- *Corresponding Author:
- Majed Nahari
Pharmaceutical Care Services, King Abdullah bin Abdulaziz University Hospital, Riyadh, Kingdom of Saudi Arabia
E-mail: aaalsharif@pnu.edu.sa
| This article was originally published in a special issue, “Integrative Approaches in Biomedical Sciences for Drug Discovery and Development” |
| Indian J Pharm Sci 2024:86(6) Spl Issue “52-57” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
There is limited data on the prevalence and risk factors of hyperkalemia in heart failure patients who received spironolactone as an add-on to standard therapy, including angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. The objective of this study is to determine the incidence of hyperkalemia and identify its risk factors among heart failure patients using spironolactone. This retrospective chart review included adult heart failure patients (≥18 y) who were initiated on spironolactone therapy, using health information system data to capture the period from treatment initiation to the incidence of hyperkalemia. The study was conducted at King Abdulaziz Medical City in Riyadh. An excel-based tool (Microsoft® Excel; version 2018) was used for systematic data sampling and analysis. A total of 349 patients met the inclusion criteria. 43 % of patients were men while 57 % were women. The mean age of patients was (64.87±14.02) y. 161 patients were received 12.5 mg spironolactone, 40 % of those patients who had incidence of hyperkalemia. 62 % of those who developed hyperkalemia were on angiotensin-converting enzyme inhibitors, 28 % on angiotensin II receptor blockers, 14 % on potassium supplements. 263 patients were received 25 mg spironolactone, 47 % of patients had incidence of hyperkalemia. 49 % of those who developed hyperkalemia were on angiotensin-converting enzyme inhibitors, 31 % on angiotensin II receptor blockers, and 22 % on potassium supplements. 17 patients were received 50 mg spironolactone, 53 % of patients had incidence of hyperkalemia. Our study showed that half of heart failure patients who used spironolactone developed hyperkalemia and were either on angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. Further study with a larger sample size is required to clarify and confirm our study findings.
Keywords
Hyperkalemia, renin-angiotensin-aldosterone system inhibitors, spironolactone, aldosterone antagonists, mineralocorticoid receptor antagonist
Heart Failure (HF) is a leading cause of death in the cardiovascular system. Worldwide, it is the primary cause of morbidity and mortality[1,2]. HF has been linked to a number of serious clinical outcomes, including atrial fibrillation, stroke, peripheral embolism, pulmonary embolism, hepatic dysfunction, pulmonary congestion, and kidney failure. Disruptions in potassium homeostasis are prevalent in patients with HF and have been linked to adverse clinical outcomes[3,4]. Potassium balance in HF is influenced by neurohormonal mechanisms and the medications used for treatment[5-7]. Chronic Kidney Disease (CKD) is common in patients with HF and increases the risk of hyperkalemia. CKD is defined as an estimated Glomerular Filtration Rate (eGFR) of <60 ml/min/1.73 m2 persisting for >3 mo, which further elevates the risk of hyperkalemia in HF patients.
Spironolactone is a Mineralocorticoid Receptor Antagonist (MRA) and a potassium-sparing diuretic. It competes with aldosterone for binding to its cytoplasmic receptor, promoting sodium secretion and reducing electrically coupled potassium secretion[8,9]. Gynecomastia, Gastrointestinal (GI) upset, and hyperkalemia are the most frequently reported adverse effects of spironolactone[10]. Hyperkalemia is a potentially fatal condition defined by a serum potassium concentration of >5 mmol/l[11,12]. The Randomized Aldactone Evaluation Study (RALES) trial established a precedent for the use of spironolactone in patients with HF. This trial enrolled patients with severe HF who had an Ejection Fraction (EF) of <35 % and discovered that adding spironolactone to standard therapy reduced morbidity and mortality. According to the American Heart Association's guidelines, spironolactone is recommended in patients with New York Heart Association (NYHA) class II-IV who have a Left Ventricular EF (LVEF) of <35 % and in patients who have had a Myocardial Infarction (MI) and have an LVEF <40 % with symptoms of HF or an LVEF <40 % with Diabetes Mellitus (DM)[13-15]. According to a population-based time series analysis, the publication of the RALES trial was associated with abrupt increases in the rate of spironolactone prescriptions and hyperkalemia-related morbidity and mortality[16]. The risk of hyperkalemia was four times greater with spironolactone and an Angiotensin-Converting Enzyme Inhibitor (ACEI)/Angiotensin Receptor Blocker (ARB) compared to placebo in patients with preserved EF enrolled in the Treatment Of Preserved Cardiac function heart failure with an Aldosterone anTagonist (TOPCAT) trial[17]. Additionally, a cohort study conducted in Brazil evaluated the risk of hyperkalemia in HF patients treated with ACEIs with or without spironolactone. The study found that the spironolactone group had a higher incidence of hyperkalemia[18]. 30 patients developed hyperkalemia in a retrospective study of 125 Congestive HF (CHF) patients. They discovered that kidney function, DM, and medications for HF are all independently associated with hyperkalemia[19]. In a nested case-control study in Germany, HF patients receiving ACE inhibitors or ARBs in combination with spironolactone had a significantly higher risk of hyperkalemia, particularly those over the age of 70 y[20]. Currently, there is limited data on the incidence and risk factors for hyperkalemia in HF patients receiving spironolactone in addition to standard therapy with ACEIs or ARBs. The purpose of this study was to determine the incidence and identify the risk factors for hyperkalemia in HF patients treated with spironolactone.
Materials and Methods
Study design:
From March 1st 2016 to March 31st 2019, this retrospective descriptive chart review was conducted at King Abdulaziz Medical City (KAMC) in Riyadh, Saudi Arabia. Both in-patients and out-patients with HF requiring spironolactone were included, identified through the BESTCare electronic health system. Patients were primarily assessed by a multidisciplinary care team, including cardiologists, internists, and family medicine providers. Eligible patients were aged 18 y or older, with the following exclusion criteria, CKD requiring dialysis, cancer, or a history of hyperkalemia (defined as two consecutive readings >5 mmol/l prior to starting spironolactone).
Data collection:
The data for this study were extracted from the patients' charts and entered into an Excel sheet using a data collection form. The demographic data extracted included the patient's age, gender, weight, EF, and baseline potassium levels, all collected from patient records prior to spironolactone initiation. Additional data gathered included comorbidities, spironolactone dose, incidences of hyperkalemia (if any), time to the first event, other medications (ACEI, ARBs, digoxin, furosemide, Beta (β)- blockers, and potassium supplements), as well as average potassium, creatinine, and Brain Natriuretic Peptide (BNP) levels.
Follow-up:
Patient follow-up in this study was retrospective, utilizing data from the BESTCare electronic HIS at KAMC. Patients were tracked from the initiation of spironolactone therapy until the first recorded incidence of hyperkalemia. Unlike the RALES trial, which implemented specific follow-up intervals, this study did not have predefined check-up schedules. Monitoring frequency and clinical assessments, including potassium and creatinine levels, were conducted based on individualized clinical needs, as documented in the patient records.
Statistical analysis:
For systematic data collection and analysis, an Excel-based tool (Microsoft® Excel; version 2018) was used. Descriptive statistics (i.e., means and frequencies) were used to present patients' demographic characteristics, clinical variables, study outcomes, and other variables. The mean and Standard Deviation (SD) summarized the study's findings, while percentages and proportions were used for categorical variables. The study was approved by the King Abdullah International Medical Research Center's (KAIMRC) Ethical Review Board in Riyadh, Saudi Arabia. Informed consent was waived due to the lack of patient interaction.
Results and Discussion
A total of 429 patient records were reviewed. 80 subjects were excluded from the study based on at least one of the following criteria, any form of cancer, a history of hyperkalemia, hemodialysis, or missing data. As a result, 349 patients met the study's inclusion criteria and were included in the statistical analysis. The baseline characteristics of these patients are summarized in Table 1. The mean age of the population was 64.87 y (SD 14.02), and 57 % were male. Prior to initiating spironolactone, the mean baseline potassium level was 4.34 mmol/l (SD 3.45). The majority of patients (75 %) had an EF level of <40 %. Additionally, 69 % of patients were diagnosed with DM, and 23 % were diagnosed with CKD (Table 1).
| Variable (n=349) | Values |
|---|---|
| Age (mean±SD) | 64.87±14.02 |
| >65 y old (%) | 194 (56) |
| <65 y old (%) | 155 (44) |
| Weight (mean±SD) | 79.72±19.68 |
| Sex | |
| Female (%) | 148 (43) |
| Male (%) | 201 (57) |
| Potassium baseline (mean±SD) | 4.15±0.5 |
| Type of HF | |
| EF ≥40 (%) | 87 (25) |
| EF <40 (%) | 260 (74) |
| EF=10-20 (%) | 22 (8) |
| EF=20-30 (%) | 176 (68) |
| EF=30-40 (%) | 61 (23) |
| Comorbidities | |
| CKD (%) | 83 (23.7) |
| DM (%) | 240 (69) |
Table 1: Study Population Baseline Characteristics
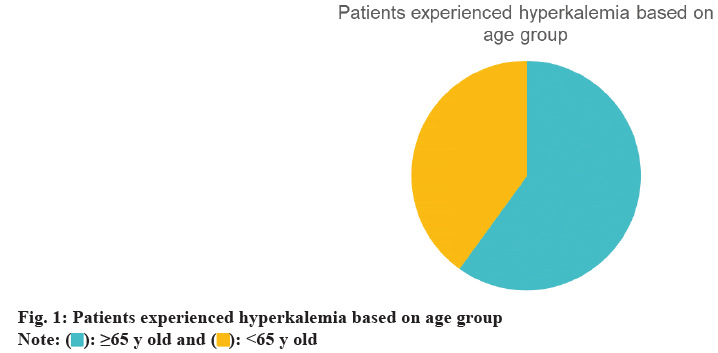
During the follow-up period, a total of 164 incidents (47 %) were recorded. 60 % of these cases occurred in patients aged 65 y or older, and 56 % were male (fig. 1 and fig. 2). We compared various doses of spironolactone (12.5 mg, 25 mg, and 50 mg). Hyperkalemia occurred in 40 % of patients receiving 12.5 mg of spironolactone. Of these patients, 62 % were also taking an ACEI, 28 % were taking an ARB, and 14 % were taking potassium supplements. A total of 263 patients received 25 mg of spironolactone, and 47 % of them developed hyperkalemia. 49 % of those who developed hyperkalemia were taking an ACEI, 31 % were taking an ARB, and 22 % were using potassium supplements. 17 patients received 50 mg of spironolactone, and 53 % of these patients developed hyperkalemia. Among those who developed hyperkalemia, 44 % were taking an ACEI, 22 % were taking an ARB, and 22 % were using potassium supplements (fig. 3).
The combination of Renin-Angiotensin-Aldosterone System (RAAS) inhibitors and spironolactone is recommended for HF patients with NYHA classes II- IV, particularly those with reduced LVEF or a history of MI, as it has been shown to improve morbidity and mortality[13-15]. However, this combination is also associated with an increased risk of hyperkalemia, a significant adverse effect[21-25]. Currently, data on hyperkalemia incidence and risk factors in HF patients receiving spironolactone in addition to RAAS inhibitors are limited. This study aimed to address this gap by assessing the incidence and contributing factors for hyperkalemia in this population.
Our findings revealed that 164 patients (53 %) developed hyperkalemia, supporting previous studies that have linked spironolactone use to a higher incidence of hyperkalemia[17,18]. 60 % of cases occurred in patients aged 65 y or older, indicating an association between older age and an elevated risk of hyperkalemia, consistent with the findings of Juurlink et al.[15,26]. Additionally, the incidence of hyperkalemia increased with higher spironolactone doses, with the highest rate observed in patients receiving 50 mg. Although only 9 patients received this dose, the dose-response pattern is in line with prior literature[21]. In this study, we defined hyperkalemia as a serum potassium level >5.0 mmol/l, which is lower than the usual clinical threshold of >5.5 mmol/l. This threshold was chosen to maintain consistency with criteria used in trials such as RALES, thereby enhancing sensitivity in detecting early hyperkalemia events. Additionally, 20 % of patients received potassium supplements, likely due to concurrent diuretic therapy, which can lead to hypokalemia. Potassium supplementation in these cases helps maintain electrolyte balance, an important aspect of HF management. The high rate of furosemide (Lasix) use (99 %) reflects its role in managing volume overload in HF. However, due to inconsistent documentation, we were unable to fully assess volume status and its relationship with hyperkalemia risk, which represents a limitation of the dataset. Overall, our findings suggest that hyperkalemia risk increases when spironolactone is used in conjunction with RAAS inhibitors, particularly in older patients and at higher doses. This study has several limitations. As a retrospective analysis, it depends on the accuracy of medical records, and our small sample size may limit the generalizability of findings to other populations. Additionally, the single-center design further limits the scope of our conclusions. Future research could expand on our findings by exploring larger, multicenter cohorts, investigating additional risk factors, and examining the relationship between spironolactone dosage and the incidence of hyperkalemia. In summary, our study enhances the understanding of hyperkalemia risk in HF patients on spironolactone, supporting previous findings and identifying areas for further investigation. Our study found that half of the HF patients treated with spironolactone developed hyperkalemia. Patients who experienced hyperkalemia were predominantly receiving ACEIs or ARBs. The highest incidence of hyperkalemia was associated with a 50 mg dose of spironolactone. Further research with a larger sample size is needed to confirm and expand upon our findings.
Authors’ contributions:
Majed Nahari drafted the paper, and all named authors critically reviewed it and gave their final approval for publication.
Data availability statement:
The data used to support the finding of this study are restricted by the KAIMRC in order to protect patient privacy. Data are available from KAIMRC for researchers who meet the criteria for access to confidential data.
Ethical approval:
The study was approved by the Institutional Review Board (IRB) of KAIMRC, National Guard Health Affairs, Riyadh, Saudi Arabia, in February 2019. The informed consent was waived due to minimal risk associated with design of retrospective studies.
Funding:
This research project was funded by the Deanship of Scientific Research, Princess Nourah bint Abdulrahman University, through the Program of Research Project Funding After Publication, Grant No: 44-PRFA-P-129.
Conflict of interests:
The authors declared no conflict of interests.
References
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;74(10):1037-147.
[Crossref] [Google Scholar] [PubMed]
- Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3(1):7.
[Crossref] [Google Scholar] [PubMed]
- Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016;13(6):368-78.
[Crossref] [Google Scholar] [PubMed]
- Watson RD, Gibbs CR, Lip GY. Clinical features and complications. BMJ 2000;320(7229):236-9.
[Crossref] [Google Scholar] [PubMed]
- Tromp J, van der Meer P. Hyperkalaemia: aetiology, epidemiology, and clinical significance. Eur Heart J Suppl 2019;21:A6-11.
[Crossref] [Google Scholar] [PubMed]
- Sarwar CM, Papadimitriou L, Pitt B, Pina I, Zannad F, Anker SD, et al. Hyperkalemia in heart failure. J Am Coll Cardiol 2016;68(14):1575-89.
[Crossref] [Google Scholar] [PubMed]
- López-Vilella R, Morillas-Climent H, Plaza-Lopez D, Cebrian-Pinar M, Sanchez-Lazaro I, Almenar-Bonet L. Hyperkalemia in heart failure patients: Current challenges and future prospects. Res Rep Clin Cardiol 2016:1-8.
- Deinum J, Riksen NP, Lenders JW. Pharmacological treatment of aldosterone excess. Pharmacol Ther 2015;154:120-33.
[Crossref] [Google Scholar] [PubMed]
- Sica DA. Mineralocorticoid receptor antagonists for treatment of hypertension and heart failure. Methodist Debakey Cardiovasc J 2015;11(4):235.
[Crossref] [Google Scholar] [PubMed]
- Lainscak M, Pelliccia F, Rosano G, Vitale C, Schiariti M, Greco C, et al. Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone. Int J Cardiol 2015;200:25-9.
[Crossref] [Google Scholar] [PubMed]
- Hollander-Rodriguez JC, Calvert Jr JF. Hyperkalemia. Am Fam Physician 2006;73(2):283-90.
[Google Scholar] [PubMed]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341(10):709-17.
[Crossref] [Google Scholar] [PubMed]
- Vizzardi E, Sciatti E, Bonadei I, Aloia A, Tartiere KL, Tartiere JM, et al. Effects of spironolactone on ventricular-arterial coupling in patients with chronic systolic heart failure and mild symptoms. Clin Res Cardiol 2015;104:1078-87.
[Crossref] [Google Scholar] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey Jr DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure-Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128(16):1810-52.
[Crossref] [Google Scholar] [PubMed]
- Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, et al. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med 2004;351(6):543-51.
[Crossref] [Google Scholar] [PubMed]
- Desai AS, Liu J, Pfeffer MA, Claggett B, Fleg J, Lewis EF, et al. Incident hyperkalemia, hypokalemia, and clinical outcomes during spironolactone treatment of heart failure with preserved ejection fraction: Analysis of the TOPCAT trial. J Card Fail 2018;24(5):313-20.
[Crossref] [Google Scholar] [PubMed]
- Cruz CS, Cruz AA, Marcilio CA. Hyperkalaemia in congestive heart failure patients using ACE inhibitors and spironolactone. Nephrol Dial Transplant 2003;18(9):1814-9.
[Crossref] [Google Scholar] [PubMed]
- Vereijken TL, Bellersen L, Groenewoud JM, Knubben L, Baltussen L, Kramers C. Risk calculation for hyperkalaemia in heart failure patients. Neth J Med 2007;65(6):208-11.
[Google Scholar] [PubMed]
- Abbas S, Ihle P, Harder S, Schubert I. Risk of hyperkalemia and combined use of spironolactone and long-term ACE inhibitor/angiotensin receptor blocker therapy in heart failure using real-life data: A population- and insurance-based cohort. Pharmacoepidemiol Drug Saf 2015;24(4):406-13.
[Crossref] [Google Scholar] [PubMed]
- Thomsen RW, Nicolaisen SK, Hasvold P, Garcia-Sanchez R, Pedersen L, Adelborg K, et al. Elevated potassium levels in patients with congestive heart failure: Occurrence, risk factors, and clinical outcomes: A Danish population-based cohort study. J Am Heart Assoc 2018;7(11):e008912.
[Crossref] [Google Scholar] [PubMed]
- Vardeny O, Claggett B, Anand I, Rossignol P, Desai AS, Zannad F, et al. Incidence, predictors, and outcomes related to hypo and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail 2014;7(4):573-9.
[Crossref] [Google Scholar] [PubMed]
- Sica DA. Mineralocorticoid receptor antagonists for treatment of hypertension and heart failure. Methodist Debakey Cardiovasc J 2015;11(4):235.
[Crossref] [Google Scholar] [PubMed]
- Alotaibi AS, Alabdan N, Alotaibi AM, Aljaafary H, Alqahtani M. The utilization of spironolactone in heart failure patients at a tertiary hospital in Saudi Arabia. Cureus 2020;12(8).
[Crossref] [Google Scholar] [PubMed]
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004;351(6):585-92.
[Crossref] [Google Scholar] [PubMed]
- Desai AS, Swedberg K, McMurray JJ, Granger CB, Yusuf S, Young JB, et al. Incidence and predictors of hyperkalemia in patients with heart failure: An analysis of the CHARM program. J Am Coll Cardiol 2007;50(20):1959-66.
[Crossref] [Google Scholar] [PubMed]
- Bielecka-Dabrowa A, Rysz J, Mikhailidis DP, Banach M. What is the risk of hyperkalaemia in heart failure? Expert Opin Pharmacother 2011;12(15):2329-38.
[Crossref] [Google Scholar] [PubMed]
 <65 y old
<65 y old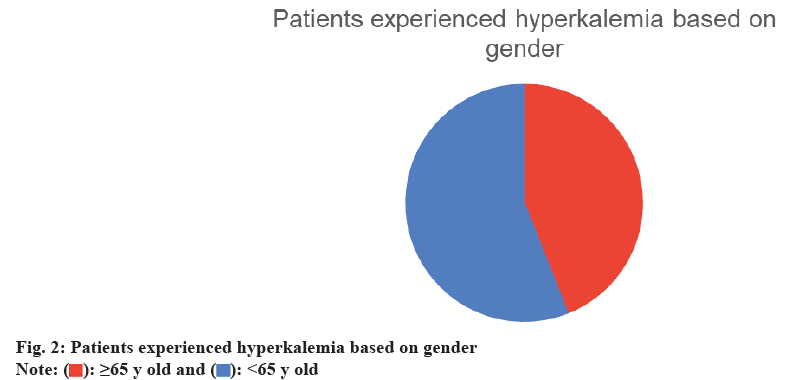
 <65 y old
<65 y old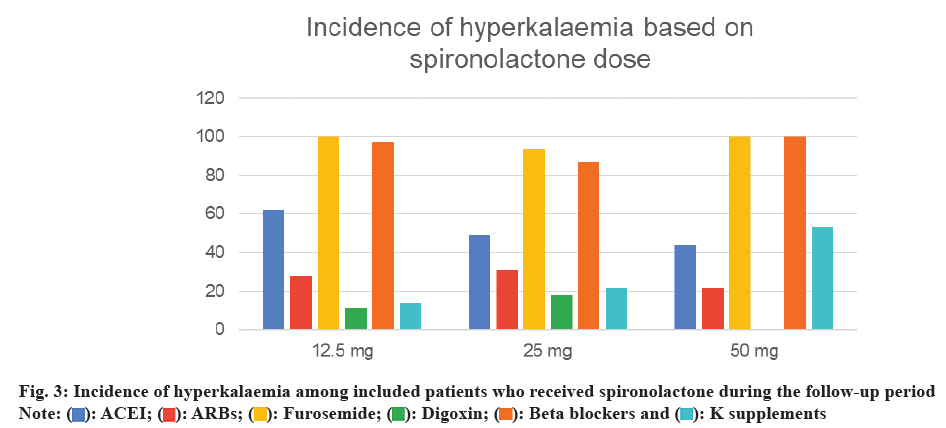
 Digoxin;
Digoxin;  K supplements
K supplements



