- *Corresponding Author:
- Kun Dong
Department of Infectious Diseases, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi, China
E-mail: dongchunqiang123@163.com
| This article was originally published in a special issue,“Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “121-128” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The pathological mechanism of liver cancer is complex. Interferon regulatory factor-1 can be used as a tumor suppressor, which has an important anti-cancer effect in liver cancer. Metformin has also shown to have therapeutic value in various cancers, but whether it influences the malignant development of liver cancer cells through interferon regulatory factor-1 remains unclear and this study finds out this association. We used cell counting kit-8 to detect the effects of metformin on hepatoma cell lines, HepG2 and Huh-7, interferon regulatory factor-1 levels, cell counting kit-8, and Transwell and flow cytometry to evaluate the role of metformin and interferon regulatory factor-1, Lentivirus interfering vectors on hepatoma cell proliferation, migration and invasion, respectively. The results showed that 10 mM metformin had a significant effect on HepG2 and Huh-7 survival; interferon regulatory factor-1 was significantly under-expressed in HepG2 and HH-7 cells, which could be up-regulated by metformin intervention. Subsequently, metformin intervention was found to validly suppress HepG2 and Huh-7 cells proliferation, migration and invasion, while significantly inducing apoptosis, but the aforementioned anti-tumor effect of metformin was offset by interferon regulatory factor-1 Lentivirus interfering vectors. This indicates that metformin has the ability to inhibit liver cancer cell growth and metastasis, and this effect is related to its regulation of interferon regulatory factor-1.
Keywords
Metformin, interferon regulatory factor-1, liver cancer, hepatoma, apoptosis
Liver Cancer (LC) is strongly associated with chronic infection with Hepatitis B (HBV) and Hepatitis C Virus (HCV). Although the incidence of LC caused by these viruses has been declining, the risk of Non-Alcoholic Steatohepatitis (NASH) is increasing[1]. In addition to the above two viruses, the risk factors for LC also includes drinking, obesity, diabetes, aflatoxin B1 infection etc.[2,3]. According to pathological classification, Hepatocellular Carcinoma (HCC) accounts for the largest proportion of LC, which can be ≥80 %, followed by intrahepatic cholangiocarcinoma[4]. There are about 800 000 new cases and nearly 750 000 deaths every year, as indicated by HCC statistics[5]. Based on this, in-depth exploration of the molecular mechanism of LC is of great value for optimizing the management of LC diagnosis and treatment, thus reducing the number of new cases and deaths.
Interferon Regulatory Factor-1 (IRF-1) is an important transcription factor of the interferon-Gamma (γ) pathway, which not only participates in the regulation of HCC Tumor Microenvironment (TME), but also mediates anti-tumor immune processes based on HCC cells[6]. It has been previously reported that its anti-tumor mechanism in HCC is related to the regulation of Programmed Cell Death-Ligand 1 (PD-L1) expression and other important immune-related inflammatory pathways[7]. However, it remains to define whether (MET) can regulate IRF-1 to affect the malignant development of LC cells.
MET is a low-cost and safe biguanide that was originally used to treat diabetes and has been found to be able to treat neoplastic diseases such as bone cancer, breast cancer and melanoma by mediating different molecular signal pathways[8-10]. It has also been used to treat LC patients, and its therapeutic mechanism is related to its inhibition of mammalian Target Of Rapamycin (mTOR) complex 1, which in turn prevents LC cell growth[11].
Given the rarity of studies on the role played by MET in the malignant development of LC cells by regulating IRF-1, this study analyzes to conduct research that is expected to shed new light on the treatment of LC.
Materials and Methods
Hepatoma cell lines (HepG2 and Huh-7) and normal hepatocytes HL-7702 were purchased from Shanghai Aoyin Biotechnology, Co., Ltd., approval no: SAc0068, SAc0188 and SAc0229. The cells were cultured in a Dulbecco’s modified eagle medium (obtained from Shanghai Advantage Biological Co., Ltd., approval no: D5030-10X1L comprising of 10 % Fetal Bovine Serum (FBS). It was purchased from Ethephon Biotechnology (Shanghai) Co., Ltd., YCL-05001). Further, 0.1 g/ml of streptomycin and 100 U/ml penicillin were obtained from Shanghai Gaochuan Chemical Technology Co., Ltd., (approval no: 15070063) which were placed in a humidified incubator with 5 % Carbon dioxide (CO2) maintained at 37°. The culture medium was changed for every 2 d, and the cells were diluted at a rate of 1:4 for every 5-6 d.
Cell transfection:
HepG2 and HH-7 were cultured into the wells of 6-well plates (1.0×105 cells/well) for 24 h. A LipofectamineTM 2000 kit was used to transfect small interfering (si) IRF-1 inhibitory sequence and its corresponding control i.e., si-Negative Control (NC) into the cells, and the transfection efficiency was verified after transfection for 12 h.
quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) for the detection of messenger Ribonucelic Acid (mRNA) expression:
Total RNA samples were isolated from the cell lines by using the Micro RNA (mir) VanaTMmiRNA isolation kit obtained from MITO Biotechnology Co., Ltd., Shanghai, China (AM1561), followed by Complementary Deoxyribonucleic Acid (cDNA) synthesis of the isolated RNA using the TapMan™MiRNA reverse transcription kit purchased from Even bridge Biotechnology Co., Ltd., Shanghai, China (4366597). The relative level of IRF-1 was determined by using the SYBR Hangzhou Kaitai Biotechnology Co., Ltd., Kit, approval no: FP205-03 in a real-time PCR detection system, using Beta (β)-actin mRNA as the standard.
Cell proliferation and viability detection:
The transfected hepatoma cells were inoculated into the wells of 6-well plates with 1.5×105 cells/ well. The suspended cells were counted every 24 h within 5 d after inoculation by a particle counter (Guangzhou Hongke Electronic Technology Co., Ltd., approval no: DR-528). The stably transfected hepatoma cells were inoculated into a 96-well plate at a density of 3000 cells and cultured overnight. Cell Counting Kit-8 (CCK-8) (Purchased from Hanheng Biotechnology (Shanghai) Co., Ltd., approval no: HB-CCK-8-500T) was used to evaluate the cell viability 72 h after inoculation, and the absorbance was measured at 450 nm using a microplate reader (Tecan (Shanghai) Trading Co., Ltd., Spark). In addition, we also intervened the cells with 0, 10, 20, 40, 80 and 160 mM MET, respectively, and we determined the optimal experimental concentration by detecting the effect of MET on cell viability.
Transwell assay of cell migration and invasion:
After 24 h of transfection, 105 hepatoma cells were placed in a 24-well plate and then transferred to the upper chamber. FBS (20 %)-supplemented with Roswell Park Memorial Institute (RPMI)-1640 medium (Shanghai Whelab Biotechnology Co., Ltd., M0202A) was added to the lower chamber. After incubation at 7º for 24 h, the cells migrating to the lower chamber were stained with 0.1 % crystal violet (Shanghai Xinyu Biotechnology Co., Ltd., approval no: XYW7320) for another 30 min. Images of cell migration were observed through a microscope. The invasion assay was the same as above, but it is necessary to use 50 mg/l Matrigel (1:8; Shanghai Fusheng Industry Co., Ltd., FS-79068) to dilute the membrane of the upper and bottom chambers.
Flow cytometry determination of apoptosis:
The transfected hepatoma cells were washed twice with cold Phosphate-Buffered Saline (PBS) and resuspended into a 1×106 cells/ml suspension. 100 μl of this culture (1×105 cells) was then transferred to a 5 ml culture tube. Subsequently, 5 μl of Fluorescein Isothiocyanate (FITC)-Annexin V and 5 μl of Propidium Iodide (PI) (Wuhan Elabscience Biotechnology Co., Ltd., approval no: E-CK-A211) were added and kept undisturbed for 15 min of incubation in a greenhouse in the dark. After the addition of 400 μl of 1× binding buffer to the tube, the apoptosis level was analyzed by using flow cytometry (Beijing Challen Biotechnology Co., Ltd., LongCyte™) and FlowJo software.
Statistical methods:
This study employed GraphPad Prism version 7.01 for statistical analysis. The inter-group comparison was conducted by the Student’s t-test. One-way Analysis of Variance (ANOVA) and Bonferroni posthoc multiple comparisons were utilized to identify multi-group differences; p<0.05 was considered to be statistically significant.
Results and Discussion
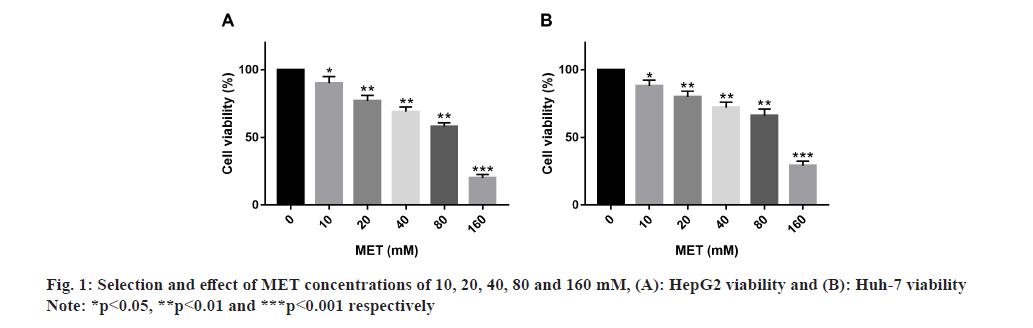
Primarily, optimum MET concentration was selected. After treating HepG2 and Huh-7 with MET concentrations of 10, 20, 40, 80 and 160 mM, respectively, it was found that 10 mM MET had a significant effect on HepG2 and Huh-7 survival, while 20, 40, 80 and 160 mM MET had a more significant inhibitory effect on cell survival, so 10 mM was selected as the optimal concentration for MET administration (fig. 1).
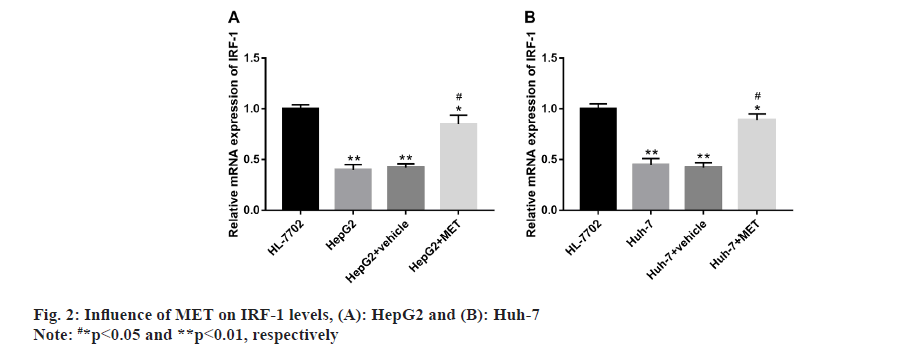
Influence of MET on IRF-1 levels in HepG2 and Huh-7 was studied. According to qRT-PCR, IRF-1 expression was abnormally low in HepG2 and Huh-7 compared with HL-7702, but were notably elevated in HepG2 and Huh-7 after MET intervention (fig. 2).
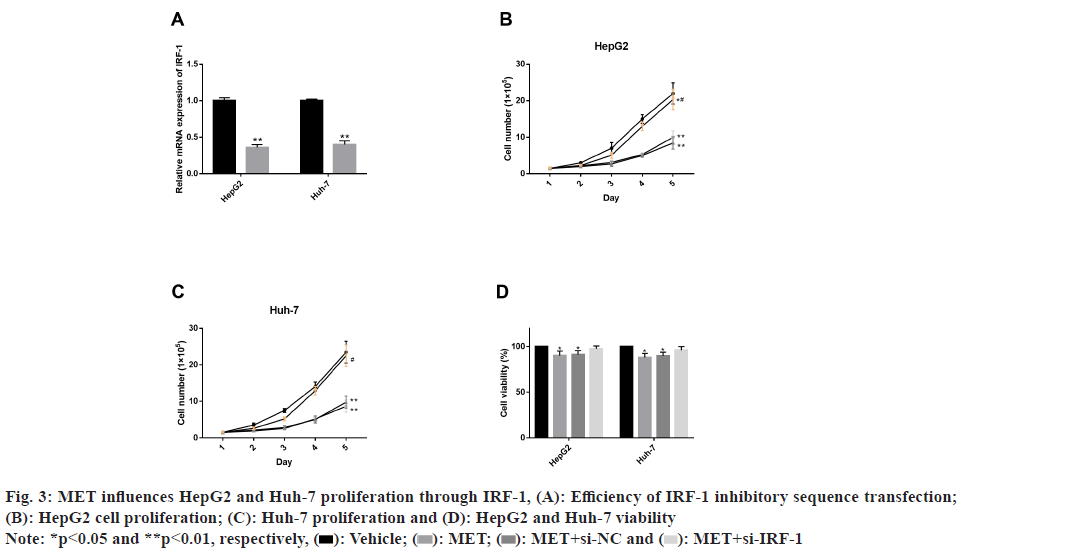
Further, influence of MET on HepG2 and Huh-7 proliferation through IRF-1 was also studied. We have verified the efficiency of IRF-1 inhibitory sequence transfection and found down-regulated IRF-1 mRNA level successfully after intervention. Next, under MET intervention, the number of HepG2 and Huh-7 cells within 5 d was significantly inhibited compared with the observation group (p<0.05). CCK-8 assay also demonstrated markedly inhibited HepG2 and Huh-7 viability by MET (p<0.05). However, after IRF-1 inhibitory sequence transfection, we found that the inhibition of MET on HepG2 and Huh-7 proliferation and vitality was significantly offset (fig. 3).
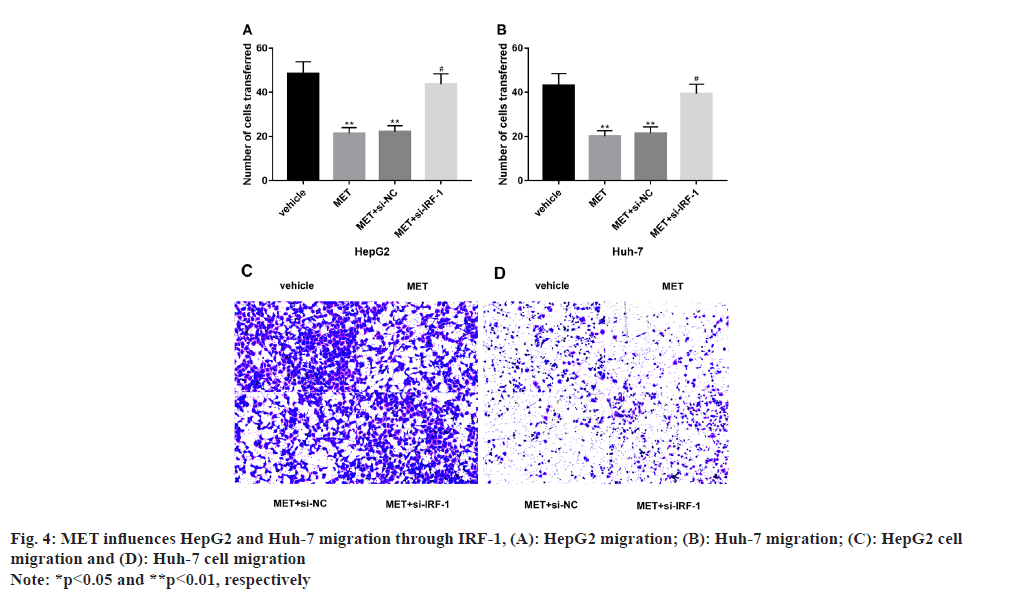
Additionally, MET also influences HepG2 and Huh- 7 migration via IRF-1 and it has been studied. MET significantly inhibited the migrating capacity of HepG2 and Huh-7 (p<0.05), which was markedly reversed by IRF-1 inhibitory sequence intervention (p<0.05) (fig. 4).
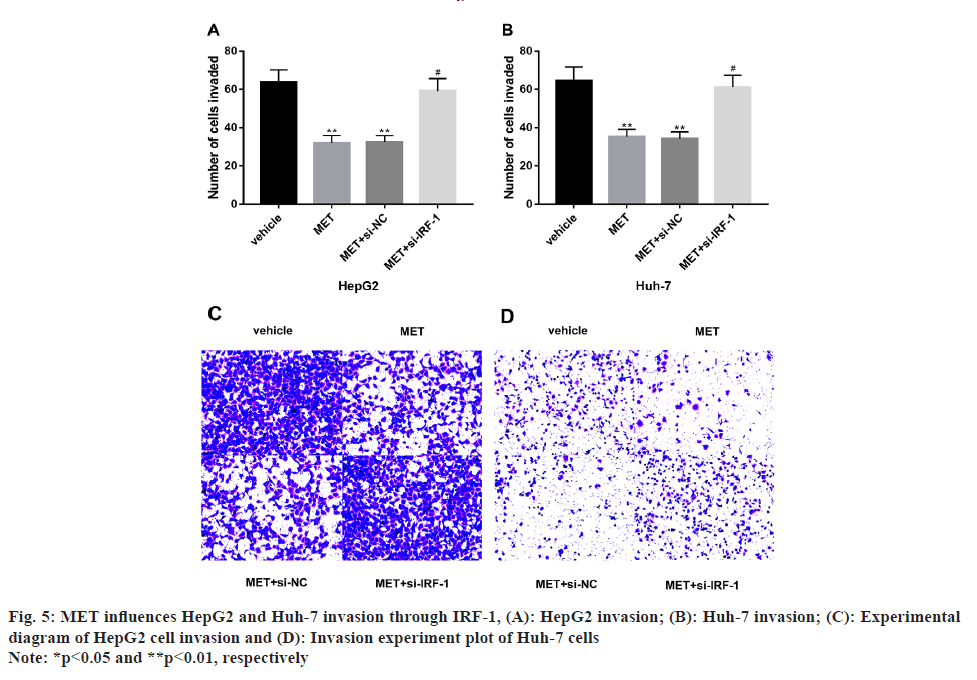
According to our study, we found that MET influences HepG2 and Huh-7 invasion through IRF-1 pathway. We found significantly suppressed invasion levels of HepG2 and Huh-7 after MET treatment compared with the observation group (p<0.05), which was significantly smoothed out after the intervention of the IRF-1 inhibitory sequence (p<0.05) (fig. 5).
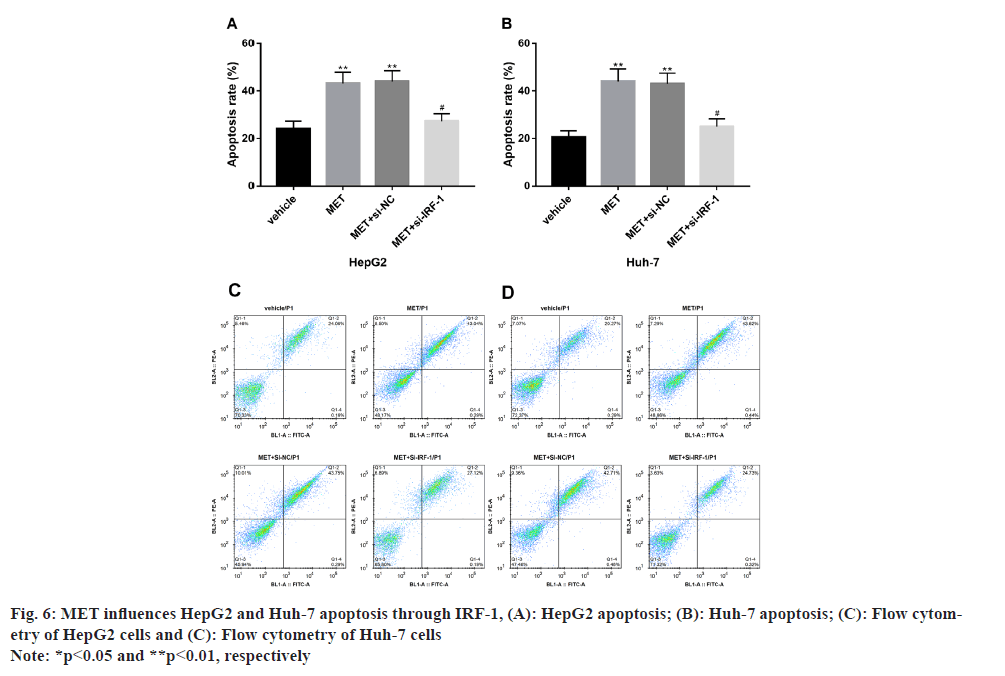
It is also found that MET influences HepG2 and Huh-7 apoptosis via IRF-1. The apoptotic level was notably increased in MET-treated HepG2 and Huh-7 vs. the observation group (p<0.05), while an apoptotic level similar to the observation group was observed in HepG2 and Huh-7 after further treatment with the IRF-1 inhibitory sequence (p>0.05) (fig. 6).
LC is a highly heterogeneous malignancy with an increasing incidence, which is invasive and fatal and often leads to unsatisfactory prognosis[12,13]. At present, surgical procedures such as hepatectomy, liver transplantation and percutaneous ablation are the most effective options for LC treatment. However, these approaches are feasible for only 20 % of LC patients due to multiple lesions and extrahepatic metastases[14]. In order to further improve the prognosis of LC patients and optimize LC diagnosis and treatment, it is necessary to conduct in-depth analysis of the molecular mechanism and biological behavior of LC.
The anti-tumor efficacy and mechanism of MET have been confirmed and explored by many scholars. For example, in the research of Feng et al.[15], the anti-LC therapeutic effect of MET, affected by Dedicator of Cytokinesis protein 1 (DOCK1) expression, is effective in prolonging the overall survival of HCC patients with low DOCK1 level, while it is ineffective for those with high DOCK1 expression. As reported by Ma et al.[16], MET validly enhances the outcomes and survival of LC patients complicated with diabetes. Sugiura et al.[17], also pointed out that the application of MET in colorectal cancer can significantly inhibit tumor occurrence and metastasis, and this anticancer mechanism is linked to its modulation of the Adenosine Monophosphate (AMP)-Activated Protein Kinase (AMPK)-mTOR axis. In this study, we have identified 10 mM as the optimal concentration for MET administration, as this concentration can exert a significant inhibitory effect on HepG2 and Huh-7 survival. Subsequently, through qRT-PCR detection, we have found that IRF-1 expression was abnormally down-regulated in HepG2 and Huh-7 and was markedly elevated by MET intervention, indicating the potential role of IRF-1 as a tumor inhibitor in HCC and the positive regulation of IRF-1 expression by MET in HepG2 and Heh-7 cells. In the report of Yan et al.[18], IRF- 1 mRNA expression is markedly under-expressed in HCC than in normal liver tissue, similar to our research results. This study also suggests that the anticancer effect of IRF-1 in HCC may be attributed to its up-regulation of PD-L1 levels in the inflammatory TME. Another study by Yi et al.[19], proposed a close correlation between the low level of IRF-1 and adverse outcomes and tumor aggressiveness of HCC, suggesting that up-regulating IRF-1 may help improve HCC patients’ outcomes by inhibiting tumor invasion. Reviewing previous studies, we found that both MET and IRF-1 have inhibitory effects on miR- 195 expression. The former down-regulates miR- 195 in Type 1 Diabetes Mellitus (T1DM) patients, thus contributing to cardioprotection, while the latter promotes the apoptosis and PD-L1 expression in HCC cells by inhibiting miR-195, suggesting that MET and IRF-1 may be closely related[20,21].
Then, we successfully achieved the down-regulation of IRF-1 mRNA level with IRF-1 inhibitory sequence for subsequent experiments. MET was found to evidently inhibit HepG2 and Huh-7 survival and vitality, but this anti-proliferation effect was basically erased by IRF-1 inhibitory sequence intervention, indicating that MET plays an anti-proliferation role in LC by up-regulating IRF-1. In the migration and invasion experiments, MET significantly prevented HepG2 and Huh-7 from migrating and invading, and IRF-1 inhibitory sequence intervention significantly offset this anti-tumor metastasis effect, demonstrating that the anti-tumor metastasis effect of MET in HCC is closely related to its up-regulation of IRF-1. IRF-1 has been reported to exert a certain regulatory effect on tumor metastasis in other cancer backgrounds such as osteosarcoma, gastric cancer, and lung adenocarcinoma, besides HCC[22-24]. For example, the regulation of IRF-1 on osteosarcoma cell migration and invasion is also shown to be influenced by the targeting of miR-4295[25]. And as reported by Zhou et al.[26], the indirect regulation of IRF-1 on peritoneal metastasis in mouse models of gastric cancer is associated with its participation in the signal transduction of Interleukin (IL)-33 mediated by Tumor Necrosis Factor Receptor 2 (TNFR2)- Nuclear Factor Kappa-B (NF-κB)-IRF-1. Vacante et al.[27], also confirmed the anti-tumor metastasis effect of MET on HCC, and the mechanism is related to its regulation of Kruppel-Like Factor 6 (KLF6)/Cyclindependent kinase inhibitor 1A (P21Cip1)/Insulin-like Growth Factor (IGF) axis. Later, the data of apoptosis revealed the significant induction of HepG2 and Huh-7 apoptosis by MET, as well as the basically disappeared apoptosis-promoting effect after IRF- 1 inhibitory sequence intervention, suggesting that the pro-apoptotic mechanism of MET in LC is also significantly related to IRF-1. Dong et al.[28], showed that IRF-1 was down-regulated in HCC cell lines in a post-transcriptional manner, and was regulated by miR-301a to participate in the regulation of HCC cell proliferation and apoptosis, which helped to partially explain the influence of MET on the growth of HepG2 and Huh-7 by up-regulating IRF-1 in this study. Yan et al.[29], also pointed out that up-regulation of IRF-1 can induce HCC cell apoptosis, which may be related to its inhibition of Checkpoint Kinase 1 (CHK1). Another study by Wang et al.[30], reported that the proapoptotic effect of MET in LC can also be positively regulated by plantainoside.
Conclusively, MET inhibits the malignant development of LC cells by up-regulating IRF-1, which is mainly manifested as significant inhibition of proliferation and metastasis of hepatoma cells and significant induction of apoptosis under MET intervention. This therapeutic role of MET is closely related to its up-regulation of IRF-1, which can provide targeted treatment options for LC treatment.
Author’s contributions:
Peiqi Wan and Chunqiang Dong equally contributed to this work and are co-first authors.
Funding:
This study was supported by the Guangxi Development and Promotion and Application (Project: Guangxi Medical and Health Appropriate Technology Development and Application Project No: S2019111).
Conflict of interests:
The authors declared no conflict of interests.
References
- Yamashita T, Kaneko S. Liver cancer. Rinsho Byori 2016;64(7):787-96.
[Google Scholar] [PubMed]
- Li L, Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett 2016;379(2):191-7.
[Crossref] [Google Scholar] [PubMed]
- Chatterjee R, Mitra A. An overview of effective therapies and recent advances in biomarkers for chronic liver diseases and associated liver cancer. Int immunopharmacol 2015;24(2):335-45.
[Crossref] [Google Scholar] [PubMed]
- de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: An international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29(23):3140-5.
[Crossref] [Google Scholar] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359-86.
[Crossref] [Google Scholar] [PubMed]
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168(4):707-23.
[Crossref] [Google Scholar] [PubMed]
- Yan Y, Zheng L, Du Q, Geller DA. Interferon-γ/IRF-1 pathway regulatory mechanisms of PD-L1 expression and relevance for immune checkpoint blockade in Hepatocellular Carcinoma (HCC). Oncotarget 2021;12(23):1-2.
[Crossref] [Google Scholar] [PubMed]
- Lv Z, Guo Y. Metformin and its benefits for various diseases. Front Endocrinol 2020;11:1-10.
[Crossref] [Google Scholar] [PubMed]
- Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, deCensi A, et al. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res 2014;7(9):867-85.
[Crossref] [Google Scholar] [PubMed]
- Morales DR, Morris AD. Metformin in cancer treatment and prevention. Annu Rev Med 2015;66:17-29.
[Crossref] [Google Scholar] [PubMed]
- Kim TS, Lee M, Park M, Kim SY, Shim MS, Lee CY, et al. Metformin and dichloroacetate suppress proliferation of liver cancer cells by inhibiting mTOR complex 1. Int J Mol Sci 2021;22(18):1-15.
[Crossref] [Google Scholar] [PubMed]
- Hu B, Lin JZ, Yang XB, Sang XT. Aberrant lipid metabolism in hepatocellular carcinoma cells as well as immune microenvironment: A review. Cell Prolif 2020;53(3):1-14.
[Crossref] [Google Scholar] [PubMed]
- Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer 2020;1873(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Li Y, Zhou T, Zheng J, Li S, Li HB. Dietary natural products for prevention and treatment of liver cancer. Nutrients 2016;8(3):1-23.
[Crossref] [Google Scholar] [PubMed]
- Feng J, Lu H, Ma W, Tian W, Lu Z, Yang H, et al. Genome-wide CRISPR screen identifies synthetic lethality between DOCK1 inhibition and metformin in liver cancer. Protein Cell 2022;13(11):825-41.
[Crossref] [Google Scholar] [PubMed]
- Ma SJ, Zheng YX, Zhou PC, Xiao YN, Tan HZ. Metformin use improves survival of diabetic liver cancer patients: Systematic review and meta-analysis. Oncotarget 2016;7(40):1-10.
[Crossref] [Google Scholar] [PubMed]
- Sugiura K, Okabayashi K, Seishima R, Ishida T, Shigeta K, Tsuruta M, et al. Metformin inhibits the development and metastasis of colorectal cancer. Med Oncol 2022;39(9):136.
[Crossref] [Google Scholar] [PubMed]
- Yan Y, Zheng L, Du Q, Yan B, Geller DA. Interferon Regulatory Factor 1 (IRF-1) and IRF-2 regulate PD-L1 expression in Hepatocellular Carcinoma (HCC) cells. Cancer Immunol Immunother 2020;69:1891-903.
[Crossref] [Google Scholar] [PubMed]
- Yi Y, Wu H, Gao Q, He HW, Li YW, Cai XY, et al. Interferon Regulatory Factor (IRF)-1 and IRF-2 are associated with prognosis and tumor invasion in HCC. Ann Surg Oncol 2013;20:267-76.
[Crossref] [Google Scholar] [PubMed]
- Ahmed FW, Bakhashab S, Bastaman IT, Crossland RE, Glanville M, Weaver JU. Anti-angiogenic miR-222, miR-195, and miR-21a plasma levels in T1DM are improved by metformin therapy, thus elucidating its cardioprotective effect: The MERIT study. Int J Mol Sci 2018;19(10):1-20.
[Crossref] [Google Scholar] [PubMed]
- Yan Y, Zheng L, Du Q, Cui X, Dong K, Guo Y, et al. Interferon Regulatory Factor 1 (IRF-1) downregulates Checkpoint Kinase 1 (CHK1) through miR-195 to upregulate apoptosis and PD-L1 expression in Hepatocellular Carcinoma (HCC) cells. Br J Cancer 2021;125(1):101-11.
[Crossref] [Google Scholar] [PubMed]
- Cheng JP, Huang B, Duan JH, Yi KJ, Zhuang ZL. miR?4295 promotes cell proliferation, migration and invasion of osteosarcoma through targeting interferon regulatory factor 1. Oncol Lett 2020;20(5): 260.
[Crossref] [Google Scholar] [PubMed]
- Yan Y, Zheng L, Du Q, Yazdani H, Dong K, Guo Y, et al. Interferon Regulatory Factor 1 (IRF-1) activates anti-tumor immunity via CXCL10/CXCR3 axis in Hepatocellular Carcinoma (HCC). Cancer Lett 2021;506:95-106.
[Crossref] [Google Scholar] [PubMed]
- Qu S, Jiao Z, Lu G, Xu J, Yao B, Wang T, et al. Human lung adenocarcinoma CD47 is upregulated by interferon-γ and promotes tumor metastasis. Mol Ther Oncolytics 2022;25:276-87.
[Crossref] [Google Scholar] [PubMed]
- Yuan J, Tan L, Yin Z, Zhu W, Tao K, Wang G, et al. miR17HG-miR-18a/19a axis, regulated by interferon regulatory factor-1, promotes gastric cancer metastasisvia Wnt/β-catenin signalling. Cell Death Dis 2019;10(6):1-16.
[Crossref] [Google Scholar] [PubMed]
- Zhou Q, Wu X, Wang X, Yu Z, Pan T, Li Z, et al. The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-α/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene 2020;39(7):1414-28.
[Crossref] [Google Scholar] [PubMed]
- Vacante F, Senesi P, Montesano A, Paini S, Luzi L, Terruzzi I. Metformin counteracts HCC progression and metastasis enhancing KLF6/p21 expression and downregulating the IGF axis. Int J Endocrinol 2019:1-15.
[Crossref] [Google Scholar] [PubMed]
- Dong K, Du Q, Cui X, Wan P, Kaltenmeier C, Luo J, et al. microRNA-301a (miR-301a) is induced in Hepatocellular Carcinoma (HCC) and down-regulates the expression of interferon regulatory factor-1. Biochem Biophys Res Commun 2020;524(2):273-9.
[Crossref] [Google Scholar] [PubMed]
- Yan Y, Zheng L, Du Q, Cui X, Dong K, Guo Y, et al. Interferon regulatory factor 1 (IRF-1) downregulates Checkpoint Kinase 1 (CHK1) through miR-195 to upregulate apoptosis and PD-L1 expression in Hepatocellular Carcinoma (HCC) cells. Br J Cancer 2021;125(1):101-11.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Zuo J, Zhang L, Zhang Z, Wei Y. Plantamajoside promotes metformin-induced apoptosis, autophagy and proliferation arrest of liver cancer cells via suppressing Akt/GSK3β signaling. Hum Exp Toxicol 2022;41:1-13.
[Crossref] [Google Scholar] [PubMed]