- *Corresponding Author:
- D. Chamundeeswari
Sri Ramachandra College of Pharmacy, Sri Ramachandra Medical College & Research Institute (Deemed University), Porur, Chennai-600 116, India.
E-mail: dvchamu@yahoo.co.in
| Date of Submission | 26 April 2005 |
| Date of Decision | 26 December 2005 |
| Date of Acceptance | 18 October 2006 |
| Indian J Pharm Sci, 2006, 68 (5): 668-670 |
Abstract
Melothria maderaspatana (Linn) Cogn, a plant drug of Siddha medicine, is an annual monoecious tendril climber, belonging to the family Cucurbitaceae, mostly prevalent in South India. It is commonly called Musumusukkai in Tamil. Preliminary phytochemical screening of the plant revealed the presence of phytochemical constituents such as coumarins, flavonoids. Hence an attempt has been made to screen the effect of Melothria maderaspatana in platelet aggregation. Successive extracts of aerial parts of Melothria maderaspatana were used in increasing polarity of solvents (i.e., hexane, chloroform, ethyl acetate and methanol). The antiplatelet activity of different fractions of Melothria maderaspatana was studied using platelet-rich plasma in presence of ADP. Each extract was tested in concentrations of 100 µg/ml, 200 µg/ml, 400 µg/ml and 500 µg/ml for the study. The ethyl acetate extract showed a dose-dependent antiplatelet activity. Hexane and methanol extracts showed antiplatelet activity only at 200 µg/ml and 400 µg/ml concentrations. Chloroform extract showed negligible antiplatelet activity. However, the inhibition of platelet aggregation was only 50% when compared to the standard Aspirin.
Melothria maderaspatana belongs to the family Cucurbitaceae. The plant is a tendril climber/prostrate herb [1]. The plant was reported to have activities such as hepatoprotective [2], antirheumatic [3], antiflatulent, antiinflammatory, antidiabetic, expectorant, diuretic, stomachic, and is used for toothache and recommended in vertigo and biliousness [4]. An attempt has been made to study the efficacy of aerial parts of Melothria maderaspatana in platelet aggregation.
Blood platelets are involved in haemostasis. The normal haemostatic system limits blood loss by precisely regulated interactions between components of vessel wall, circulating blood platelets and plasma proteins. Platelets can adhere to the walls of the blood vessels, release bioreactive compounds and aggregate to each other. These properties increase to a well established level in conditions of arterial thrombosis and atherogenesis. Several agonists such as ADP, thrombin, collagen and serotonin induce the release of arachidonic acid, after phospholipase activation through calcium mobilization [5]. Several drugs have been developed to block the different steps in platelet activation pathways. Inhibition of platelet function by Aspirin has been very well established [6].
One of the most potent mechanisms by which flavonoids appear to inhibit platelet aggregation is by mediating increase in platelets’ cyclic AMP levels by either stimulation of adenylate cyclase or inhibition of cAMP phosphodiesterase activity5. Therefore the experiments in this study were designed to evaluate the antiplatelet activity of various fractions of Melothria maderaspatana and try to elucidate the inhibitory mechanism of coumarins or flavonoids on platelet aggregation.
The aerial parts of Melothria maderaspatana were collected from the herbal garden of Sri Ramachandra Medical College and Research Institute, Chennai, and were cleaned, air dried and powdered. The powdered aerial parts were subjected to exhaustive maceration with hexane, chloroform, ethyl acetate and methanol [7]. The filtrates were collected, distilled and concentrated to get pure and dry extracts. The various fractions of the successive extracts were weighed and preliminary phytochemical screening [8,9] was performed. The result is shown in Table 1.
| Phyto constituents | Extracts | ||||
|---|---|---|---|---|---|
| Hexane | Chloroform | Ethyl acetate | Methanol | ||
| Steroid | + | + | - | - | |
| Triterpenes | + | - | + | - | |
| Flavonoids | + | - | + | + | |
| Flavonones | - | - | - | - | |
| Anthocyanins | - | - | - | - | |
| Coumarins | - | - | + | + | |
| Proteins | - | + | + | + | |
| Reducing Sugars | + | + | + | + | |
| Alkaloids | - | + | + | + | |
| Tannins | - | - | + | + | |
| Phenolics | - | - | + | + | |
| Glycosides | + | - | + | + | |
| Quinones | - | - | - | - | |
| Saponins | - | - | - | - | |
| Anthraquinones | - | - | - | - | |
Table 1: Preliminary Phytochemical screening
Various fractions of Melothria maderaspatana in different concentrations in DMSO were used. ADP, platelet-rich plasma and Tyrode buffer were used [10]. Tyrode buffer was prepared using sodium chloride 149 mM, potassium chloride 2.6 mM, sodium bicarbonate 9.5 mM, glucose 5.5 mM, sodium dihydrogen phosphate 0.5 mM, magnesium chloride 0.6 mM and gelatin 0.25%.
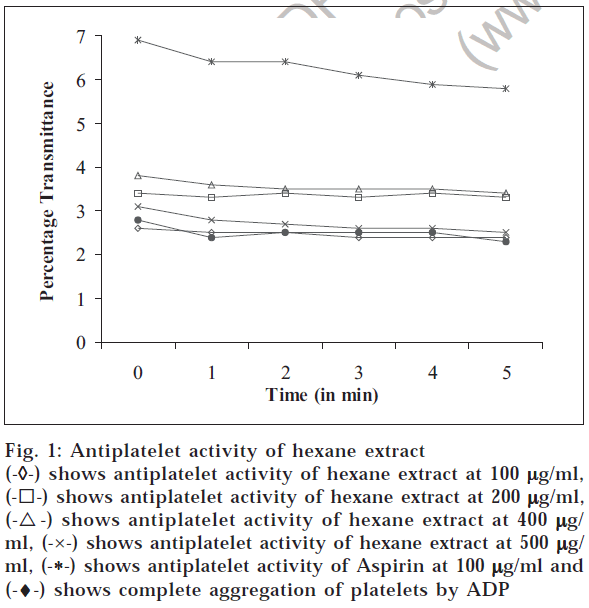
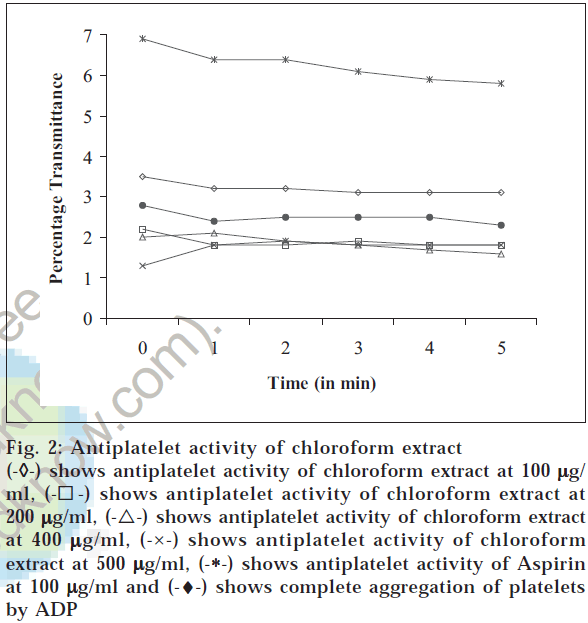
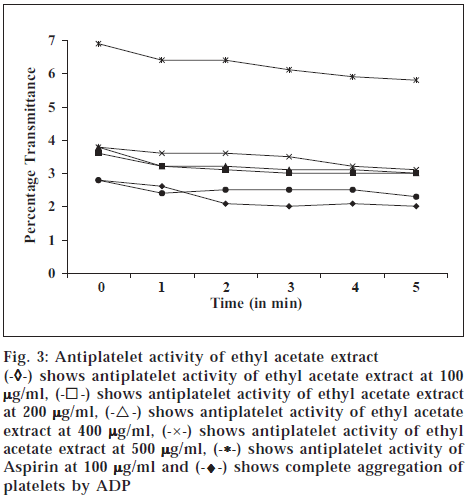
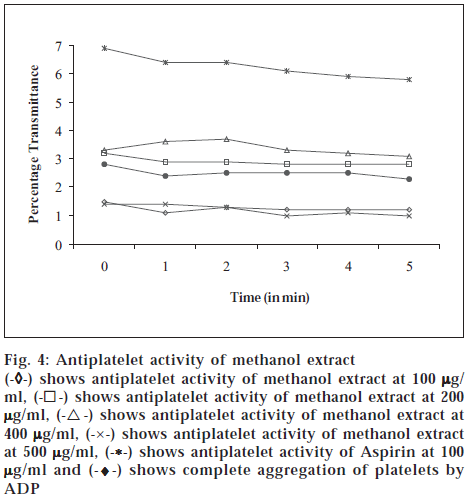
The platelet-rich plasma 0.13 × 10-7 for each assay was resuspended in Tyrode buffer (pH adjusted to 7.4 with 0.25 M HCl). Aggregation of the platelets was induced by ADP at a final concentration of 5 μM. Platelet aggregation was recorded by increasing transmittance value of spectrophotometric measurements. To determine the in vitro antiplatelet aggregation property, 100 μl of the various fractions of the plant, in different concentrations (100 μg/ ml, 200 μg/ml, 400 μg/ml, 500 μg/ml), respectively was added to the platelet suspension at different concentrations for 1 min exposure at 37° before treatment with platelet aggregating agents. Aspirin at 100 μg/ml was used as standard. The results are depicted in figs. 1, 2, 3 and 4.
Fig. 1: Antiplatelet activity of hexane extract
(-
◊-) shows antiplatelet activity of hexane extract at 100
µg/ml, (-⌈-) shows antiplatelet activity of hexane extract at 200
µg/ml, (-Δ-) shows antiplatelet activity of hexane extract at 400
µg/ ml, (-×-) shows antiplatelet activity of hexane extract at 500
µg/ ml, (-*-) shows antiplatelet activity of Aspirin at 100
µg/ml and (-♦-) shows complete aggregation of platelets by ADP
Fig. 2: Antiplatelet activity of chloroform extract
(-
◊-) shows antiplatelet activity of hexane extract at 100
µg/ml, (-⌈-) shows antiplatelet activity of hexane extract at 200
µg/ml, (-Δ-) shows antiplatelet activity of hexane extract at 400
µg/ ml, (-×-) shows antiplatelet activity of hexane extract at 500
µg/ ml, (-*-) shows antiplatelet activity of Aspirin at 100
µg/ml and (-♦-) shows complete aggregation of platelets by ADP
Fig. 3: Antiplatelet activity of ethyl acetate extract
(-
◊-) shows antiplatelet activity of hexane extract at 100
µg/ml, (-⌈-) shows antiplatelet activity of hexane extract at 200
µg/ml, (-Δ-) shows antiplatelet activity of hexane extract at 400
µg/ ml, (-×-) shows antiplatelet activity of hexane extract at 500
µg/ ml, (-*-) shows antiplatelet activity of Aspirin at 100
µg/ml and (-♦-) shows complete aggregation of platelets by ADP
Fig. 4: Antiplatelet activity of methanol extract
(-
◊-) shows antiplatelet activity of hexane extract at 100
µg/ml, (-⌈-) shows antiplatelet activity of hexane extract at 200
µg/ml, (-Δ-) shows antiplatelet activity of hexane extract at 400
µg/ ml, (-×-) shows antiplatelet activity of hexane extract at 500
µg/ ml, (-*-) shows antiplatelet activity of Aspirin at 100
µg/ml and (-♦-) shows complete aggregation of platelets by ADP
The hexane and methanol fractions showed maximum antiplatelet activity at 400 μg/ml concentration, whereas ethyl acetate fraction showed a dose-dependent antiplatelet activity. However, the prevention of platelet aggregation was lesser when compared to standard aspirin at 100 μg/ml. Chloroform fraction did not show protection against platelet aggregation. The coumarins and flavonoids present in ethyl acetate extract and flavonoids in hexane extract might have prevented the adhesion and aggregation of platelets, besides release of cytoplasmic calcium that stimulates the release of ADP and 5HT. The above result proves that Melothria maderaspatana prevents aggregation of platelets.
Acknowledgements
The authors thank Dr. Panicker, in charge, Blood Bank, SRMC and RI (DU), for providing platelet-rich plasma; and the management of SRMC and RI (DU) for encouragement and support.
References
- The Wealth of India, A dictionary of Raw materials and industrialproducts, Vol VI, Council of Scientific and Industrial Research, NewDelhi, 2003, 164.
- Thabrew, M.I., Gove, C. D., Hughes, R. D., Mefarlane, I. G., andWilliams, R., Phytother. Res., 1995, 9, 513.
- Ramakrishnamacharya, C. H., Krishnaswamy, M. R., Rao, R. B. andViswanathan, S., Clin. Rheumatol., 1996, 15, 214.
- Chopra, R.N., In; Glossary of Indian Medicinal Plants, NationalInstitute of Science Com unication and information Resources(CSIR), New Delhi, 2002, 165.
- Guyton, A.C., In; Textbook of Medical Physiology, 8th Edn., Saunders,An imprint of Elsevier, The Curtis center Independence Square west,fromPhiladelphia, Pennsylvania, 1991, 390.
- Patrono, C., New Engl. J. Med., 1994, 330, 1287.
- Harborne, J.B., In; Phytochemical methods, Chapmann& Hall,London, 1973, 1.
- Kokate, C.K., In; Textbook of Pharmacognosy, NiraliPrakashan, Pune,1985, 112.
- Evans, W.C., In; Trease and Evans’ Pharmacognosy 13th Edn.,BailliereTindall&Cansell Ltd., London, 1994, 13.
- Subramanian, A., and Sathyanarayana, M.N., J. Food Safety, 1989, 9, 201.