- *Corresponding Author:
- P. Subramanian
Laboratory of Aquabiotics/Nanoscience, Department of Animal Science, School of Life Sciences, Bharathidasan University, Tiruchirappalli-620 024, India
E-mail: subbus1952@gmail.com
| Date of Submission | 23 October 2013 |
| Date of Revision | 29 October 2014 |
| Date of Acceptance | 14 March 2015 |
| Indian J Pharm Sci 2015;77(2):156-162 |
Abstract
Atrazine, a herbicide is one the most toxic and sustaining pollutants in aquatic environment. It is detectable in surface water and in underground sources of drinking water. Many studies indicate that atrazine might be a potent endocrine disrupting xenobiotic. There are limited studies have revealed that the effects of atrazine on sex steroids hormones, vitellogenin and induction of aromatase, gonadosomatic index and hepatosomatic index. In this study, juvenile Poecilia sphenops fish was exposed to three different (0.83, 1.25 and 2.5 ppm) concentration of atrazine for 100 d. Changes in plasma and gonadal content and concentrations of sex steroids and vitellogenin protein in poecilia sphenops under laboratory conditions were assessed. The low level of the atrazine show estrogenic effect in males, as determined by a shortage of testosterone induction. Present study suggests that low induction of plasma vitellogenin and aromatase in male fish become suitable biomarkers of exposure to estrogenic chemicals.
Keywords
Poecilia sphenops, atrazine, sex steroid disruption, vitellogenin and aromatase
Herbicides are extensively used in agriculture, sanitation, gardens and weed control. Terrestrial and aquatic environments are mainly polluted by indiscriminately used agriculture products [1]. They contaminate the food chains and display toxic effects in animals and the human population. In the last 60 years, atrazine (2‑chloro‑4‑ethylamino‑ 6‑isopropylamino‑s‑triazine) has been one of the most extensively used herbicides in agriculture and roadways which, has been considered as an endocrine disruptor, causing adverse effects on reproductive function mainly by altered sex hormone levels and gonadal abnormalities [2‑6]. Possible interactions of atrazine with numerous endocrine functions have been the subject of various studies [7‑12].
Environmental compounds that disrupt endocrine function have been associated to adverse effects on the reproductive system in wildlife and humans. Although there are several mechanisms through which, environmental chemicals might alter the endocrine system, chemicals that mimic steroid hormones through an interaction with the estrogen receptor continue to receive considerable attention. Numerous environmental chemicals can bind to the estrogen receptor and initiate transcription of the estrogen receptor regulated genes in vitro [13].
Induction of vitellogenin protein in male fish has been extensively used as a biomarker for exposure of xenoestrogens in field and in laboratory studies. Vitellogenin (VTG) is a yolk precursor protein produced in the liver under the stimulation of ovarian estradiol and is normally found in female blood, whereas VTG levels in male fish are normally very low or absent [14]. However, if exposed to an exogenous estrogen, male fish are capable to synthesis VTG protein equivalent to that of mature female fish [15,16]. The enzyme aromatase, (P450 arom), is a member of the P450 cytochrome family complex and encoded by the CYP19 gene. The aromatase enzyme converts testosterone (T) to estradiol (E2) and is found in the brains of most male and female vertebrates. The enzyme is important for male sexual behaviour in a variety of species including rodents [17,18]. The aromatization of androgens to estrogens takes place in the endoplasmic reticulum and is classified as several functions of oxidation reactions [19]. A central function of aromatization is to play a very important and limiting role by T in the control of many physiological processes connected to reproduction, maintenance of masculinization of sexual behaviour in males, steroid hormone feedback on secretion of gonadotropic steroid hormone levels in the blood plasma [20].
Environment pollutants have been causing major impact on the hypothalamic pituitary gonad and hypothalamic pituitary thyroid axis as demonstrated in rare minnow [21]. The effect of atrazine on aromatase was treated as increase in estrogen and reduction in T levels described in fish [11]. Sanderson [22] and Hayes [9] found that abnormal elevated plasma concentration of E2 coupled with a decrease of the hormonal precursor T are the basis of atrazine. In adults, male goldfish exposed to high doses of atrazine displayed a hormonal imbalance in a time, dose related manner [11]. Mature male atlantic salmon are affected by atrazine with a significant reduction of 11‑KT. male sex steroid metabolism seems to be disrupted by atrazine exposure [23]. However, only limited information is available on the effect of atrazine on VTG production, aromatase activity and sex hormone levels in fish. Hence, fish can be used as experimental models in studies of endocrine disruption effects and also can serve as an early warning signal. Therefore, the objectives of this study were to elucidate the effects of atrazine on plasma and gonadal hormones, Gonadosomatic index and hepatosomatic index in Poecilia sphenops. Further the present study probe on sensitive markers like VTG and aromatase in fish Poecilia sphenops. The information obtained may be useful to explore the potential biomarkers for atrazine biomonitoring in aquatic environments.
Materials and Methods
VTG and aromatase enzyme linked immunosorbent assay (ELISA) kits were purchased from Cusabio, Italy and steroid hormone ELISA kit was purchased from Dia Metra, China and all other chemicals used in the study were obtained from Sigma Chemicals (USA).
Animals and experimental design
Sexually mature males and females poecilia sphenops were obtained from local ornamental fish dealer and maintained in the aquarium separately and intended for breeding within 7 d. The laboratory temperature, 28±2° and normal illumination (approx 12 h light and 12 h dark) with the ration of pieces of earthworm twice a day were ideal with continuous aeration. The newly hatched fries were separated from their respective mother and maintained in 100 l tank. A total of 200 fry (0 d old fry) were collected and separated into four equal treatment groups. Fifty individuals in each group were exposed to three different concentrations (1/10 of LC50‑2.5 ppm, 1/20 of LC50‑1.25 ppm and 1/30 of LC50‑0.83 ppm) of atrazine and a control was also maintained simultaneously. They were used for the experiment in the same day hatching. The young ones are fed with commercial feed throughout the experimental period. The experiments were repeated three times. The aquarium system was static and bathing medium was changed once in 7 d with same concentration of atrazine. Fries were captured and removed from each group after different exposure periods (60, 80 and 100 d). On respective sampling period, the blood was collected from the caudal vein with a heparinized microsyringe and transferred to plastic eppendorf tubes. Blood samples were centrifuged at 10 000×g for 15 min and the resulting plasma was analysed. Gonadosomatic indices (GSI) and hepatosomatic indices (HSI) were calculated. During each sampling ten fishes were sacrificed and dissected out carefully the liver, and gonads for analysis. The gonadosomatic index (GSI) was calculated as the percentage of the ovary or testis weight to the whole body weight. The liver somatic index (LSI) was calculated as the percentage of the liver weight to the whole body weight.
Determination of VTG, steroids hormones and aromatase
VTG concentrations were determined using ELISA with a polygonal antibody to fish VTG [24,25] plasma and gonadal T and E2 in both sexes were measured using radioimmunoassay (RIA) method adapted to small volume of sample [26,27]. Aromatase activity was determined using ELISA with a goat antirabbit antibody [28].
Statistical analysis
The values were expressed as mean±SEM (n=5). Differences between groups were assessed by one‑way analysis of variance (ANOVA) using the statistical package for social sciences (SPSS) software package for windows (version 16.0). Post hoc testing was carried out for intergroup comparisons using the least significant difference test and the values of P<0.05 were considered as statistically significant.
Results
Plasma and gonadal reproductive steroids and aromatase
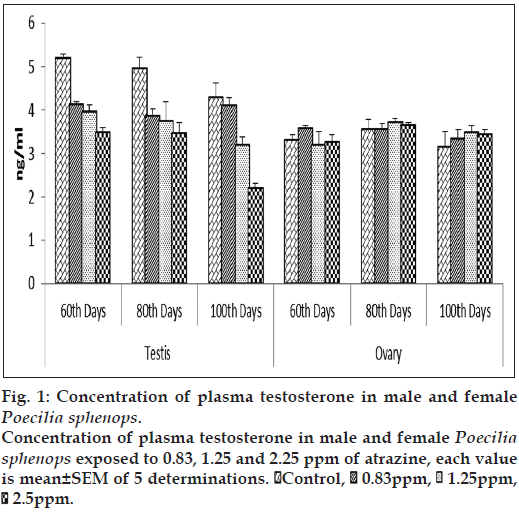
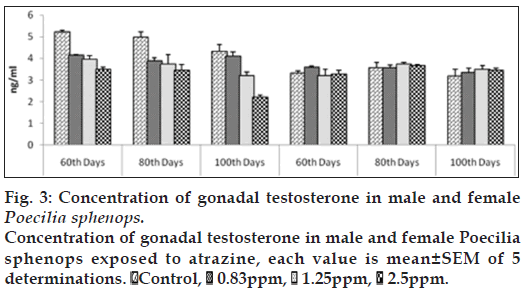
Both male and female Poecilia sphenops had detectable concentrations of T and E2 in their plasma and gonads. The atrazine treated male fish showed a significant (P<0.05) reduction in plasma T concentrations (fig. 1) and a significant (P<0.05) increase in plasma E2 concentrations (fig. 2) compared to control male fish. Plasma concentrations of T in control males ranged between 17.503±0.813 and 19.374±0.246 ng/ml over the study period. At the highest concentrations of atrazine exposure to fish, there was a reduction in plasma content of T at all sampling points (P<0.05). In control female fish, mean concentrations of testosterone in the ovary were lower than in the plasma. In control females, plasma T concentrations were lower to that of males (3.320 to 13.763 ng/ml). Although a gradual decrease in plasma T from 17.503±0.813 to 12.383±0.233 ng/ml in male fish. The atrazine exposed fish testis showed (fig. 3) a significant (P<0.05) reduction in T concentration when compared to control testis.
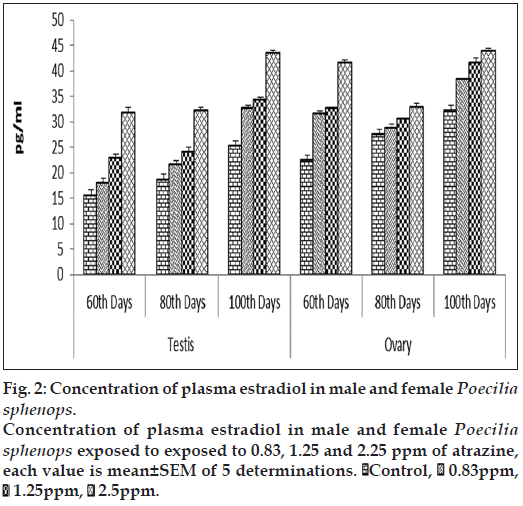
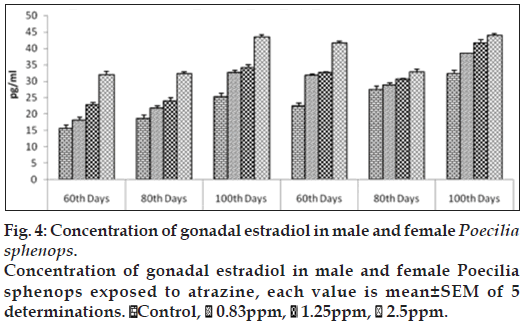
E2 concentrations in testis were slightly affected by three doses of atrazine exposure when compared to that of control plasma. Plasma E2 concentrations was increased significantly (P<0.05) in the highest atrazine concentration (fig. 2). E2 concentrations in testis ranged between 15.593±0.652 to 37.531±0.5747 pg/ml (fig. 4). E2 levels in female fishes were not affected by atrazine exposure, either in plasma or in the ovary. E2 concentrations in the ovary were measured in the range between 22.372±0.258 and 43.959±0.541 pg/mg of tissue while, in plasma, E2 were higher in females than in males, ranging between 29.563±0.894 and 55.959±0.731 pg/ml. No dosage or time related effects of atrazine occurred in female.
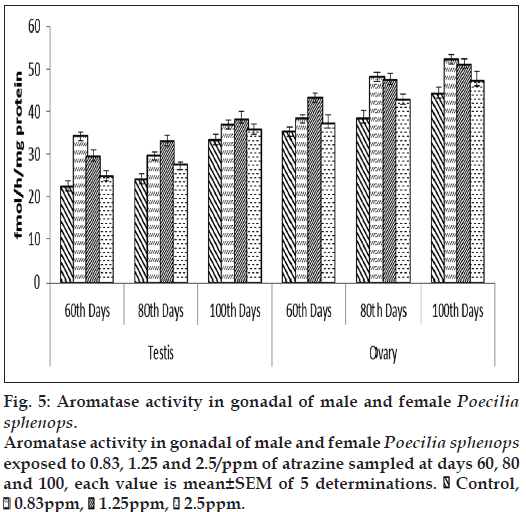
Both male and female Poecilia sphenops showed altered gonadal aromatase activity in the atrazine treatments. Gonadal aromatase activity was increased in fish treated with a low concentration of atrazine, but lower alteration in higher concentrations when compared to control. Gonadal aromatase activity increased and showed variations in between three groups (fig. 5). Aromatase activity was significantly (P<0.05) stimulated upon the atrazine treatment in the gonads of treated fishes as compared to the control fish.
Figure 5: Aromatase activity in gonadal of male and female Poecilia sphenops.
Aromatase activity in gonadal of male and female Poecilia sphenops exposed to 0.83, 1.25 and 2.5/ppm of atrazine sampled at days 60, 80 and 100, each value is mean±SEM of 5 determinations.  Control,
Control,  0.83ppm,
0.83ppm,  1.25ppm,
1.25ppm,  2.5ppm.
2.5ppm.
VTG determination
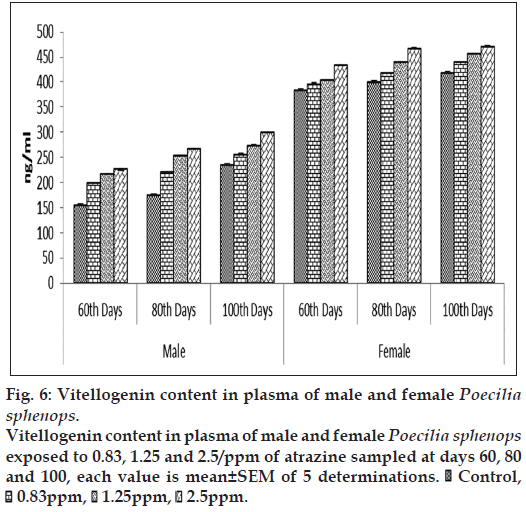
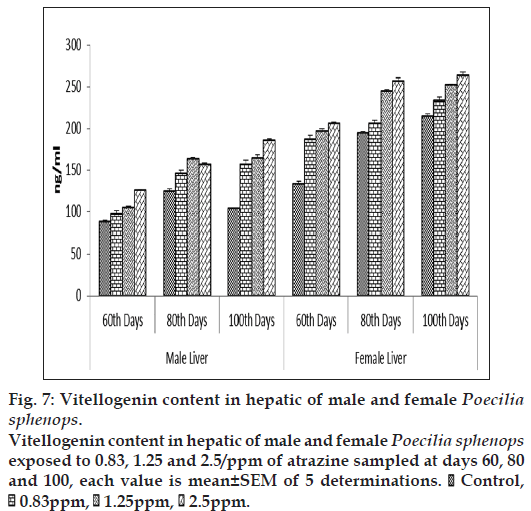
At the first sampling (60 d) of the experiment plasma VTG concentrations in the male Poecilia sphenops were 82.62±2.88 ng VTG/ml and 204.26±1.98 ng/ml in the female Poecilia sphenops (fig. 6). The plasma VTG levels were higher when compared to that of hepatic tissue. The plasma concentrations of VTG had increased by more than threefold in the atrazine treated male fish (299.425±1.85 ng/ml) and compared to liver (185.09±2.45ng/ml), plasma VTG level were high. Plasma VTG concentrations in atrazine exposed male were significantly higher than control males (P<0.05). Hepatic VTG production was significantly (P<0.05) increased in atrazine treatment in dose dependent manner in male and females when compared to control (fig. 7). Levels of VTG were higher in higher concentration of atrazine treated fish. The females plasma VTG concentrations around 10‑fold higher than the males. On exposure of atrazine, plasma and hepatic VTG concentrations in the females were slightly higher than in unexposed females. In this experiment however there was significant increase in E2 concentration in plasma and gonad of male and female Poecilia sphenops and low level induction of VTG in male fish. The concentration (μg/ml) of plasma VTG in atrazine exposed male fish was three to four orders of magnitude lower than that of fish exposed to female.
Figure 6: Vitellogenin content in plasma of male and female Poecilia sphenops.
Vitellogenin content in plasma of male and female Poecilia sphenops exposed to 0.83, 1.25 and 2.5/ppm of atrazine sampled at days 60, 80 and 100, each value is mean±SEM of 5 determinations.  Control,
Control,  0.83ppm,
0.83ppm,  1.25ppm,
1.25ppm,  2.5ppm.
2.5ppm.
Figure 7: Vitellogenin content in hepatic of male and female Poecilia sphenops.
Vitellogenin content in hepatic of male and female Poecilia sphenops exposed to 0.83, 1.25 and 2.5/ppm of atrazine sampled at days 60, 80 and 100, each value is mean±SEM of 5 determinations.  Control,
Control,  0.83ppm,
0.83ppm,  1.25ppm,
1.25ppm,  2.5ppm.
2.5ppm.
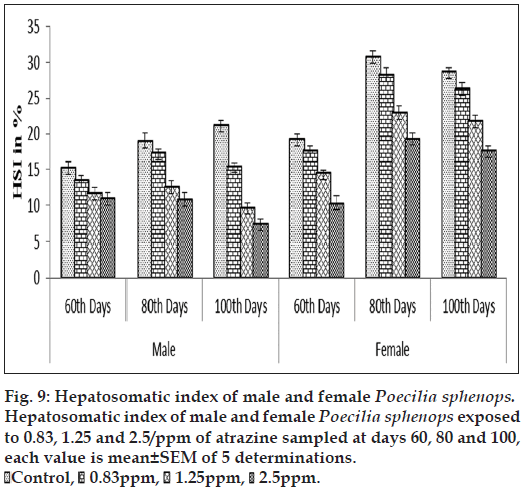
Gonadosomatic index and hepatosomatic index
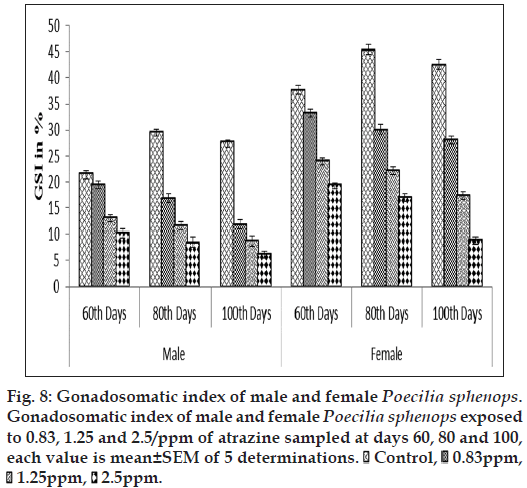
The atrazine exposure groups showed significantly lower GSI and HSI (P<0.05) during the experimental period than control. Figs. 8 and 9 show a concentration‑related tendency for decrease of GSI and HSI in the atrazine exposed fish. In the primary concentration, the fish exposed to 0.83 ppm of atrazine had significantly lesser (P<0.05) GSI and HSI values compared with the fish exposed to 2.5 ppm of atrazine. Although the control fish had a higher mean GSI and HSI than the fish exposed to 2.5 ppm of atrazine, there was a significant (P<0.05) difference between control fish and the fish exposed to atrazine.
Discussion
The purpose of this study was to explore the effects of atrazine on the reproductive system of Poecilia sphenops, with an emphasis on sex steroid hormone concentrations (in both plasma and gonads), plasma and hepatic VTG concentrations and gonadal aromatase. The atrazine also altered T and E2 levels in the plasma and testis of male fish resulting its effects on GSI and HSI. Atrazine at doses of 0.83 ppm had low dose or day related effects on gonad and hepatic growth in either males or females over the study period. Effects of higher concentration of atrazine 1.25 and 2.5 ppm on gonad and hepatic growth had high effects on dose or days‑related manner.
In the current study, atrazine decreases the T levels in the plasma and testis at all tested concentration on all exposure days. Supporting previous data [11], we found that reduction in plasma T in male fish. Plasma E2 levels in male showed a significant increase in the highest exposure of atrazine. Decrease in plasma T in males and connected increase in plasma E2 levels. Atrazine effects on the sex steroids T and E2 can be pronounced by a stimulatory effect on aromatase activity increasing the change of T into E2 [29]. However there was noticeable increase in E2 concentration in male plasma and testis, and no induction of T in female fish. The effect of atrazine on sex steroid has been shown in a variety of species like rats [30] and fish [11].
The present study suggested reproductive effects in fish on exposure to atrazine. It is a new trend related with atrazine for plasma concentrations of VTG in male and female fish. The result indicated that atrazine induce low level of VTG in male fish which supports a report from a study on plasma VTG in fathead minnow [31]. Induction of VTG has been reported in rainbow trout hepatocytes after atrazine exposure [32]. However no effects were described on VTG protein concentrations in adult goldfish (Carassius auratus) and male carp hepatocytes [11,33,34].
Atrazine also produced a low induction of aromatase in gonads. Our results support the current hypothesis that the mechanism of atrazine induces the aromatase activity. Moreover aromatase activities in gonads of Poecilia sphenops were altered by atrazine. However, our results are similar to the results stated for female zebrafish where atrazine significantly induces aromatase expression [35]. It has been reported that induction of aromatase in male juvenile alligators when exposed to atrazine [36]. In addition, some other studies have found no effect of atrazine on aromatase activity in zebrafish [37]. The current upshots are in agreement with the clarifications of the earlier reports. We have established for the first time that atrazine also produced a low level induction of aromatase activity in gonads, decreases T and increases E2 concentration in male Poecilia sphenops. The female Poecilia sphenops was not affected by atrazine. Increased estradiol was largely due to reduction in T which, was predictable by altered physiological measurements of endocrine disruptions like steroid hormones, aromatase, VTG, gonado somatic index and hepatosomatic index. These findings have shown that atrazine disrupts steroidogenesis in fish. Measured plasma hormone levels were highly variable in atrazine exposed males and females fishes. Hence, atrazine herbicide can carefully be suggested for weed controlling in aquatic media. Plasma E2 and T hormone levels, VTG protein content may be good, early biomarkers for atrazine toxicity studies.
References
- Oruc EO, Uner N. Effects of 2,4-diamine on some parameters of protein and carbohydrate metabolisms in the serum, muscle and liver of Cyprinuscarpio. Environ Pollut 1999;104:267-72.
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jr, et al. Male reproductive health and environmentalchemicals with estrogenic effects. Ministry of Environment and Energy, Denmark, Danish Environmental Protection Agency, Miljoprojekt nr 1995;290:1-166.
- Tyler CR, Jobling S, Sumpter JP. Endocrine disruption in wildlife: A critical review of the evidence. Crit Rev Toxicol 1998;28:319-61.
- Tyler CR, Van AR, Hutchinson TH, Maddix S, Trip H. An in vivo testing system for endocrine disruptors in fish early life stages using induction of vitellogenin. Environ ToxicolChem 1999;18:337-47.
- Vos JG, Dybing E, Greim HA, Lagefoged O, Lambre C, Tarazona JV, et al. Health effects on endocrine-disrupting chemicals on wildlife,with special reference to European situation. Crit Rev Toxicol2000;30:71-133.
- Hashimoto S, Bessho H, Hara A, Nakamura M, Iguchi T, Fujita K. Elevated serum vitellogenin levels and gonadal abnormalities in wild male flounder (Pleuronectesyokohamae) from Tokyo Bay, Japan. MarEnviron Res 2000;49:37-53.
- Carr JA, Gentles A, Smith EE, Goleman WL, Urquidi LJ, Thuett K, et al. Response of larvalXenopuslaevisto atrazine: Assessmentof growth, metamorphosis, and gonadal and laryngeal morphology. Environ ToxicolChem 2003;22:396-405.
- Cooper RL, Stoker TE, Tyrey L, Goldman JM, McElroy WK. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. ToxicolSci 2000;53:297-307.
- Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, et al. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine, at low ecologically relevant doses. ProcNatlAcadSci USA 2002;99:5476-80.
- Hecker M, Giesy JP, Jones PD, Jooste AM, Carr JA, Solomon KR, et al. Plasma sex steroid concentrations and gonadal aromataseactivities in African clawed frogs (Xenopuslaevis) from the corn-growing region of South Africa. Environ ToxicolChem2004;23:1996-2007.
- Spano L, Tyler CR, Van AR, Devos P, Mandiki SN, Silvestre F, et al. Effects of atrazine on sex steroid dynamics, plasma vitellogeninconcentration and gonad development in adult goldfish (Carassiusauratus). AquatToxicol 2004;66:369-79.
- Stoker TE, Parks LG, Gray LE, Cooper RL. Endocrine disrupting chemicals: Prepubertal exposures and effects on sexual maturation and thyroid function in the male rat. A focus on the EDSTAC recommendations. Crit Rev Toxicol 2000;30:197-252.
- Bolger R, Wiese TE, Ervin K, Nestich S, Checovich W. Rapid screening of environmental chemicals for estrogen receptor binding capacity. Environ Health Perspect 1998;106:551-7.
- Kime DE, Nash JP, Scott AP. Vitellogenesis as a biomarker of reproductive disruption by xenobiotics. Aquacult 1999;177:345-52.
- Maitre JL, Mercier L, Dole L, Valotaire Y. Characterization of estradiol specific receptors and induction of vitellogenin mRNA in the rainbow trout liver (Salmogairdneri). Biochimie 1985;67:215-25.
- Le Guellec K, Lawless K, Valotair Y, Kress M, Tenniswood M. Vitellogenin gene expression in male rainbow trout (Salmogairdneri). Gen Comp Endocr 1988;71:359-71.
- Bakker J, Honda S, Harada N, Bathazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. HormBehav 2004;46:1-10.
- Timonin ME, Wynne-Edwards KE. Aromatase inhibition during adolescence reduces adult sexual and paternal behavior in the biparentaldwarf hamster Phodopuscampbelli. HormBehav 2008;54:748-57.
- Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev 1996;22:1-26.
- Dessl-Fulgheri F. Odour of male and female rats changes hypothalamic aromatase and 5a-reductase activity and plasma sex steroid levels inunisexually reared male rats. PhysiolBehav 1982;28:231-5.
- Li W, Zha J, Li Z, Yang L, Wang Z. Changes of thyroid hormone levels and related gene expression in chinese rare minnow (Gobiocyprisrarus) during 3-amino-1,2,4-triazole exposure and recovery. AquatToxicol 2009;92:50-7.
- Sanderson JT, Boerma J, Lansbergen GW, Van den Berg M. Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol App Pharmacol 2002;182:44-54.
- Moore A, Waring CP. Mechanistic effects of a triazine pesticide on reproductive endocrine function in mature male atlantic salmon (SalmosalarL.) parr. Pest BiochemPhysiol 1998;62:41-50.
- Parks LG, Cheek AO, Denslow ND, Heppell SA, McLachlan JA, LeBlanc GA, et al. Fathead minnow PinephalesPromelasvitellogenin:the Detection of Estrogenic Compounds. Comp Biochem Physiol1999;123:113-25.
- Korte JJ, Kahl MD, Jensen KM, Pasha MS, Parks LG, LeBlanc GA, et al. Fathead minnow vitellogenin: cDNA sequence and mRNA andprotein expression after 17ß-estradiol treatment. Environ ToxicolChem2000;19:972-81.
- Jensen KM, Korte JJ, Kahl MD, Pasha MS, Ankley GT. Aspects of basic reproductive biology and endocrinology in the fathead minnow (Pimephalespromelas). Comp BiochemPhysiol C ToxicolPharmacol 2001;128:127-41.
- USEPA (U.S. Environmental Protection Agency). A Short-Term method for assessing the reproductive and developmental toxicity of endocrine-disrupting chemicals using the fathead minnow (Pimephalespromelas). EPA-2001;600/R-01/067. Duluth, MN. Office of Research andDevelopment, Environmental Monitoring Systems Laboratory; 2002.
- Numazawa M, Mutsumi A, Asano N, Ito Y. A time-dependent inactivation of aromatase by 19-substituted androst-4-ene-3,6,17-triones. Steds 1993;58:40-6.
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S et al. Aromatase cytochrome P450, the enzyme responsible for estrogen byosinthesis. Endocr Rev 1994;15:342-55.
- Stoker TE, Laws SC, Guidici DL, Cooper RL. The effect of atrazine on puberty in male wistar rats: An evaluation in the protocol for the assessment of pubertal development and thyroid function. ToxicolSci2000;58:50-9.
- Bringolf RB, Jason BB, Summerfelt RC. Effects of atrazine on fathead minnow in a short-term reproduction assay. Environ ToxicolChem2004;23:1019-25.
- Salaberria I, Hansen BH, Asensio V, Olsvik PA, Andersen RA,Jenssen BM. Effects of atrazine on hepatic metabolism and endocrine homeostasis in rainbow trout (Oncorhynchusmykiss). ToxicolApplPharmacol 2009;234:98-106.
- Chang LW, Toth GP, Gordon DA, Graham DW, Meier JR, Knapp CW, et al. Responses of molecular indicators of exposure in mesocosms:Common carp (Cyprinuscarpio) exposed to the herbicides alachlor and atrazine. Environ ToxicolChem 2005;24:190-7.
- Sanderson JT, Letcher RJ, Heneweer M, Giesy JP, van den Berg M. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ Health Perspect 2001;109:1027-31.
- Suzawa M, Ingraham HA. The herbicide atrazine activates endocrine gene networks via non-steroidal NR5A nuclear receptors in fish and mammalian cells. PLoS One 2008;3:e2117.
- Spiteri ID, Guillette LJ Jr, Crain DA. The functional and structural observations of the neonatal reproductive system of alligators exposed in ovo to atrazine, 2,4-D, or estradiol. Toxicol Indus Health 1999;15:180-6.
- Kazeto Y, Place AR, Trant JM. Effects of endocrine disrupting chemicals on the expression of CYP19 genes in zebrafish (Daniorerio) juveniles. AquatToxicol 2004;69:25-34.