- Corresponding Author:
- Mala D. Menon
Bombay College of Pharmacy, Kalina, Santacruz (E), Mumbai-400 098, India
Email: maladmbcp@yahoo.co.in
| Date of Submission | 27 August 2010 |
| Date of Revision | 16 December 2011 |
| Date of Acceptance | 25 December 2011 |
| Indian J Pharm Sci, 2011, 73 (6): 656-662 |
Abstract
Etoposide is a semisynthetic compound, widely used in treatment of non small cell lung cancer. However, frequent dosing and adverse effects remain a major concern in the use of etoposide. Liposomal systems for pulmonary drug delivery have been particularly attractive because of their compatibility with lung surfactant components. In the present investigation, pulmonary liposomal delivery system of etoposide was prepared by film hydration method. Various parameters were optimized with respect to entrapment efficiency as well as particle size of etoposide liposomes. For better shelf life of etoposide liposomes, freeze drying using trehalose as cryoprotectant was carried out. The liposomes were characterized for entrapment efficiency, particle size, surface topography, and in vitro drug release was carried out in simulated lung fluid at 37° at pH 7.4. The respirable or fine particle fraction was determined by using twin stage impinger. The stability study of freeze dried as well as aqueous liposomal systems was carried out at 2-8° and at ambient temperature (28±4°). The freeze dried liposomes showed better fine particle fraction and drug content over the period of six months at ambient as well as at 2-8° storage condition compared to aqueous dispersion of liposomes.
Keywords
Etoposide, inhalation, liposome, pulmonary delivery, twin stage impinger
Inhalation aerosols are attractive delivery platforms as compared to conventional systemic therapy, which include its non invasive patient friendly mode of administration, reduction in dose, minimized adverse effects. Pulmonary system provides large surface area for absorption and the relatively low metabolic activity of the lungs make this organ system a potential route for the systemic delivery of drugs not easily delivered by other means [1-7].
Etoposide (ETP) is an antineoplastic agent, which acts by forming a ternary complex with topoisomerase II and DNA, causing DNA breaks and cell death. It is part of the first line therapy for small cell lung carcinoma and drug-resistant testicular cancer. However, strong lipophilicity is a major limitation in the administration of ETP, and vehicles needed as solubilizers have often been associated with adverse effects. Liposomal encapsulation of ETP has been found to be effective when administered by i.v. route in animals and a reduction in haematological toxicity is reported [8,9].
Liposomal delivery systems are advantageous to improve the solubilization of poorly soluble agents, and can act as a sustained release reservoir. It is also useful potentially target specifically to alveolar macrophages to ensure better uptake of drug. It is compatible with lung surfactant components. [3,10,11].
Sustained release formulations of ETP in the lung provides better retention of drug as well as reduces biodistribution of drug throughout the systemic circulation and hence reduced incidence of side effects. Thus, sustained release etoposide formulations can provide prolonged drug release and hence, better anticancer activity [12,13]. The objective of the present work was to develop an effective ETP liposomal aerosol formulation for lung delivery, and thereby achieve improved therapy in lung cancer
Materials and Methods.
Phospholipon 90 G and Phospholipon 90 H were kindly gifted by Natterman GmbH Germany. Etoposide (ETP) was gifted by Dabur Research Foundation Pvt. Ltd. Gaziabad, India; while trehalose was a kind gift from Hyashibara, Japan. Cholesterol was purchased from s.d. fine-chem, Mumbai, India. All the other solvents and chemicals were of analytical grade and were obtained from s.d. fine-chem, Mumbai, India.
Preparation of ETP–liposomes:
Liposomes were prepared by lipid film hydration method [14]. The hydration of the lipid film was carried out with hydroxy propyl β-cyclodextrin (HPβCD) solution at 600. Vesicle size was reduced by subjecting the liposomes to mechanical shaking for upto 6 h on a horizontal shaker bath (Expo, India) followed by intermittent sonication (30 s) carried out after every 2 h, in a bath sonicator (Expo, India) at room temperature. The unentrapped drug was removed by centrifugation (20,000 g, Beckman, U.S.A.). Various parameters such as cholesterol concentration, lipid proportion and shaking time were optimized with respect to particle size and entrapment efficiency. The liposomal dispersion of ETP was freeze dried using trehalose as cryoprotectant (Labconco, England). The freeze dried ETP-liposomes were stored in a refrigerator in sealed vials until use. Thermal behaviour of ETP liposomes before and after freeze drying was studied using DSC (DT40, Shimadzu Japan).
Characterization of freeze dried liposomes:
The particle size distribution of the liposomes was assessed on a Malvern Mastersizer (Malvern Instruments, USA) after rehydration with saline. Transmission electron microscopy (TEM) images were visualized using the electron microscope (Jeol JEM-1010, Japan) and photographed. Surface topography of the freeze dried liposomes was determined using environmental scanning electron microscope (Quanta-200, FEI). To visualize the reformation of liposomes in presence of saline, the dry powder was wetted with a drop of saline, and observed over a period of 5 min under ESEM and photographed.
Determination of entrapment efficiency (EE):
The EE was determined using reverse phase HPLC. The chromatographic system consisted of JASCO PU 980 Intelligent Pump, JASCO UV 975, a UV-VIS detector Version 1.50 Build 15 (Jasco-BorwinTM, Japan) and Rheodyne injector valve bracket fitted with a 20 ml sample loop. HPLC separations were performed on a stainless-steel Technochrom kromacil; C-18 analytical column (250×4.6 mm) packed with 5 mm diameter particles. Data was processed using Chromatography Software, Hercule 2000 chromatography interface star 800 interface module interface Version 2.0 (Jasco-BorwinTM, Japan). The composition of the mobile phase was methanol: water: glacial acetic acid 0.1%, (60:40:1). The mobile phase was delivered at a flow rate of 1 ml/min. The injection volume was 20 ml and the retention time was found to be 4.3 min.
For EE of ETP liposomes, 0.25 ml of liposomal preparation was diluted to 4 ml with distilled water and centrifuged at 20000 g at 8°±0.5° for 30 min using Allegra TM 64 R centrifuge (Beckman, U.S.A.). The supernatant was appropriately diluted in distilled water and the amount of drug present in the supernatant was determined by reverse phase HPLC. Entrapment efficiency was calculated as the difference between the initial amount of drug added and that present in the supernatant as free drug, and was expressed as μg of drug entrapped per mM of total phospholipids (μg/mM of lipid).
In vitro drug release
In vitro drug release profile of freeze dried ETP liposomes was assessed in simulated lung fluid (pH 7.4) at 370 by static method. After periodic intervals of time, the liposomal aliquots were centrifuged and supernatant was evaluated for the drug content by HPLC.
In vitro drug deposition studies by twin stage impinger (TSI)
The respirable fine particle fraction (FPF) of the ETP liposomes was determined using a twin-stage impinger, apparatus A (TSI), official in Indian Pharmacopoeia. The liposomes reconstituted in saline was nebulized using the nebulizer (Omron CX3) attached to the TSI by means of rubber collars. The vacuum pump was operated at 28 l/min. The fractions collected in each stage were analyzed for ETP content by HPLC using the chromatographic conditions as described above. Fine particle fraction (FPF) i.e., respirable fraction is expressed as the ratio of the amount of drug recovered from the lower stage of the impinger to the sum of drug recovered from the capsule shells, the inhaler device and the upper and lower stages of the twin stage impinger.
Stability studies
Stability evaluation of aqueous liposome suspension and freeze dried liposomes was carried out at 2-8º and ambient condition (28±4°) for a period of six months. Samples withdrawn at specified time intervals (1, 3 and 6 month) were visually observed for appearance, ease of reconstitution, and sedimentation. Also the liposomes were evaluated for ETP content, particle size and FPF.
Results and Discussion
The advantage provided by liposomes as drug carrier is based on their ability to alter favuorably the pharmacokinetic profile of the encapsulated species and thus provide selective and prolonged pharmacological effects at the site of administration.
ETP liposomes were prepared by lipid film hydration method. ETP, being practically insoluble in water, may precipitate out on hydration of liposomes. This would lower the entrapment efficiency and possibly destabilize the liposomes. Many studies report the use of HPβCD drug complexes to improve the entrapment and impart stability to liposomes [15,16]. To determine the effect of hydration medium on entrapment efficiency of ETP, liposomes were prepared by lipid film hydration using distilled water or HPβCD solution. HPβCD when used as hydration medium increased the entrapment efficiency of ETP by 50%. Improved entrapment efficiency of ETP may be attributed to complexation of drug with HPβCD. The solution of ETP HPβCD complex is able to travel inside the liposomes during hydration and lead to increase in entrapment efficiency.
Entrapment efficiency also depends on the formulation variables i. e., lipid composition used. To study this aspect, several batches of ETP liposomes were prepared with varying lipid compositions. Lipid composition of various batches, their EE and particle size observed are listed in Table 1. Decreased EE, after increasing cholesterol may be attributed to the lipophilic nature of cholesterol and hence possibly competing with ETP in the lipid bilayer and reducing the entrapment of ETP. Glavas-Dodov et al., have reported similar observations for 5-flourouracil [17].
| Batch code | Composition | Drug content | Mean particle size (µm) | ||||
|---|---|---|---|---|---|---|---|
| (µg/mM of lipid) | |||||||
| CH | PL | PL | Before freeze | After freeze | Before freeze | After freeze | |
| 90 G | 90 H | drying | drying | drying | drying | ||
| EL-1 | 2 | 1 | 9 | 514.7 | 485.07±77.8 | 4.13±0.29 | 5.55±0.57 |
| EL-4 | 2 | 3 | 7 | 797.4 | 811.55±56.98 | 3.83±0.08 | 6.05±0.90 |
| EL-7 | 2 | 5 | 5 | 1229.2 | 1170.99±43.06 | 3.37±0.12 | 5.98±0.74 |
| EL-10 | 6 | 1 | 9 | 607.8 | 652.38±66.63 | 5.13±0.22 | 6.15±0.83 |
| EL-13 | 6 | 3 | 7 | 566.3 | 618.06±68.01 | 4.54±0.42 | 6.72±0.43 |
| EL-16 | 6 | 5 | 5 | 1031.2 | 972.22±117.98 | 4.82±0.15 | 6.76±0.59 |
| EL-19 | 10 | 1 | 9 | 258.9 | 242.24±42.32 | 6.80±0.25 | 7.35±0.98 |
| EL-22 | 10 | 3 | 7 | 381.8 | 333.59±60.95 | 6.17±0.15 | 7.54±1.15 |
| EL-25 | 10 | 5 | 5 | 764.8 | 736.54±44.77 | 5.48±0.15 | 7.57±0.95 |
There was decrease in entrapment efficiency with increasing cholesterol as well as PL 90H, and decreasing PL 90G. Slight increase in particle size is evident on freeze drying
Table 1: Effect Of Freeze Drying On Drug Content And Particle Size
Increasing the amount of Phospholipon 90 G increased the EE of ETP. This could be attributed to the fact that unsaturated phospholipids are relatively more flexible in nature, and thereby provide less hindrance for ETP to be retained in lipid bilayer. Increasing the saturated lipid, Phospholipon 90 H resulted in reduced EE; this could be due to the saturated lipid behaving in the same way as cholesterol. Better hydration was achieved by increasing shaking time, leading to increased EE. This could be attributed to better solubilization of ETP in HPβCD and subsequent increased EE. Further investigations were carried out on batch EL 7 as it had shown better drug content as well as particle size.
Liposomal delivery systems are highly susceptible to degradation. Various approaches have been developed and evaluated to impart long term stability to liposomes and one of them is lyophilization [18]. To maintain the same particle size distribution and to avoid leakage of the encapsulated drug from liposomes after the freeze-drying/rehydration cycle, suitable cryoprotectants like disaccharides need to be added, which prevent fusion, aggregation and leakage of encapsulated compounds [17]. In the present study, liposomes were freeze dried using various cryoprotectants. Several disaccharides like lactose, mannitol, maltodextrin, and trehalose were evaluated for their cryoprotective effects at varying ratios. Lipid: trehalose at ratio of 1:10 was found to be most suitable, as less drug leakage and minimal change of particle size was observed after freeze drying.
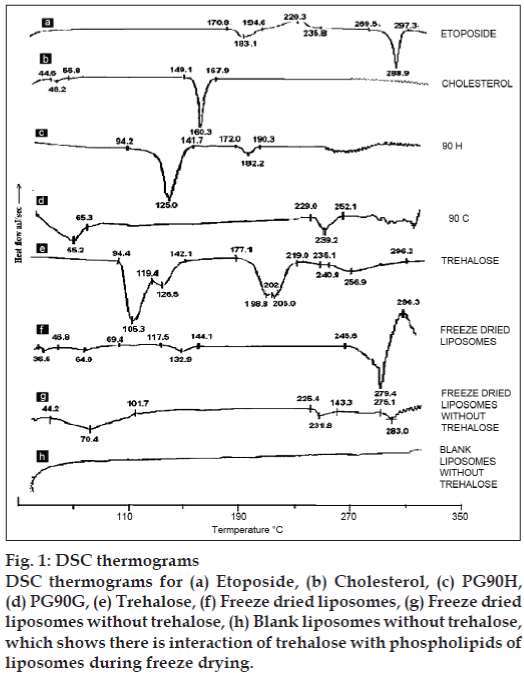
DSC spectra of various batches are given in fig. 1. DSC spectra of ETP as in fig. 1 showed one sharp endotherm at 288.9º with onset temperature 269.5º and recovery temperature 297.3º. Freeze dried liposomes gave thermal events in the range of 117.5-144.1º, and a big endothermic peak at 279.4º immediately followed by exotherm with peak temperature at 296.5º. This event indicated the interaction of trehalose with phospholipids of liposomes during freeze drying.
Fig. 1: DSC thermograms
DSC thermograms for (a) Etoposide, (b) Cholesterol, (c) PG90H, (d) PG90G, (e) Trehalose, (f) Freeze dried liposomes, (g) Freeze dried
liposomes without trehalose, (h) Blank liposomes without trehalose,
which shows there is interaction of trehalose with phospholipids of
liposomes during freeze drying.
The sharp endotherm of ETP at 288.9º was masked when freeze dried ETP-loaded liposomes were analyzed. The broad endotherm started at 245.6º with peak of endotherm at 279.4º and followed immediately by exotherm at 297.3º. So to detect the presence of ETP in liposomes, a DSC scan was carried out on freeze dried blank and ETP loaded liposomes without trehalose. Blank liposomes did not show any thermal event for the entire temperature range studied, while a small broad endotherm was observed at 283º in ETP loaded liposomes indicating presence of the drug. The shift in the endotherm from 288.9º may be due to the presence of lipids.
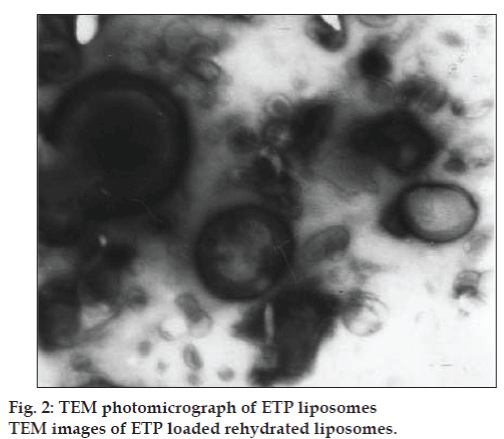
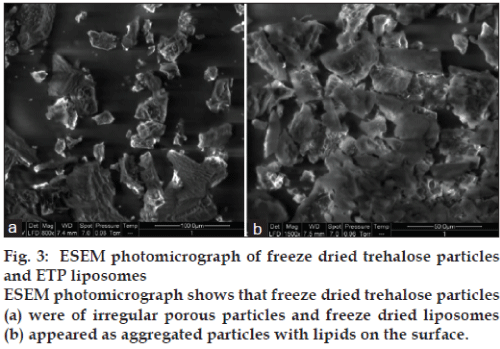
The TEM images of ETP loaded rehydrated liposomes are shown in fig. 2. The liposomes appeared as bright spheres surrounded by dark thick layer showing large internal aqueous core by one or two lamelle. The ESEM analysis showed the freeze dried trehalose particles as irregular porous particles as shown in fig. 3a, whereas the freeze dried liposomes fig. 3b appeared as aggregated particles with lipids on the surface.
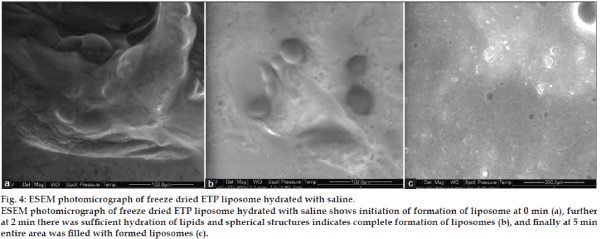
ESEM was used to study liposome formation from freeze dried liposomes and to provide an alternative method to observe liposome formation after rehydration. Dynamic formation of liposomes was monitored by placing drop of saline on the tab and analyzing over a period of 5 min. The ESEM photographs of hydrated freeze dried liposomes at 0 min, 2 min and after complete hydration (5 min) are shown in figs. 4a-4c respectively. At 0 min, after adding few drops of saline on tab with freeze dried liposomes, trehalose started dissolving in saline and liposome formation was initiated due to budding off mechanism. Following sufficient hydration of the lipids on trehalose particles, spherical structures were clearly seen, after 2 min liposomes were completely formed, and after 5 min entire area was filled with formed liposomes.
Fig. 4: ESEM photomicrograph of freeze dried ETP liposome hydrated with saline.
ESEM photomicrograph of freeze dried ETP liposome hydrated with saline shows initiation of formation of liposome at 0 min (a), further at 2 min there was sufficient hydration of lipids and spherical structures indicates complete formation of liposomes (b), and finally at 5 min
entire area was filled with formed liposomes (c).
The particle size increased in all the liposomes irrespective of the formulation variables indicating aggregation of liposomes after freeze drying. However, no significant difference was observed in drug content after freeze drying of ETP liposomes indicating minimum leakage. Table 1 shows the result of drug content and particle size before and after freeze drying. These results indicate the protection provided by trehalose.
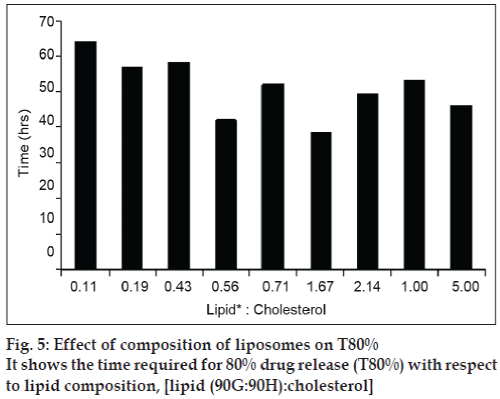
The drug release was sustained for more than 70 h for all batches but there was significant difference in release rate of the drug between the batches as shown in fig 5. The formulation variables like cholesterol content, and lipid content (90 G and 90 H), played a major role in the drug release. Fig. 5 shows the time required for 80% drug release (T80%) with respect to lipid ratio [lipid (90G:90H):cholesterol]. It clearly showed that increase in the cholesterol content reduced T80%. Thus it can be concluded that, the permeability of the bilayer is strongly influenced by its constituents. It can be seen that high amount of cholesterol significantly reduced the T80%.The FPF of both liposomal dispersions as well as freeze dried liposomes ranged around 50%, revealing good respirable properties.
Stability of freeze dried liposomes and aqueous dispersion of ETP liposomes was assessed at 2-8° and at ambient conditions (28±4°). Stored samples were evaluated periodically for change in colour, appearance, particle size and drug leakage. The change in particle size as well as drug leakage from the freeze dried liposomes and aqueous suspension of liposomes in stability studies are as given in Table 2.
| Aq. dispersion | Freezd dried liposome | |||||
|---|---|---|---|---|---|---|
| (After six month) | (After six month) | |||||
| Initial | 2-8° | Ambient | Initial | 2-8° | Ambient | |
| Mean particle size (µ) | 3.12±0.32 | 10.54±0.84 | 17.58±1.88 | 5.14±0.46 | 8.88±1.10 | 7.94±0.47 |
| Drug leakage (%) | - | 18.70±2.38 | 24.14±4.27 | - | 4.95±1.90 | 4.32±1.14 |
| Fine particle fraction (FPF) (%) | 51.21±1.89 | 42.23±2.57 | 37.16±3.75 | 49.23±2.12 | 44.19±2.71 | 45.81±2.44 |
Table 2: Drug Leakage and Particle Size Of Stability Samples Of Etp Liposomes
Sedimentation of lipid phase was observed in aqueous liposomes which upon gentle shaking easily get redispersed, and no colour change was evident upon storage. Microscopic observation of aqueous liposomes showed absence of drug precipitation; however the particle size was markedly increased.
At 2-8º, aqueous liposomes showed lesser aggregates compared to the ones stored at ambient conditions in which aggregates of liposomal vesicles were observed. Particle size distribution of aqueous liposomes stored at different conditions showed shifting towards increasing the particle size and the range of particle size was also increased as the time increased. Several reports on light scattering studies of the coalescence kinetics of liposomes have revealed that the average cluster size grows with time following a characteristic behaviour. After the aggregation, liposomes tend to form large vesicles via coalescence [3].
Liposomes were easily formed when stored samples of freeze dried liposomes were mixed with saline on gentle shaking, and no color change was seen upon storage. Microscopic observation of rehydrated freeze dried liposomes did not show drug crystals. No significant difference was observed in the particle size of freeze dried liposomes stored for a period of six month at different conditions. The drug leakage was significantly less in freeze dried liposomes compared to aqueous liposomes, and was observed to be in accordance with the increase in particle size. It was observed that increase in particle size was directly associated with the drug leakage.
The effect on FPF of the freshly prepared liposomes was compared with stored freeze dried and aqueous liposomes. FPF was determined using TSI. For freeze dried liposomes, increase in storage time did not significantly change the FPF in six months storage at both the storage conditions. On the other hand, aqueous dispersion showed decrease in FPF at the different storage conditions. This decrease in the FPF can be attributed to the increase in particle size.
Drug delivery to or via the respiratory tree has been a long standing pharmaceutical objective. For locally acting agents it is desirable to confine the action of the drug to the lungs in order to eliminate unintended side effects, which might result following absorption and distribution to other extra vascular sites. Oral inhalation is often the preferred route in order that such effects can be minimized. Here, ETP-loaded liposomes were developed optimized and evaluated for its suitability for pulmonary sustained drug delivery for treatment of cancer. Liposomes were successfully developed and freeze dried using trehalose as cryoprotectant. Further in vivo safety and deposition studies in suitable animal models will give better insight for clinical applications of the inhalable ETP liposomes.
Acknowledgements
The authors are thankful to BRNS-DAE for providing financial assistance for the present investigation (Sanction no. 2004/35/5/BRNS).
References
- Courrier HM, Butz N, Vandamme TF. Pulmonary drug delivery systems: Recent developments and prospects. Crit Rev Ther Drug Carrier Syst 2002;19:425-98.
- Groneberg DA, Witt C, Wagner U, Chung KF, Fischer A. Fundamentals of pulmonary drug delivery. Respir Med 2003;97:382-7.
- Zeng XM, Martin GP, Marriott C. The controlled delivery of drugs to the lung. Int J Pharm 1995;124:149-64.
- Witek TJ. The Fate of Inhaled Drugs: The pharmacokinetics and pharmacodynamics of drugs administered by aerosol. Respir Care 2000;45:826-30.
- Clark AR. Pulmonary Delivery Technology: Recent Advances and Potential for the New Millennium. In: Hickey AJ, editor. Pharmaceutical Inhalation Aerosol Technology. 2nd ed. New York: Marcel Dekker Inc.; 2004. p. 563-84.
- Byron PR. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J Pharm Sci 1986;75:433-8.
- Shenfield GM, Evans ME, Paterson JW. Absorption of drugs by the lung. Br J Clin Pharm 1976;3:1218-23.
- Chabner BA, Allegra CJ, Curt GA, Calabresi P. Antineoplastic agents. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s:The Pharmacological Basis of Therapeutics. 9th ed. New York: The McGraw-Hill Companies Inc.; 1996. p. 1262.
- Product Information: VePesid®, Etoposide. Princeton, NJ: Bristol Laboratories; 2000.
- Misra A, Jinturkar K, Patel D, Lalani J, Chougule MB. Recent advances in liposomal dry powder formulations: Preparation and evaluation. Expert Opin Drug Deliv 2009;6:71-89.
- Kellaway IW, Farr SJ. Liposomes as drug delivry to the lung. Adv Drug Deliv Rev 1990;5:149-61.
- Joshi MR, Mishra A. Liposomal etoposide for dry powder inhaler: Preparation and stabilization. AAPS PharmSciTech 2001;2:25.
- Smolensky MH, D’Alonzo GE, Kunkel G, Barnes PJ. Day-night patterns in bronchial patency and dyspnoea: Basis for one daily and unequally divided twice daily dosing schedules. ChronobiolInt 1987;4:303-7.
- Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across lamellae of swollen phospholipids. J MolBiol 1965;13:238-52.
- Fatouros D, Hatzidimitiou K, Antimisiaris S. Liposomes encapsulating prednisolone and prednisolone-cyclodextrin complexes: comparisonof membrane integrity and drug release. Eur J Pharm Sci 2001;13: 287-96.
- McCormack B, Gregoriadis G. Entrapment of cyclodextrin-drug complexes into liposomes: Potential advantages in drug delivery. J Drug Target 1994;2:449-54.
- Glavas-Dodov M, Fredro-Kumbaradzi E, Goracinova K, Simonoska M, Calis S, Trajkovic-Jolevska S, et al. The effects of lyophilization on the stability of liposomes containing 5-FU. Int J Pharm 2005;291: 79-86.
- Zingel C, Sachse A, Robling LG, Muller HR. Lyophilization and rehydration of iopromide-carrying liposomes. Int J Pharm 1996;140: 13-24..