Agric Sci 2007;13(6):683.
- Singh D, Tanwar A, Agrawal P. An overview on coriander. J Biomed Pharm Res 2015;4(2):67-70.
- Remington BC, Westerhout J, Meima MY, Blom WM, Kruizinga AG, Wheeler MW, et al. Updated population minimal eliciting dose distributions for use in risk assessment of 14 priority food allergens. Food Chem Toxicol 2020;139:111259..
[Crossref] [Google Scholar] [PubMed]
- Ahmad N, Singh S, Rashid Megna HM. Walnut. Fruit Production of India 2018:660-72.
- Anderson KJ, Teuber SS, Gobeille A, Cremin P, Waterhouse AL, Steinberg FM. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J Nutr 2001;131(11):2837-42.
[Crossref] [Google Scholar] [PubMed]
- Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jerónimo C, et al. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol 2010;48(1):441-7.
[Crossref] [Google Scholar] [PubMed]
- Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation 2004;109(13):1609-14.
[Crossref] [Google Scholar] [PubMed]
- Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Bare M, et al. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes care 2004;27(12):2777-83.
[Crossref] [Google Scholar] [PubMed]
- Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary α-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr 2004;134(11):2991-7.
[Crossref] [Google Scholar] [PubMed]
- Jaradat NA. Medical plants utilized in Palestinian folk medicine for treatment of diabetes mellitus and cardiac diseases. Al-Aqsa Univ J 2005;9(1):1-28.
- Kale AA, Gaikwada SA, Kamble G. In vitro anthelmintic activity of stem bark of Juglans regia L. J Chem Pharm Res 2011;3(2):298-302.
- Tagarelli G, Tagarelli A, Piro A. Folk medicine used to heal malaria in Calabria (Southern Italy). J Ethnobiol Ethnomed 2010;6:1-6.
[Crossref] [Google Scholar] [PubMed]
- Ali‐Shtayeh MS, Abu Ghdeib SI. Antifungal activity of plant extracts against dermatophytes. Mycoses 1999;42(11‐12):665-72.
[Crossref] [Google Scholar] [PubMed]
- Robbers JE, Tyler VE. Tyler's herbs of choice. The therapeutic use of phytomedicinals. 1999
- Arslan J, Gilani AU, Jamshed H, Khan SF, Kamal MA. Edible nuts for memory. Curr Pharm Des 2020;26(37):4712-20.
[Crossref] [Google Scholar] [PubMed]
- Jahanban-Esfahlan A, Ostadrahimi A, Tabibiazar M, Amarowicz R. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk. Int J Mol Sci 2019;20(16):3920.
[Crossref] [Google Scholar] [PubMed]
- Chen F, Chen J, Wang Z, Zhang J, Li X, Lin M, et al. Genomics: Cracking the mysteries of walnuts. J Genet 2019;98:1-3.
[Crossref] [Google Scholar] [PubMed]
- Bunge A, Diemont SA, Bunge JA, Harris S. Urban foraging for food security and sovereignty: Quantifying edible forest yield in Syracuse, New York using four common fruit-and nut-producing street tree species. J Urban Ecol 2019;5(1):28.
- Poggetti L, Ferfuia C, Chiabà C, Testolin R, Baldini M. Kernel oil content and oil composition in walnut (Juglans regia L.) accessions from north‐eastern Italy. J Sci Food Agric 2018;98(3):955-62.
[Crossref] [Google Scholar] [PubMed]
- Li X, Guo M, Chi J, Ma J. Bioactive peptides from walnut residue protein. Molecules 2020;25(6):1285.
[Crossref] [Google Scholar] [PubMed]
- Jahanban-Esfahlan A, Davaran S, Moosavi-Movahedi AA, Dastmalchi S. Investigating the interaction of juglone (5-hydroxy-1, 4-naphthoquinone) with serum albumins using spectroscopic and in silico methods. J Iran Chem Soc 2017;14:1527-40.
- Roufegarinejad L, Amarowicz R, Jahanban-Esfahlan A. Characterizing the interaction between pyrogallol and human serum albumin by spectroscopic and molecular docking methods. J Biomol Struct Dyn 2019;37(11):2766-75.
[Crossref] [Google Scholar] [PubMed]
- Junaid M, Basak B, Akter Y, Afrose SS, Nahrin A, Emran R, et al. Sakuranetin and its therapeutic potentials-a comprehensive review. Z Naturforsch C J Biosci 2023;78(1-2):27-48.
[Crossref] [Google Scholar] [PubMed]
- Jahanban-Esfahlan A, Jahanban-Esfahlan R, Tabibiazar M, Roufegarinejad L, Amarowicz R. Recent advances in the use of walnut (Juglans regia L.) shell as a valuable plant-based bio-sorbent for the removal of hazardous materials. RSC Adv 2020;10(12):7026-47.
[Crossref] [Google Scholar] [PubMed]
- Salimi M, Ardestaniyan MH, Mostafapour KH, Saeidnia S, Gohari AR, Amanzadeh A, et al. Anti‐proliferative and apoptotic activities of constituents of chloroform extract of Juglans regia leaves. Cell Prolif 2014;47(2):172-9.
[Crossref] [Google Scholar] [PubMed]
- Pereira JA, Oliveira I, Sousa A, Valentão P, Andrade PB, Ferreira IC, et al. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem Toxicol 2007;45(11):2287-95.
[Crossref] [Google Scholar] [PubMed]
- Rather MA, Dar BA, Dar MY, Wani BA, Shah WA, Bhat BA, et al. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine 2012;19(13):1185-90.
[Crossref] [Google Scholar] [PubMed]
- Li CY, Du HJ, Su XH, Zhong YJ, Yuan ZP, Li YF, et al. Juglanones A and B: Two novel tetralone dimers from walnut pericarp (Juglans regia). Helv Chim Acta 2013;96(6):1031-5.
- Colaric M, Veberic R, Solar A, Hudina M, Stampar F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J Agric Food Chem 2005;53(16):6390-6.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Meng M, Li C, Huang X, Di D. Simultaneous determination of three diarylheptanoids and an α-tetralone derivative in the green walnut husks (Juglans regia L.) by high-performance liquid chromatography with photodiode array detector. J Chromatogr A 2008;1190(1-2):80-5.
[Crossref] [Google Scholar] [PubMed]
- Tan B, Wang Y, Zhang X, Sun X. Recent studies on protective effects of walnuts against neuroinflammation. Nutrients 2022;14(20):4360.
[Crossref] [Google Scholar] [PubMed]
- Rezaeizadeh H, Rahimi R, Abbasi M. Fatigue due to multiple sclerosis: A comparison between Persian medicine and conventional medicine. Galen Med J 2019;8:e1139.
[Crossref] [Google Scholar] [PubMed]
- Beigh S, Rashid H, Sharma S, Parvez S, Raisuddin S. Bleomycin-induced pulmonary toxicopathological changes in rats and its prevention by walnut extract. Biomed Pharmacother 2017;94:418-29.
[Crossref] [Google Scholar] [PubMed]
- Saadat S, Beigoli S, Khazdair MR, Amin F, Boskabady MH. Experimental and clinical studies on the effects of natural products on noxious agents-induced lung disorders, a review. Front Nutr 2022;9:867914.
[Crossref] [Google Scholar] [PubMed]
- Jahanban-Esfahlan A, Ostadrahimi A, Tabibiazar M, Amarowicz R. A comparative review on the extraction, antioxidant content and antioxidant potential of different parts of walnut (Juglans regia L.) fruit and tree. Molecules 2019;24(11):2133.
[Crossref] [Google Scholar] [PubMed]
- Sadogh A, Gorji N, Moeini R. Herbal foodstuffs in Avicenna’s recommended diet to improve sperm quality and increase male fertility; an evidence-based approach. J Complement Integr Med 2022;19(1):47-70.
[Crossref] [Google Scholar] [PubMed]
- Robbins WA, Xun L, FitzGerald LZ, Esguerra S, Henning SM, Carpenter CL. Walnuts improve semen quality in men consuming a Western-style diet: Randomized control dietary intervention trial. Biol Reprod 2012;87(4):101-.
[Crossref] [Google Scholar] [PubMed]
- Robbins W, Kim H, Houman J, Lee GW. Randomized clinical trial: effect of walnuts on semen parameters and male fertility (P18-042-19). Curr Dev Nutr 2019;3:nzz039-P18.
[Crossref] [Google Scholar] [PubMed]
- Santos HO, Price JC, Bueno AA. Beyond fish oil supplementation: the effects of alternative plant sources of omega-3 polyunsaturated fatty acids upon lipid indexes and cardiometabolic biomarkers-An overview. Nutrients 2020;12(10):3159.
[Crossref] [Google Scholar] [PubMed]
- Fitzgerald E, Lambert K, Stanford J, Neale EP. The effect of nut consumption (tree nuts and peanuts) on the gut microbiota of humans: A systematic review. Br J Nutr 2021;125(5):508-20.
[Crossref] [Google Scholar] [PubMed]
- Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017;8(2):172-84.
[Crossref] [Google Scholar] [PubMed]
- So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am J Clin Nutr 2018;107(6):965-83.
[Crossref] [Google Scholar] [PubMed]
- Lichtenberger LM, Zhou Y, Jayaraman V, Doyen JR, O'Neil RG, Dial EJ, et al. Insight into NSAID-induced membrane alterations, pathogenesis and therapeutics: Characterization of interaction of NSAIDs with phosphatidylcholine. Biochim Biophys Acta 2012;1821(7):994-1002.
[Crossref] [Google Scholar] [PubMed]
- An JM, Kim EH, Lee H, Lee HJ, Hahm KB. Dietary walnut as food factor to rescue from NSAID-induced gastrointestinal mucosal damages. Arch Biochem Biophys 2020;689:108466.
[Crossref] [Google Scholar] [PubMed]
- Rusu ME, Gheldiu AM, Mocan A, Moldovan C, Popa DS, Tomuta I, et al. Process optimization for improved phenolic compounds recovery from walnut (Juglans regia L.) septum: Phytochemical profile and biological activities. Molecules 2018;23(11):2814.
[Crossref] [Google Scholar] [PubMed]
- Park JM, An JM, Han YM, Surh YJ, Hwang SJ, Kim SJ, et al. Walnut polyphenol extracts inhibit Helicobacter pylori-induced STAT3Tyr705 phosphorylation through activation of PPAR-γ and SOCS1 induction. J Clin Biochem Nutr 2020;67(3):248-56.
[Crossref] [Google Scholar] [PubMed]
- Reiter RJ, Tan DX, Manchester LC, Korkmaz A, Fuentes-Broto L, Hardman WE, et al. A walnut-enriched diet reduces the growth of LNCaP human prostate cancer xenografts in nude mice. Cancer Invest 2013;31(6):365-73.
[Crossref] [Google Scholar] [PubMed]
- Figueroa F, Marhuenda J, Zafrilla P, Villaño D, Martínez-Cachá A, Tejada L, et al. High-performance liquid chromatography-diode array detector determination and availability of phenolic compounds in 10 genotypes of walnuts. Int J Food Prop 2017;20(5):1074-84.
- Zhang YY, Zhang F, Zhang YS, Thakur K, Zhang JG, Liu Y, et al. Mechanism of juglone-induced cell cycle arrest and apoptosis in Ishikawa human endometrial cancer cells. J Agric Food Chem 2019;67(26):7378-89.
[Crossref] [Google Scholar] [PubMed]
- Adams LS, Zhang Y, Seeram NP, Heber D, Chen S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev Res 2010;3(1):108-13.
[Crossref] [Google Scholar] [PubMed]
- Zhang YG, Kan H, Chen SX, Thakur K, Wang S, Zhang JG, et al. Comparison of phenolic compounds extracted from Diaphragma juglandis fructus, walnut pellicle, and flowers of Juglans regia using methanol, ultrasonic wave, and enzyme assisted-extraction. Food Chem 2020;321:126672.
[Crossref] [Google Scholar] [PubMed]
- Khalil AA, Khan MR, Shabbir MA. In vitro antioxidant activity and punicalagin content quantification of pomegranate peel obtained as agro-waste after juice extraction. Pak J Agric Sci 2018;55(1).
- Pandareesh MD, Chauhan V, Chauhan A. Walnut supplementation in the diet reduces oxidative damage and improves antioxidant status in transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis 2018;64(4):1295-305.
[Crossref] [Google Scholar] [PubMed]
- Solomon A, Shmueli O, Harari R, Ovadia H, Erdinest N. Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Invest Ophthalmol Vis Sci 2012;53(14):4194.
[Crossref] [Google Scholar] [PubMed]
- Reifen R, Karlinsky A, Stark AH, Berkovich Z, Nyska A. α-Linolenic Acid (ALA) is an anti-inflammatory agent in inflammatory bowel disease. J Nutr Biochem 2015;26(12):1632-40.
[Crossref] [Google Scholar] [PubMed]
- Muthaiyah B, Essa MM, Lee M, Chauhan V, Kaur K, Chauhan A. Dietary supplementation of walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer's disease. J Alzheimers Dis 2014;42(4):1397-405.
[Crossref] [Google Scholar] [PubMed]
- Mousavi SM, Karimi E, Hajishafiee M, Milajerdi A, Amini MR, Esmaillzadeh A. Anti-hypertensive effects of cinnamon supplementation in adults: A systematic review and dose-response meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr 2020;60(18):3144-54.
[Crossref] [Google Scholar] [PubMed]
- Badimon L, Chagas P, Chiva-Blanch G. Diet and cardiovascular disease: Effects of foods and nutrients in classical and emerging cardiovascular risk factors. Curr Med Chem 2019;26(19):3639-51.
[Crossref] [Google Scholar] [PubMed]
- Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal K, et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr 2019;59(7):1071-90.
[Crossref] [Google Scholar] [PubMed]
- White-Williams C, Rossi LP, Bittner VA, Driscoll A, Durant RW, Granger BB, et al. Addressing social determinants of health in the care of patients with heart failure: A scientific statement from the American Heart Association. Circulation 2020;141(22):e841-63.
[Crossref] [Google Scholar] [PubMed]
- Kurihara A, Okamura T, Sugiyama D, Higashiyama A, Watanabe M, Okuda N, et al. Vegetable protein intake was inversely associated with cardiovascular mortality in a 15-year follow-up study of the general Japanese population. J Atheroscler Thromb 2019;26(2):198-206.
[Crossref] [Google Scholar] [PubMed]
- Zhao LG, Zhang QL, Liu XL, Wu H, Zheng JL, Xiang YB. Dietary protein intake and risk of type 2 diabetes: A dose-response meta-analysis of prospective studies. Eur J Nutr 2019;58:1351-67.
[Crossref] [Google Scholar] [PubMed]
- Leape LL, Berwick DM, Bates DW. What practices will most improve safety?: Evidence-based medicine meets patient safety. Jama 2002;288(4):501-7.
[Crossref] [Google Scholar] [PubMed]
- Bober JR, Beisel CL, Nair NU. Synthetic biology approaches to engineer probiotics and members of the human microbiota for biomedical applications. Annu Rev Biomed Eng 2018;20(1):277-300.
[Crossref] [Google Scholar] [PubMed]
- Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, et al. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKCα in human intestinal epithelial cells. Gastroenterology 2005;128(4):962-74.
[Crossref] [Google Scholar] [PubMed]
- Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, et al. Best practices for analysing microbiomes. Nat Rev Microbiol 2018;16(7):410-22.
[Crossref] [Google Scholar] [PubMed]
- Song Y, Ammar S, Shaoqi LI, Yang W, Inventors; Shandong University of Technology, Assignee, et al. Heart-protective plant preparation for preventing and improving coronary heart disease. United States patent US 11,077,162.2021.
- Bhagat MT, Inventor; Jiwaji University, Gwalior, Assignee. Method for preparation of synergistic herbal extract of Juglans regia for treatment of cerebral stroke. India patent IN201921025805 01-2021.
- Fang Lv HZ, Qingtao Tang, Inventor; Infinitus (China) Company Ltd., Assignee. Powder formulation, method for preparing the same and use thereof. United States of America patent US20190111098:2019.
- Wei L, Inventor; Beijing Lu De Kai Qi Co., Ltd, Assignee. Pharmaceutical product for treating tumor and combined immune defect, and preparation and application thereof. Europe Patent EP3441076:2019.
- Xingfeng W, Inventor; Shenyang Wanrun Biological Technology Co., Ltd., Assignee. Juglans regia paste for health care and using method thereof. China Patent CN107913302:2018.
- Wei L, Inventor; Beijing Lu De Kai Qi Co., Ltd., Assignee. Pharmaceutical product for treating lupus erythematosus and combined immune defect, and preparation and application thereof. Europe Patent EP3441078:2019.
- Bitkauskas G, Inventor; Ekologiska Energija, Assignee. Energy drink for men and women. Patent WO2015174808:2015.
- Patil CR, Jadhav PS, Lokhande MV, Abraham KG, Farhaan MM, Kakde UB, et al. Oral care composition. India Patent IN1102/MUM/2012.
- Pavlov A, Inventor; Pavlov, Aleksanda, Assignee. Medical cosmetic herbal composition on the basis of herbal mixture and the procedure for its obtention. Patent WO2012033422:2010.
- Fang Z, Dang M, Zhang W, Wang Y, Kord-Varkaneh H, Nazary-Vannani A, et al. Effects of walnut intake on anthropometric characteristics: a systematic review and dose-response meta-analysis of randomized controlled trials. Complement Ther Med 2020;50:102395.
- Mirzababaei A, Daneshvar M, Abaj F, Daneshzad E, Hosseininasab D, Clark CC, et al. The effect of walnut (Juglans regia) leaf extract on glycemic control and lipid profile in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized clinical trials. Clin Nutr Res 2022;11(2):120.
[Crossref] [Google Scholar] [PubMed]
- Neale EP, Guan V, Tapsell LC, Probst YC. Effect of walnut consumption on markers of blood glucose control: A systematic review and meta-analysis. Br J Nutr 2020;124(7):641-53.
[Crossref] [Google Scholar] [PubMed]
- Arabi SM, Bahrami LS, Milkarizi N, Nematy M, Kalmykov V, Sahebkar A. Impact of walnut consumption on cardio metabolic and anthropometric parameters in metabolic syndrome patients: GRADE-assessed systematic review and dose-response meta-analysis of data from randomized controlled trials. Pharmacol Res 2022;178:106190.
[Crossref] [Google Scholar] [PubMed]
- Cahoon D, Shertukde SP, Avendano EE, Tanprasertsuk J, Scott TM, Johnson EJ, et al. Walnut intake, cognitive outcomes and risk factors: A systematic review and meta-analysis. Ann Med 2021;53(1):972-98.
[Crossref] [Google Scholar] [PubMed]
- Fatahi S, Haghighatdoost F, Larijani B, Azadbakht L. Effect of weight reduction diets containing fish, walnut or fish plus walnut on cardiovascular risk factors in overweight and obese women. Arch Iran Med 2019;22(10):574-83.
[Google Scholar] [PubMed]
- Domènech M, Serra-Mir M, Roth I, Freitas-Simoes T, Valls-Pedret C, Cofán M, et al. Effect of a walnut diet on office and 24-hour ambulatory blood pressure in elderly individuals: Findings from the WAHA randomized trial. Hypertension 2019;73(5):1049-57.
[Crossref] [Google Scholar] [PubMed]
- Li J, Jiang B, O. Santos H, Santos D, Singh A, Wang L. Effects of walnut intake on blood pressure: A systematic review and meta‐analysis of randomized controlled trials. Hypertension 2020;34(11):2921-31.
[Crossref] [Google Scholar] [PubMed]
- Yang L, Guo Z, Qi S, Fang T, Zhu H, Santos HO, et al. Walnut intake may increase circulating adiponectin and leptin levels but does not improve glycemic biomarkers: A systematic review and meta-analysis of randomized clinical trials. Complement Ther Med 2020;52:102505.
[Crossref] [Google Scholar] [PubMed]
- Creedon AC, Hung ES, Berry SE, Whelan K. Nuts and their effect on gut microbiota, gut function and symptoms in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2020;12(8):2347.
[Crossref] [Google Scholar] [PubMed]
- Petran M, Dragos D, Gilca M. Historical ethnobotanical review of medicinal plants used to treat children diseases in Romania (1860s-1970s). J Ethnobiol Ethnomed 2020;16:1-33.
[Crossref] [Google Scholar] [PubMed]
- Catanzaro E, Greco G, Potenza L, Calcabrini C, Fimognari C. Natural products to fight cancer: A focus on Juglans regia. Toxins 2018;10(11):469.
[Crossref] [Google Scholar] [PubMed]
- Mohammadi-Sartang M, Bellissimo N, de Zepetnek JO, Bazyar H, Mahmoodi M, Mazloom Z. Effects of walnuts consumption on vascular endothelial function in humans: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN 2018;28:52-8.
[Crossref] [Google Scholar] [PubMed]
- Ros E, Izquierdo-Pulido M, Sala-Vila A. Beneficial effects of walnut consumption on human health: Role of micronutrients. Curr Opin Clin Nutr Metab Care 2018;21(6):498-504.
[Crossref] [Google Scholar] [PubMed]
- Sari TP, Sirohi R, Krishania M, Bhoj S, Samtiya M, Duggal M, et al. Critical overview of biorefinery approaches for valorization of protein rich tree nut oil industry by-product. Bioresour Technol 2022;362:127775.
[Crossref] [Google Scholar] [PubMed]
- Chauhan A, Chauhan V. Beneficial effects of walnuts on cognition and brain health. Nutrients 2020;12(2):550.
[Crossref] [Google Scholar] [PubMed]