- *Corresponding Author:
- J. Sun
Beijing Key Laboratory for Green Catalysis and Separation, Department of Chemistry and Chemical Engineering, Beijing University of Technology, Beijing 100124, P. R. China
E-mail: jhsun@bjut.edu.cn
| Date of Submission | 28 December 2016 |
| Date of Revision | 12 June 2017 |
| Date of Acceptance | 02 February 2018 |
| Indian J Pharm Sci 2018;80(2):298-306 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The effects of cations and pH on the in vitro ibuprofen release from dextran-poly(acrylic acid) copolymer were investigated at varying pH and Na+ or Ca2+ concentrations. These results revealed that ibuprofen release was strongly depended on the acidic and basic environments, in which, the cumulative amount released in 24 h reached up to 87 % at pH 2.0 but only 32 % at pH 7.4, respectively, demonstrating a good pH-dependency. However, cations in release solution exerted a remarkable influence on ibuprofen release, especially in the acidic media. Meanwhile, ibuprofen release rate decreased with increasing strength of Na+, Ca2+ and NaCl concentration. The zeta potential profiles and particle distributions at different ionic strengths indicated that cations strongly influence ibuprofen release performance, through surface charge, hydrogen bonding, and electrostatic interactions in and between the polymer and ibuprofen. These results suggested that Na+ and Ca2+ strength exerted a profound influence on the pH-sensitive in vitro ibuprofen release from the dextran-poly(acrylic acid) copolymer.
Keywords
Dextran-poly(acrylic acid) copolymer, dextran, ibuprofen, pH-sensitivity, salt effect
During the past few decades, new polymer-based acrylic particles have attracted extensive attentions due to its pH-dependent shrinking-swelling behaviours [1]. Quintero et al. [2] investigated the swelling behaviour of polyvinyl alcohol (PVA) and polyacrylic acid (PAA) hydrogels in buffer solutions at different pH values and found that its swelling performance increased with pH value, evidently demonstrating that PVA, PAA hydrogels were a promising candidate for the pH sensing drug carrier. Meanwhile, Abd El-Rehim et al. [3] prepared a PAA-based drug carrier with polyvinylpyrrolidone (PVP)/PAA as a template polymer and then investigated its swelling properties and release behaviours. They found that the caffeine release from obtained PVP/PAA copolymer was slower than that from PAA, whereas, the carboxylic groups of PAA in the PVP/PAA polymer led to a lower release rate of caffeine at lower pH. These results clearly indicate that that the copolymers are suitable carriers for drug delivery systems with stimuli responsibility to pH. Since then, numerous preparations and potential applications of the pH responding swelling/ release behaviours of various copolymers have been extensively explored [4].
The drug-releasing behaviours from stimuliresponsible copolymer are greatly dependent on various interactions caused by aqueous property, copolymer skeleton, and drug structure [5]. Based on the variables of the gastrointestinal (GI) fluids [6] in the human body, it has been concluded that the pH environments and cation charge are two major parameters considered in the design of oral extended release formulations [7]. Several investigators have sought to examine the effects of ionic strength on drug-release performances. Lapidus and Lordi early noted this effect and demonstrated rapid release of chlorpheniramine in 0.2 M solutions of sodium and magnesium sulphates from hydroxypropyl methylcellulose type 2208 matrices [8]. Mitchel et al. further investigated the effects of various ions and concentrations on the disintegration time and dissolution of drugs from these matrices [9]. Therefore, numerous studies have been focused on the evaluations of their pH-responsibility and salt effects on these polymers under the abovementioned biological conditions [10]. Fu et al. [11] prepared the poly(acrylamide-co-acrylic acid) hydrogels and elucidated the salt effect on swelling ratios. They found that the swelling rate of the hydrogels abruptly declined with the swelling media changed from distilled water to NaCl solution, indicating the unique property of the polymers. Awasthi and Singhal [12] synthesized a series of poly(acrylamide-co-hydroxyethylacrylamideco- acrylic acid) hydrogels and investigated the effect of NaCl concentration on swelling behaviours. The experimental results demonstrated that the swelling ratios of all hydrogels apparently decreased with the increase of NaCl concentration due to the decrement in the expansion of the gel networks. In addition, the equilibrium swelling ratio of the hydrogels in various salt solutions decreased with the increase of cationic charges, following the order of Na+>Ca2+>Fe3+.
Our previous work [13] has clearly demonstrated that the vinyl triethoxylsilane-modified polymethylacrylic acid copolymer is a highly pH-sensitive material. However, further investigation found that its swelling-shrinking properties were significantly declined with the increase of NaCl concentration. All these studies have demonstrated the profound effects of salt solutions on the swelling ratio of the copolymers. However, their influences on the drug-release performance of the copolymers are still vague.
Dextran is a water-soluble polysaccharide [14] and is clinically used in food and medical fields due to its lack of toxicity, biodegradability and biocompatibility [15-17]. Dextran-based nanoparticles exhibited a pronounced pH-sensitivity because of the carboxy groups in the nanoparticles [18]. Zhang et al. reported a novel pH- and ionic-strength-sensitive carboxymethyl dextran hydrogel membrane, in which the acidic groups caused the pH-dependent swelling [19]. However, pH-sensitive polymer based on dextran has not been used in the drug loading and controlleddelivery [20]. More recently, we synthesized a modifiedcopolymer, dextran-poly(acrylic acid) copolymer (D-A copolymer) by grafting a synthetic PAA onto dextran and demonstrated its non-toxicity, biocompatibility, excellent biodegradability, and pH-sensitivity [21]. Its drug loading and releasing performance in phosphate buffer solutions (PBS) at pH 2.0 and 7.4 was investigated with ibuprofen (IBU) as model drug. The results show that the prepared copolymer is sensitive to the pH of its releasing environment, and its pH-sensitivity is increased with the increase of the molar ratio of AA/dextran. However, the influences of salt concentration and species on its drug-release performance are unknown. The present work has been focused on the tolerance of the in vitro drug-release performance of the D-A copolymers to salts using IBU as a model drug. IBU is a well-known nonsteroidal antiinflammatory drug that has been extensively used to treat rheumatoid arthritis and atherosclerosis [22]. However, IBU cannot be effectively absorbed due to its relatively poor water-solubility. In addition, its halflife is only 1.5 or 2 h, which requires frequent dosing to maintain effective blood drug concentration [23]. Therefore, it is suitable as a model drug to evaluate performance of novel drug delivery systems. The effects of different salt species and concentrations on the zeta potential and particle distribution of D-A copolymer were also investigated in the present work.
Materials and Methods
Dextran, acrylic acid, cerium (IV) ammonium nitrate (CAN), N,N-methylene bisacrylamide (MBA) was obtained from Sinopharm Chemical Reagent Co. Ltd, China. MBA was re-crystallized from methanol before using. Hydrochloric acid (HCl), ammonia solution (NH3·H2O), sodium chloride (NaCl), sodium hydroxide (NaOH), nitric acid (HNO3) and ethanol all of analytical grade were purchased from Beijing Chemical Factory. Analytical grade anhydrous calcium chloride (CaCl2) was obtained from Tianjin Fuchen Chemical Reagents Factory. IBU was provided by Zibo Beikang Biotechnology Co. Ltd. PBS was made from disodium hydrogen phosphate, sodium dihydrogen phosphate and phosphoric acid in a particular proportion. Deionized water was used throughout all experiments.
Preparation of dextran-PAA polymer
Synthesis of dextran-PAA was based on a reported procedure, originally as described by Guo et al. [21]. About 1.25 g dextran was dissolved in 50 ml deionized water with mild magnetic stirring and nitrogen bubbling, then the solution of a specific amount of CAN dissolved in 1.25 ml dilute HNO3 (0.1 mol/l) and ten moles of acrylic acid per mole of dextran Tunit (glucose) were added into the flask. After 0.5 h, MBA was added to the reaction mixture and then kept on stirring at 30° for 4 h. Thereafter, 1 M NaOH was added to neutralize the reaction system. Finally, the reaction solution was precipitated with ethanol to remove the un-reactive monomers. The obtained products were filtered and washed with ethanol for several times and dried at 40°, denoted as D-A.
Drug loading
About 0.3 g D-A was dissolved in 30 ml IBU ethanol solution (30 mg/ml) containing in a flask and the mixture was stirred using magnetic stirring apparatus for 48 h at room temperature. Then the solution was filtered and washed with ethanol to remove excess IBU adsorbed on the outer surface of D-A. Finally, the product was dried under vacuum and denoted as I/D-A. To evaluate the amount of drug loaded by D-A copolymer, high-performance liquid chromatography (HPLC) was used for analysis. First of all, the calibration curve of IBU was prepared by measuring absorbance of IBU concentration between 0.1 and 18 mg/ml, and the calibration curve was fitted as follows: C = 0.0031A+0.0117, R2= 0.9999, where, A is the peak area and C is the concentration (mg/ml).
After adsorption, the IBU solution (1 ml) was extracted and diluted to 50 ml, and then analysed using a HPLC (Agilent Technologies 1200 series), set with the following parameters, wavelength was 272 nm, chromatographic column was Extend-C18, and the mobile phase was anhydrous methanol- pH 3.0 phosphate buffer (volume ratio of 3:1), the flow rate was 1.000 ml/min and injection volume was 6 μl. The retention time of a characteristic peak in the HPLC of the IBU was 6.6 min. The loading amount (LA) was calculated using the following Eqn., LA (%) = m0–m1/m2+(m0–m1)×100, where, m0 is the weight of IBU added initially, m1 is the weight of IBU in the filtrate, and m2 is the weight of copolymer D-A. The LA % was 7.2 through the measurement and calculation.
In vitro drug release
Release test of IBU was carried out as follows. The release medium was PBS solution (pH value of 2.0 and 7.4), 1.0 M HCl solution (pH 2.0) and 1.2 M NH3·H2O (pH 9.3). CaCl2 anhydrous and NaCl were used to regulate the ionic strength and ionic concentration at pH 2.0 and 9.3. Firstly, samples were pressed into slice (10 mg) and put into dialysis bag with a 14 000 cutoff molecular weight, and then immersed into different release mediums (10 ml) at 37°. In the course of the drug release, 1 ml of this solution was taken out at certain time intervals for drug measurement. The total volume of the solution was kept constant by adding 1 ml of the fresh medium after each sampling. The concentration of the drug-released was determined by UV/Vis spectroscopy monitored at 272 nm because of low concentration of IBU in the release solutions. The calibration curve of IBU was determined by taking absorbance vs. IBU concentration between 0.0005 and 0.5 mg/ml, and the calibration curve was fitted as follows: C = 0.7012A–0.00329, R2= 0.9999, where A is the peak area and C is the concentration (mg/ml).
All measurements were made two times and standard deviations (R2) from the average value were more than 0.999. The released drug content was calculated according to the following formula, Cc = Ct+v/V× Σt–10×Ct, where, Cc (mg/ml) is the corrected concentration at time t (min), Ct (mg/ml) is the apparent concentration at time t (min), v (ml) is the volume of sample taken, and V (ml) is the total volume of the released fluids.
Characterizations
Fourier transform infrared spectroscopy (FTIR) spectrum was measured using KBr discs in the region of 4000-400 cm-1 by a Nicolet Nexus 470 spectrometer to analyse the composition of the resultant copolymer. Thermogravimetric analysis (TGA) were carried out between 25 and 800o using a Seiko TG/DTA 320 with an N2 flow rate 100 ml/min and a heating rate of 10°/min. Scan electron microscopy (SEM, Hitachi S-4300, Japan) images were obtained by a Hitachi S-4300 electron microscope at an acceleration voltage of 15 kV. Agilent Technologies HPLC-1200 series was used to measure the amount of IBU loaded. A Shimadzu UV-2450 spectrophotometer was used to measure the amount of IBU released from the samples. Zeta-potential was measured by particle sizing systems (Nicomp 380/ZLS) and the particle size distribution of the copolymer was carried out by Zetasizer Nano Series (Nano-ZS) instrument.
Results and Discussion
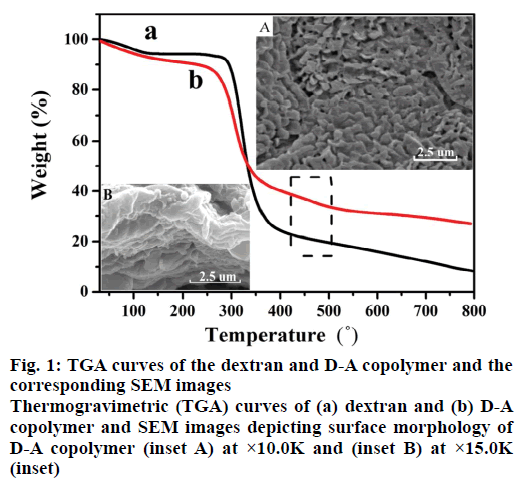
Figure 1 shows the TGA profiles of dextran and D-A copolymer. As can be seen, both polymers subjected two decomposition stages. The 7 % weight loss at temperatures below 100° was ascribed to the evaporation of the physically absorbed water, and the 52 % weight loss at temperature range of 250-360° was assigned to the degradation of their saccharide structures [24]. Interestingly, as profiled in Figure 1b, D-A copolymer subjected an addition decomposition stage in the temperature range of 450-480° [25], which was attributed to the presence of PAA (Figure 1a vs. b). Meanwhile, the surface morphology of D-A copolymer is a thick sheet structure (Figure 1 inset A) and the SEM image with high magnification (Figure 1 inset B) exhibits the highly porous cross-linked networks, which can promote the swelling rate and drug delivery.
In addition, two new absorption peaks at 1419 cm-1 and 1547 cm-1 appeared in the FTIR spectrum of D-A copolymer, which were attributed to asymmetric and symmetric stretching vibrations of carboxyl groups of PAA [26] as compared with that of pure dextran. While the typical C-O-C asymmetrical stretching of dextran at 1152 cm-1 and 1104 cm-1 [27], and C-H stretching vibration at 2918 cm-1 were also present in the FTIR spectrum of D-A copolymer. Based on these results, it can be concluded that AA has been successfully grafted to the dextran skeletons.
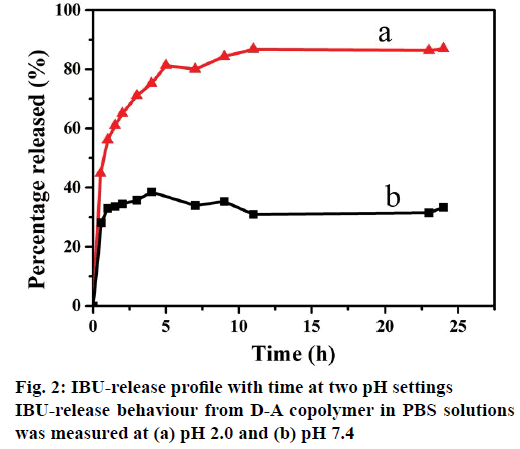
The drug release tests were conducted by soaking I/D-A copolymer in PBS medium with pH of pH 2.0 and 7.4 at 37°, as shown in Figure 2. It can be seen clearly that the IBU-release rate at pH 2.0 was obviously faster than that at pH 7.4. Accordingly, the cumulative release for D-A copolymer reached up to about 65 % within 2 h in the pH 2.0 PBS solution, while, the released amount was around 34 % of cumulative total within 2 h in the pH 7.4 PBS solution. The cumulative release reached 87 and 32 %, respectively in 24 h. These phenomena could be explained on the basis of pH influence on the structures of both D-A copolymer and IBU. The pKa of PAA and IBU are 4.75 and 4.91, respectively [28], and thereafter both PAA and IBU were protonated in the acidic solution (pH 2.0). D-A copolymer was in a neutral state and its internal electrostatic repulsion was negligible. The predominant hydrogen-bonding interactions via intra-molecular and inter-molecular interactions in the D-A copolymer forms a compact and ultra-coil conformation [29], which can easily shrink to release the loaded IBU. In the alkaline solution (pH 7.4), the carboxyl groups inside the polymer were deprotonated as -COO- [5], which increased the charge density on the polymer chains, weakened or even broken the hydrogen-bonding interactions, and thus enhanced the electrostatic repulsion [30]. The electrostatic repulsion among the -COO- groups of the D-A copolymer maintained its swelling status, leading to slow IBU-release and a small cumulated amount of IBU. These results evidently confirm that the D-A copolymer has an excellent pH-sensitivity in physiological and biological environments due to its dissociable acid groups (-COOH). Therefore, it is necessary to further investigate the influence of the salt solution on the in vitro IBU-release performance from resultant D-A copolymer.
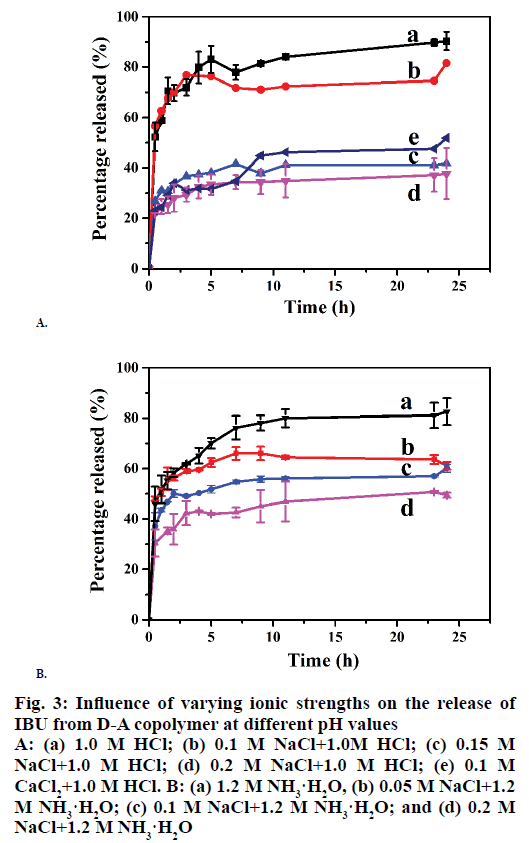
It has been reported that cation charge plays an important role in drug delivery. In the present work, the influences of the cation concentration on the release behaviours of I/D-P were determined at first. As can be seen from Figure 3A, the cumulative release decreased with the increasing of NaCl concentration from 0.0 to 0.2 M. The 24 h cumulative release reached to ~85 % in 1.0 M HCl, 73 % in 0.1 M NaCl , 40 % in 0.15 M NaCl, and 30 % in 0.2 M NaCl (Figure 3A), respectively, in 24 h. It can be explained that both D-A copolymer and IBU molecules have numerous hydroxide groups. The hydrogen-bonding interactions are one of the major bindings for the IBU adsorption on the D-A copolymer skeletons. The introduction of cations can significantly effect on the hydrogen-bonding between the copolymer and IBU and also among the copolymers molecules, which is favourable for the volume swelling of the copolymer. Therefore, IBU can be effectively confined inside the copolymer skeletons. In all, cations reduce the IBU-release performance of the copolymer by weaken the hydrogen-bonding between the copolymers and IBU.
The IBU-release profiles of the D-P copolymer in 0.1 M NaCl and 0.1 M CaCl2 are depicted in Figure 3A. As can be seen, the balanced cumulative release reached 73 % in the NaCl solution and 46 % in the CaCl2 solution in 10 h, indicating that the IBU-release was decreased with the increase of cation charge. It might be attributed to the stronger “ionic cross-linking density” [7] between Ca2+ and -COO-. While, Na+ is surrounded with a large numbers of water molecules, which leads to a longer hydration radius, and thus weaker binding ability of Na+ [31].
Figure 3B shows the effects of Na+ concentration on the IBU-release performance of the D-A polymer in 1.2 M NH3·H2O solutions. The cumulative release reached 76 % in 1.2 M NH3·H2O solutions in 24 h. The additions of 0.05 M, 0.1 M, and 0.2 M NaCl (Figure 3B) led to cumulative release amounts of 64, 54, and 43 %, respectively, indicating that the cumulative IBU-release from the copolymer was decreased with the increase of Na+ concentration under alkali conditions. This is attributed to the weak hydrogen-bonding interactions between IBU and the copolymer caused by the high cation concentration.
Figure 3: Influence of varying ionic strengths on the release of IBU from D-A copolymer at different pH values
A: (a) 1.0 M HCl; (b) 0.1 M NaCl+1.0M HCl; (c) 0.15 M NaCl+1.0 M HCl; (d) 0.2 M NaCl+1.0 M HCl; (e) 0.1 M CaCl2+1.0 M HCl. B: (a) 1.2 M NH3·H2O, (b) 0.05 M NaCl+1.2 M NH3·H2O; (c) 0.1 M NaCl+1.2 M NH3·H2O; and (d) 0.2 M NaCl+1.2 M NH3·H2O
The results presented above evidently demonstrate that the IBU-release rate of the D-A copolymer is decreased with the increase of ion concentration and ionic strength. The salt cation has a great influence on the drug release properties of the D-A copolymer and significantly reduces the controlled-release sensitivity. The swelling and shrinking properties of obtained copolymer are affected by its surface charge and particle distribution. Therefore, Zeta potential and particle distribution of the D-A copolymer was investigated in the following work. To further evaluate the release performance of the D-A copolymer, the kinetics of the IBU-release was determined at first by fitting the release profiles with the following theoretical models: Higuchi model [32], Mt/ M∞ = kHt0.5, where, kH is the Higuchi release constant; Mt is the cumulative mass of IBU released at time t; and M∞ is the total amount of the IBU loaded in the D-A copolymer. Korsmeyer-Peppas model [33]: Mt/M∞ = ktn. Modified Korsmeyer-Peppas model [34], Mt/M∞ = ktn+C, where, k is a release constant; n is the diffusional exponent that varies with the solute mode of transport; and C is a constant that represents the initial burst effect. Based on these models, the drug release from polymer in a solvent in thermodynamic equilibrium is Fickian if n<0.43 [35] and is non-Fickian or anomalous if 0.43<n<0.85. Otherwise, the drug release is dominated by the corrosion of the polymer [36].
The Eqns for each model and the kinetic parameters are listed in Table 1, and the fitted plots of the IBU-release profiles of D-A copolymer using Korsmeyer-Peppas model under different cation concentrations. As can be seen in Table 1, the squared correlation coefficient (R2) values obtained with the Higuchi model are in the range of 0.76-0.87, suggesting that the IBU-release profiles of D-A polymer do not fit this model well. Much higher R2 values (˃0.99) were obtained with the Korsmeyer-Peppas model, (except that in the 1.0 M HCl solution), indicating that it is suitable to elucidate the IBU-release kinetics. Particularly, R2= 0.9994 was obtained for the IBU-release rate of D-A copolymer in 1.0 M HCl in the first 1 h with the modified Korsmeyer-Peppas model for the pronounced burst effect.
| IBU-release media | Higuchi | Korsmeyer-Peppas model | |||
|---|---|---|---|---|---|
| Mt/M∞ = kHt0.5 | Mt/M∞ = ktn | ||||
| KH | R2 | k | n | R2 | |
| 1.0 M HCl pH=2.0 |
0.37 | 0.8242 | — | — | — |
| 0.1 M NaCl+1.0 M HCl pH= 2.0 |
0.43 | 0.8363 | 0.60 | 0.21 | 0.9942 |
| 0.15 M NaCl+1.0M HCl pH= 2.0 |
0.18 | 0.7931 | 0.31 | 0.13 | 0.9944 |
| 0.2 M NaCl+1.0 M HCl pH= 2.0 |
0.15 | 0.8265 | 0.25 | 0.16 | 0.9921 |
| 0.1 M CaCl2+1.0 M HCl pH= 2.0 |
0.16 | 0.7677 | 0.26 | 0.16 | 0.9902 |
| 1.2 M NH3·H2O pH= 9.3 |
0.19 | 0.7965 | 0.52 | 0.17 | 0.9993 |
| 0.05 M NaCl+1.2 M NH3·H2O pH= 9.3 |
0.30 | 0.8016 | 0.31 | 0.18 | 0.9965 |
| 0.1 M NaCl +1.2 M NH3·H2O pH= 9.3 |
0.22 | 0.8155 | 0.29 | 0.15 | 0.9910 |
| 0.2M NaCl+1.2M NH3·H2O pH= 9.3 |
0.21 | 0.8759 | 0.29 | 0.29 | 0.9911 |
Table 1: The Mathematical Model Used to Describe Drug Release from D-A Polymers
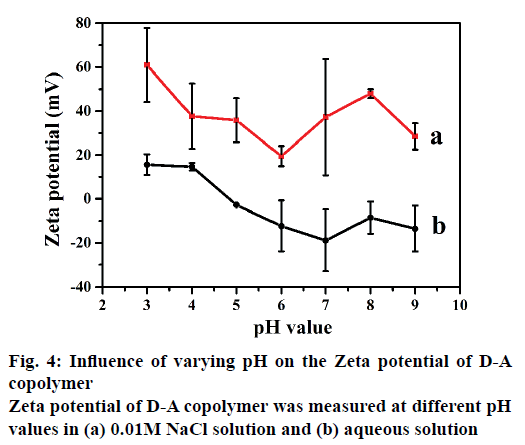
The IBU-release kinetics also indicates that the rate constant k decreases with the increase of cation charge. The release exponent n is in the range of 0.13- 0.29 (<0.43), suggesting the diffusion-controlled release is by Fickian diffusion mechanism instead of corrosion. These results are in a good agreement with the previous reports of Hahn et al. [37] and Parfenyuk et al. [38]. Evidently, two factors including salt cation and pH should be considered as the release forces. Cations can weaken the hydrogen-bonding interaction between dextran and PAA, especially in alkaline medium promoting the swelling volume of the copolymer and reducing the IBU-release rate. The enhanced electrostatic repulsions delay the IBU diffusion from D-A copolymer. In addition, thermodynamics equilibrium concentrations in the carrier and various release media are usually reached in 10 h (Figures 2 and 3). The effects of salt on the zeta potential of the D-A copolymer at different pHs are shown in Figure 4. As can be seen in Figure 4b, the D-A copolymer showed negative zeta potentials ranging from –13.5 to –2.7 (mV) in pure aqueous solutions with pH of 5.0-9.0, indicating that most carboxyl groups were deprotonated to -COO-. In this regard, the PAA easily leads to an overcharged surface and subsequently stronger electrostatic repulsion between -COO- groups, which cause higher negative zeta-potential [39]. The copolymer has an isoelectric point between pH 4.0 and pH 5.0 due to the pKa of PAA at 4.75 [40]. Therefore, the D-A copolymer displayed a positive zeta potential at pHs below 4.0 due to the protonation of PAA in the acidic media. They interact with each other mainly through hydrogenbonding, which leads to positive zeta-potential and voluminous aggregation [41]. Moreover, the D-A copolymer also showed positive zeta potentials in the certain concentrations of NaCl. However, the zeta potential profile in 0.1 M NaCl presented huge fluctuations at different pH, and thus the obtained data were unreasonable and unrepeatable. Therefore, we chose 0.01 M NaCl to evaluate the effects of salt concentration on the zeta potential of the D-A copolymer. As depicted in Figure 4a, the absolute value of the zeta potential in the NaCl solution is higher than that in pure aqueous solution, indicating that Na+ ions can more significantly increase the surface charge of D-A copolymers due to hydrogen binding ability of the carboxylate groups. Consequently, the electrostatic repulsion force of copolymer is increased, leading to a decrease in its controlled-release ability. In addition, the zeta potential is positive in the alkaline solution due to the counter ions on the copolymer shielded by the bound ionic charges [31,32].
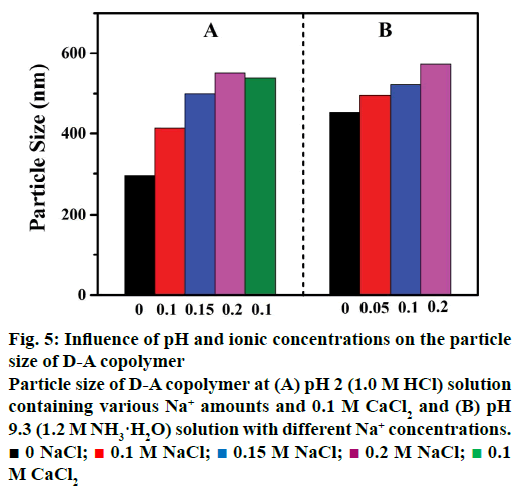
The particle size of the D-A copolymer varies with Na+ concentrations and salt species in the HCl solution (Figure 5). As can be seen in Figure 5A, the D-A copolymer displayed a smaller size of ~294 nm in 1.0 M HCl. The average particle size gradually increased from 415 to 500 nm, and 548 nm (statistical error, ~3 %) as the ion concentration increased from 0.1 to 0.15 M, and 0.2 M, respectively, suggesting the structural transformation of the copolymer from shrinkage at low ion concentration to swelling at high ionic concentration. The average particle size of D-A copolymers in 0.1 M CaCl2 solution is ~534 nm, larger than that in 0.1M NaCl (about 415 nm), clearly indicating a strong influence of cation charge on the copolymer particle size. It has been reported that hydrogen-bonding and surface charge can impact on the particle size of the D-A copolymer [30]. The variation of copolymer particle size and volume at different Na+ concentration and cation species is the key factor for its sensitivity as discussed above that the IBU-release rate of the D-A copolymer decreased with the increasing of NaCl concentration (Figure 3A). The increment in the expansion of the copolymer volume at high NaCl concentrations impacts adversely on the controlled-release. Therefore, it is inferred here that the cation charge could also play an important role in the drug-release performance of the D-A copolymer in an acidic medium.
Figure 5: Influence of pH and ionic concentrations on the particle size of D-A copolymer
Particle size of D-A copolymer at (A) pH 2 (1.0 M HCl) solution containing various Na+ amounts and 0.1 M CaCl2 and (B) pH 9.3 (1.2 M NH3·H2O) solution with different Na+ concentrations. ■ 0 NaCl;  0.1 M NaCl;
0.1 M NaCl;  0.15 M NaCl;
0.15 M NaCl;  0.2 M NaCl;
0.2 M NaCl;  0.1 M CaCl2
0.1 M CaCl2
As shown in Figure 5B, the particle size of the D-A copolymer increased from 453 to 573 nm (statistical error, ~3 %) as the NH3·H2O concentration increased to 0.2 M. This indicates that cations have a remarkable influence on the drug release in alkaline solution, consisting with the results discussed above (Figure 3B).
Based on above discussions, we propose a possible mechanism as follows: the high ion concentration of the releasing solution could easily cause the enhancement of the surface charge of the D-A copolymer, and increasing the particle size, obvious leading to the lower IBU-release rate, particularly, in acidic aqueous solutions than that in alkaline medium. In all, the high cation concentration and ionic strength could be helpful to increase particle size of the D-A copolymer, leading to its poor drug-release performance.
In addition, according to the electrolyte composition of GI fluids deriving from literature [42,43], it is unlikely that the low ionic strengths of 0.01-0.16 mEq/l for gastric and 0.070-0.166 meg/l for intestinal fluids would undergo in vivo influences on the pH-sensitive release performances using D-A copolymer as a drug carrier. In this way, the obtained D-A copolymers should be a good candidate as a drug carrier for potential drug delivery applications. But, the salt cationic concentration (up to 0.2 M/l) and charge (Na+ or Ca2+) of the solution used in this study exceeded the physiological ionic strength conditions in the human GI tract, which have a great impact on its drug release properties and significantly reduce the controlled-release sensitivity, as shown in Figures 3-5.
The effects of cations in the release solution on the IBU-release performance of D-A copolymers were evaluated in different solutions with different pH and containing various concentrations of Na+ or Ca2+ cations. The results indicate that the copolymer was pH-sensitive and showed a fast IBU release in low pH solutions and slow IBU release in high pH solutions. Increasing the ionic concentration of the release solutions from 0.05 to 0.20 M resulted in a decrease in the cumulative release rate. The tendency to decline is more pronounced in an acidic solution than that in an alkaline medium.
The IBU cumulative release rate of the D-A copolymer in the Ca2+ solution is significantly lower than that in the Na+ solution, indicating that ionic strength can also impact on the IBU-release performance of the D-A copolymer. The ion concentration and ionic strength effect on the zeta potential and particle distribution of the D-A polymer by changing the hydrogen-bonding, electrostatic interaction, and surface charge, which further impacts on its swollen/shrunken volume and IBU-release performance. In summary, the cations in the release solution can significantly influence on the drug-release profile of the D-A copolymer.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (21576005), and the Beijing Municipal Natural Science Foundation (2152005).
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Dittgen M, Durrani M, Lehmann K. Acrylic polymers-A review of pharmaceutical applications. STP Pharma Sci 1997;7:403-37.
- Quintero SMM, Ponce FRV, Cremona M, Triques ALC, d’Almeida AR, Braga AMB. Swelling and morphological properties of poly(vinyl alcohol) (PVA) and poly(acrylic acid) (PAA) hydrogels in solution with high salt concentration. Polymer 2010;5:1953-8.
- Abd El-Rehim HA, Hegazy EA, Khalil FH, Hamed NA. Radiation preparation of drug carriers based polyacrylic acid (PAAc) using poly(vinyl pyrrolidone) (PVP) as a template polymer. Nucl Instrum Methods Phys Res B 2007;B254:105-12.
- Bury K, Neugebauer D. Novel self-assembly graft copolymers as carriers for antiinflammatory drug delivery. Int J Pharm 2014;460:150-57.
- Wilson CG, Washington N, Greaves JL, Kamali F, Rees JA, Semplk AK, et al. Bimodal release of ibuprofen in a sustained-release formulation-a scintigraphic and pharmacokinetic open study in healthy-volunteers under different conditions of food-intake. Int J Pharm 1989;50:155-61.
- Charman WN, Porter CJH, Mithani SD, Ressman JB. Physicochemical and physiological mechanisms for the effects of food on drug absorption: The Role of lipids and pH. J Pharm Sci 1997;86:269-82.
- Sasa B, Odon P, Stane S, Julijana K. Analysis of surface properties of cellulose ethers and drug release from their matrix tablets. Eur J Pharm Sci 2006;27:375-83.
- Lapidus H, Lordi NG. Drug release from compressed hydrophilic matrices. J Pharm Sci 1968;57:1292-301.
- Mitchel K, Ford JL, Armstrong DJ, Elliott PNC, Rostron C, Hogan JE. The influence of additives on the cloud point, disintegration and dissolution of hydroxypropyl methylcellulose gels and matrix tablets. Int J Pharm 1990;66:233-42.
- Johnson JL, Holinej J, Williams MD. Influence of ionic strength on matrix integrity and drug release from hydroxypropyl cellulose compacts. Int J Pharm 1993;90:151-59.
- Fu Q, Rao GVR, Ista LK, Wu Y, Andrzejewski BP, Sklar LA, et al. Control of molecular transport through stimuli-responsive ordered mesoporous materials. Adv Mater 2003;15:1262-66.
- Awasthi S, Singhal R. A mathematical study on effect of 2-hydroxyl ethyl acrylate on controlled drug diffusion from smart hydrogels based on poly(acrylamide-co-hydroxy ethyl acrylate-co-acrylic acid). J Macromol Sci Pure Appl Chem 2012;49:397-413.
- Bai SY, Zhang H, Sun JH, Han J, Guo YY. Preparation and pH-responsive performance of silane-modified poly(methylacrylic acid). J Appl Polym Sci 2014;131:40403-10.
- Thoren L. The dextrans-clinical data. Dev Biol Stand 1981;48:157-67.
- Cristallini C, Barbani N, Giusti P, Lazzeri L, Cascone MG, Ciardelli G. Polymerisation onto biological templates, a new way to obtain bioartificial polymeric materials. Macromol Chem Phys 2001;202:2104-13.
- Chauvierre C, Labarre D, Couvreur P, Vauthier C. Radical Emulsion polymerization of alkylcyanoacrylates initiated by the redox system dextran-cerium(IV) under acidic aqueous conditions. Macromolecules 2003;36:6018-27.
- Heidrick GW, Pippitt Jr CH, Morgan MA, Thurnau GR. Efficacy of intraperitoneal sodium carboxymethyl cellulose in preventing postoperative adhesion formation. J Reprod Med 1994;39;575-8.
- Tang MH, Dou HJ, Sun K. One-step synthesis of dextran-based stable nanoparticles assisted by self-assembly. Polymer 2006;47:728-34.
- Zhang RS, Tang MG, Bowyer A, Eisenthal R, Hubble J. A novel pH and ionic-strength-sensitive carboxymethyl dextran hydrogel. Biomaterials 2005;26:4677-683.
- Heidrick GW, Pippitt CH, Morgan MA, Thurnau GR. Efficacy of intraperitoneal sodium carboxymethylcellulose in preventing postoperative adhesion formation. J Reprod Med 1994;39:575-8.
- Guo YY, Sun JH, Bai SY, Zhang YN, Wu X. Preparation of pH-sensitive Dextran-Poly (acrylic acid) copolymer and its application as a controlled ibuprofen delivery. Int J Polym Mater Polym Biomater 2017;66(17):900-06.
- Cai X, Wang N, Lin X. Chemo-enzymatic synthesis of optically active polymeric prodrug of naproxen, ketoprofen and ibuprofen. Polymer 2006;47:6491-95.
- Chen HB, Wen T, Chang XL, Yang YJ, Yang XL. Preparation of ibuprofen microemulsion and its transdermal absorption. Chin Pharm J 2004;39:43-5.
- Peniche C, Arguelles-Monal W, Davidenko N, Sastre R, Gallardo A, San Roman J. A novel mucoadhesive polymer prepared by template polymerization of acrylic acid in the presence of chitosan. Biomaterials 1999;20:1869-78.
- Barbani N, Bertoni F, Ciardelli G, Cristallini C, Silvestri D, Coluccio ML, et al. Bioartificial materials based on blends of dextranand poly(vinyl alcohol-co-acrylic acid). Eur Polym J 2005;41:3004-10.
- Tang HY, Guo J, Sun Y, Chang BS, Ren QG, Yang WL. Facile synthesis of pH sensitive polymer-coated mesoporous silica nanoparticles and their application in drug delivery. Int J Pharm 2011;42:388-96.
- Shukla R, Goyal A. A novel dextran from pediococcus pentosaceus CRAG3 isolated from fermented cucumber with anti-cancer properties. Int J Biol Macromol 2011;62:352-57.
- Ahn JS, Choi HK, Cho CS. A novel mucoadhesive polymer prepared by template polymerization of acrylic acid in the presence of chitosan. Biomaterials 2001;22:923-28.
- Dou HJ, Sun K, Yang WH. The self-assembly of hydroxypropylcellulose and carboxy-ended surfactants to multi-morphological nanoparticles. Macromol Chem Phys 2006;207:1899-904.
- Hu Y, Chen Y, Chen Q, Zhang LY, Jiang XQ, Yang CZ. Synthesis and stimuli-responsive properties of chitosan/poly(acrylic acid) hollow nanospheres. Polymer 2005;46:12703-10.
- Sadeghi M, Hosseinzadeh H. Synthesis and super swelling behaviour of carboxymethylcellulose-poly(sodium acrylate-co-acrylamide) hydrogel. J Appl Polym Sci 2008;108:1142-51.
- Higuchi T. Mechanism of sustained action medication, theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 1963;52:1145-49.
- Korsemeyer RW, Gurny R, Doelker E, Pierre NB, Peppas A. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 1983;15:25-35.
- Kim H, Fassihi R. Application of a binary polymer system in drug release rate modulation.1. Characterization of release mechanism. J Pharm Sci 1997;86:316-22.
- Katime I, Valderruten N, Quintana JR. Controlled release of aminophylline from poly (N-isopropylacrylamide-co-itaconic acid) hydrogels. Polym Int 2001;50:869-79.
- Lynch I, Dawson KA. Release of model compounds from "plum-pudding"-type gels composed of microgel particles randomly dispersed in a gel matrix. J Phys Chem B 2004;108:10893-98.
- Hahn A, Brandes G, Wagener P, Barcikowski S. Metal ion release kinetics from nanoparticle silicone composites. J Control Release 2011;154:164-70.
- Parfenyuk EV, Dolinina ES. Design of silica carrier for controlled release of molsidomine: Effect of preparation methods of silica matrixes and their composites with molsidomine on the drug release kinetics in vitro. Eur J Pharm Biopharm 2014;88:1038-45.
- Joksimovic R, Prevost S, Schweins R, Appavou MS, Gradzielski M. Interactions of silica nanoparticles with poly(ethylene oxide) and poly(acrylic acid): Effect of the polymer molecular weight and of the surface charge. J Colloid Interface Sci 2013;394:85-93.
- Ahn JS, Choi HK, Lee KH, Nahm JH, Cho CS. Novel mucoadhesive polymer prepared by template polymerization of acrylic acid in the presence of silk sericin. J Appl Polym Sci 2001;80:274-80.
- Arias JL, Lopez-Viota M. Stability of fenbendazole suspensions for veterinary use Correlation between zeta potential and sedimentation. Eur J Biochem 2008;34:257-62.
- Collins RD. Illustrated Manual of Fluid and Electrolyte Disorders. 2nd ed. Philadelphia: Lippincott; 1983. p. 190.
- Goldberger E, Brensilver JM. A Primer of Water, Electrolyte, and Acid-Base Syndromes, 7th ed. Philadelphia: Lea & Febiger; 1986. p. 74.