- *Corresponding Author:
- N. Ramaswamy
Plant Biotechnology Research Group, Department of Biotechnology, Kakatiya University, Warangal-506 009, India
E-mail: swamynr.dr@gmail.com
| Date of Submission | 28 April 2014 |
| Date of Revision | 31 October 2014 |
| Date of Acceptance | 18 March 2015 |
| Indian J Pharm Sci 2015; 77(2):170-177 |
Abstract
Cajanus cajan (L.) Millsp is one of the second most dietary legume crops. The leaf extracts may be used as a potential source of natural antioxidant. The ash values, extractive values, total phenolic and flavonoid content, in vitro antioxidant activity of various leaf extracts as well as anatomical investigation of Cajanus cajan were carried out. Physicochemical parameters such as total, acid-insoluble and water-soluble ash values and moisture content of the leaf powder of C. cajan were found to be 9.50%, 1.40 g/100 g, 4.15 g/100 g drug and 6.72%, respectively. Percent yield of acetone, aqueous, ethanol, ethyl acetate and chloroform leaf extracts were 9.0, 10.6, 13.75, 8.7 and 5.8 g/100 g, respectively. Significant amount of phenolic and flavonoid content were observed. The results of the antioxidant activity were found to be concentration-dependent. The IC 50 values for DPPH assay determined for aqueous and ethanol extracts were 0.69 and 0.79 mg/ml, respectively. Reducing power is increased with increasing amount of concentration in both aqueous and ethanol leaf extracts. The highest hydroxyl radical scavenging activity reached up to 83.67% in aqueous and 78.75% in ethanol extracts and in phosphomolybdenum assay the aqueous extract showed strong antioxidant capacity up to 55.97 nM gallic acid equivalents/g. It was found that the aqueous extract possessed highest antioxidant activity in all the assays tested.The antioxidant characteristics of leaf extracts are possibly because of the presence of polyphenols. Microscopic study showed the presence of collenchyma, fibres, xylem, phloem, epidermis, trichomes, palisade tissue, basal sheath, pith and cortex in leaf, petiole and pulvinus.
Keywords
Cajanus cajan, total phenolics, flavonoid, antioxidant, free radicals, hydroxyl radical scavenging activity
Cajanus cajan (L.) Millsp is a perennial member of the family Leguminosae, commonly known as Pigeon pea and red gram (in English); kardis, gandule bean, tropical green pea, kadios, Congo pea, gungo pea, gunga pea, fio‑fio, mgbụmgbụ, no‑eye pea, toor dal, arhar dal, togari bele (Kannada), thuvaram paruppu (Tamil), thuvara parippu (Malayalam) and kandi pappu (Telugu). It is one of the second most important dietary legume crops grown in the tropical regions. When compared with the other grain legumes India contributes about 90% of the world production, ranks sixth position in production and area [1]. Pigeon pea is a multipurpose plant as it is eaten as dhal which is rich in proteins. Its leaves are used for rearing silk worms; pods are used as vegetable, green leaves and husk are used as fodder and green manure [2]. The leaves of pigeon pea have been widely used to kill worms, relieve pain and arrest blood, as a Chinese medicine [3]. Chemical constituent investigations have indicated that pigeon pea leaves are rich in flavonoids and stilbenes, which are considered responsible for beneficiaries of pigeon pea leaves on human health [4,5]. Boiled leaves are given orally to nullify the effect of intoxication and as a laxative in eastern Rajasthan. The leaf paste is applied for inflammations and oral ulcers [6]. Leaf, seeds and young stems of pigeon pea are used to cure gingivitis, stomatitis and as a tooth brush in some parts of India and Tamil Nadu [7]. Flavonoids were isolated from leaf extracts (isorhamnetin, luteolin, apigenin, quercetin) [4,8] and roots of pigeon pea. Genistein and genistin isoflavonoids isolated from the roots of C. cajan were found to possess antioxidant activity [8‑10]. Antioxidants are known to possess antiinflammatory, anticardiovascular disease, anticancer and antineurogenerative properties. These are important in biological and industrial processes. Antioxidants are used widely as food additives. To improve flavours various types of spices are added which are well known for their antioxidant capacities since ancient times [11]. Many of the disorders are linked to oxidative stress due to free radicals [12]. Antioxidants possess the ability to protect body from damage caused by free radical induced oxidative stress. Many free radical scavenging antioxidants are present within the body, of which many are derived from dietary sources like teas, vegetables and fruits [13]. It is prerequisite to know about pigeon pea plant in view of its anatomical, genetical and morphological for a successful improvement of program. Biological phenomena of pigeon pea are known to some extent but the information regarding anatomical characters is very scanty [14]. Some works have been carried out on the root, stem, leaf, petiole and pulvinus anatomy of pigeon pea [15]. There is limited information regarding the biological activities of C. cajan plant. Hence, In view of importance of C. cajan as ethanomedicinal plant, the present work was aimed to assess the possible antioxidant activities of acetone, aqueous, ethanol, ethyl acetate and chloroform leaf extracts of pigeon pea through various in vitro models and physicochemical studies as well as microscopic studies of leaf, petiole and pulvinus were carried out. Pharmacognostic studies help in identification and standardization of crude drug which is helpful to determine the contaminants present in the plant mixture.
Materials and Methods
Plant material
The leaves of C. cajan variety ICP‑26 were collected from the field of Department of Biotechnology, Kakatiya University, warangal. The leaves were washed, cleaned and air‑dried under shade for about 2‑3 months. Fresh leaves of C. cajan were used for microscopic studies.
Sample extraction
The shade dried leaves were ground to a fine powder using a mechanical blender. Fifteen grams of the sample was extracted by maceration with 150 ml of acetone, aqueous, ethanol, ethyl acetate and chloroform, separately. The extracts were then evaporated to dryness at room temperature and were stored for further use.
Determination of physicochemical studies
The dried and powdered leaf material was subjected to determination of physicochemical parameters such as, acid‑insoluble, water‑soluble, total ash content and moisture content [16]. Extractive values of various leaf extracts viz., acetone, aqueous, ethanol, ethyl acetate and chloroform were determined [17].
Estimation of total phenolic content
Total phenolic content of various leaf extracts of C. cajan were determined by using Folin‑Ciocalteu reagent according to the method of Singleton and Rossi [18]. One millilitre of Folin‑Ciocalteu reagent was mixed with 1 ml of the leaf extract. Then 1 ml of saturated sodium carbonate (35%) was added to the mixture after 3 min and the mixture was made up to 10 ml by addition of sterilized distilled water and incubated at room temperature for 90 min in the dark. After incubation, the absorbance was measured at 725 nm against blank. Gallic acid was used as standard and results were showed as mg of gallic acid equivalents (GAE) per gram of dry leaf extract.
Estimation of total flavonoid content
The total flavonoid content of various leaf extracts of C. cajan was determined using the method [19]. A small quantity of various leaf extracts (0.25 ml) were diluted with 1.25 ml of sterile distilled water and 75 μl of 5% sodium nitrite solution were added and incubated for 6 min. Then 150 μl of 10% AlCl3.H2O was added and mixed well. Then immediately the absorbance was measured against blank at 510 nm. The flavonoid content was showed as mg of rutin equivalents (RE) per gram of dry extract.
DPPH free radical scavenging activity
DPPH free radical scavenging activity of dried crude extracts of aqueous and ethanol leaf extracts of C. cajan were examined [20]. A small quantity of DPPH (4.3 mg) was dissolved in 3.3 ml of methanol and it was protected from light by keeping the test tubes in dark. For control 3 ml of methanol was taken in a test tube and 150 μl of DPPH solution was added to it and absorbance was taken immediately at 517 nm. Different concentrations of leaf extracts (0.2, 0.4, 0.6, 0.8, 1 mg/ml) and ascorbic acid as standard were taken. To these extracts 150 μl of methanol was added. Then each sample was diluted further with methanol up to 3 ml and 150 μl of DPPH was added to each tube. Then the tubes were kept in dark for 15 min and absorbance was taken at 517 nm using blank as methanol on UV/Vis spectrometer. The IC50 values for aqueous and ethanol extracts were estimated. The DPPH activity was calculated using the formula, % inhibition=(absorbance of control‑absorbance of test sample)/absorbance of control)×100.
Determination of reducing power
The reducing power of aqueous and ethanol leaf extracts of C. cajan were determined according to the method of Oyaizu [21]. Two and half millilitres of different concentrations of C. cajan leaf extracts (0.4, 0.8, 1.2, 1.6, 2 mg/m l) were taken and 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide were taken and mixed well. Then they were incubated at 500 for 20 min and 2.5 ml of 10% trichloroacetic acid at 5000 g for 10 min. Later 2.5 ml of supernatant was collected and mixed with 2.5 ml deionised water, 0.5 ml of 0.1% ferric chloride and incubated for 10 min. After incubation, absorbance at 700 nm was measured against blank. Increased absorbance showed enhanced reducing power.
Hydroxyl radical scavenging activity
Hydroxyl radical scavenging activity of aqueous and ethanol leaf extracts of C. cajan were determined using the method of Smirnoff and Cumbes [22]. Three millilitres of reaction mixture contained about 1 ml of 1.5 mM FeSO4, 0.3 ml of 20 mM sodium salicylate, 0.7 ml of 6 mM hydrogen peroxide and different concentrations of leaf extract (250, 500, 750 and 1000 μg/ml). Then the reaction mixtures were kept in water bath at 37° for 1 h. After incubation, the absorbance of the solution was measured at 562 nm. The scavenging activity of hydroxyl radical was calculated using the following: [1‑(A1‑A2)/A0]×100, where A1 is absorbance in the presence of extract, A2 is absorbance without sodium salicylate and A0 is absorbance of the control.
Phosphomolybdenum assay
Phosphomolybdenum activities of the acetone, aqueous, ethanol, ethyl acetate and chloroform leaf extracts of C. cajan were determined by the method [23]. One millilitre of reagent solution (0.6 M H2SO4, 28 mM Na3PO4 and 4 mM (NH4)2MoO4) and 0.1 ml of sample solution. The samples were incubated at 95° for 90 min by capping them with silver foil. After incubation the tubes were cooled to room temperature and absorbance was measured at 695 nm against blank. Ascorbic acid was used as standard. Total antioxidant activity was expressed nM GAE/gram of dry extract.
Microscopic studies
The fresh leaves of C.cajan were excised from the plants and fixed for at least 24 h in ethanol: chloroform: acetic acid (ECA, 60:30:10) v/v. After fixation, the leaf material was dehydrated by alcohol solution and sections of leaf, petiole and pulvinus were done by free hand transverse section method at a thickness of 9‑11 μ [24,25]. The sections done were stained with saffranin and were arranged on to a glass slide [26]. The anatomical sections were observed under compound microscope at a projection of 10X and 40X.
Statistical analysis
The experiments were performed in triplicate where ever neceessary and the data were statistically analysed as mean±SE. All graphs were plotted using MS Excel® software 2010. The values of correlation coefficient, intercept, slope and standard errors were obtained by non linear and linear regression analysis applying this program.
Results and Discussion
C. cajan leaf extracts showed varied physicochemical parameters such as total ash content of 9.50%, while water soluble ash is greater than that of acid insoluble ash at 4.15 and 1.40 g/100 g, moisture content 6.72%, respectively (Table 1). These results were supportive with the results of Portulaca quadrifida [27]. Ash value is useful in finding out genuineness and purity of drug and the values are significant in quantitative standards [28]. The results of extraction yield, colour, and consistency were presented (Table 2). The extraction yield of various solvents ranged from 5.8 to 13.75 g/100 g and could be ranked from high to low in the order: ethanol>aqueous>acetone>ethyl acetate>chloroform extracts. Crude extract of C. cajan exhibited a wide range of colour. Acetone, ethyl acetate, chloroform and ethanol extracts were dark green, yellowish green, greenish black and green in colour, whereas aqueous is dark brown in colour. Consistency of these extracts varied from sticky (ethyl acetate and acetone), flakes (chloroform), resin (ethanol) and amorphous (aqueous), respectively. Evaluation of physicochemical parameters like ash values is useful in finding out the non physiological and physiological ash as well as presence of impurities [29]. Pharmacognostic studies basically deal with authentication of commonly used traditional medicinal plants through morphological and physicochemical properties. This helps in identification of adulterants and also to identify controversial and closely related plant species [30].
| Parameters | Ash values |
|---|---|
| Total Ash | 9.50 ± 0.29 |
| Acid-insoluble ash | 1.40 ± 0.09 |
| Water soluble ash | 4.15 ± 0.15 |
| Moisture content | 6.72 ± 0.21 |
Table 1: Ash Values of Leaf Powder of C.Cajan
| Parameter | Colour | Consistency | Yield |
|---|---|---|---|
| Acetone | Dark green | Sticky | 9.0 ± 0.20 |
| Aqueous | Dark brown | Amorphous | 10.6 ± 0.26 |
| Ethanol | Green | Resin | 13.75 ± 0.32 |
| Ethyl acetate | Yellowish green | Sticky | 8.7 ± 0.14 |
| Chloroform | Blackish green | Flakes | 5.8 ± 0.06 |
Table 2: Extractive values of leaf extracts of C. Cajan
Total phenolic content of C. cajan leaf extract was evaluated by using the Folin‑Ciocalteu colorimetric method. The phenolic contents in the analysed leaf extracts ranged from 37.54 to 57.20 mg GAE/g (Table 3). The highest concentration of phenols was assessed in aqueous, acetone and ethanol leaf extracts. Ethyl acetate and chloroform showed considerably smaller concentration of phenols. Similar results have been reported in Marrubium peregrinum extracts [31]. The total phenol contents in leaf extracts depend on the type of extract i.e. the polarity of solvent utilized in extraction. Many investigations on qualitative composition of plant extracts revealed the presence of higher concentrations of phenols in the plant extracts using polar solvents [32]. Aqueous and ethanol leaf extracts of C. cajan showing highest antioxidant activity have the highest phenolic concentration. Phenols are important constituents of plant due to their scavenging ability on free radicals because of their hydroxyl groups. Thus, the phenol content of plants may lead directly to their antioxidant activity [33].
| Name of Extract | Total phenolic content (mg GAE/g dry extract) | Total flavonoid content (mg RE/g dry extract) |
|---|---|---|
| Acetone | 50.95 ± 0.78 | 36.17 ± 1.12 |
| Aqueous | 57.20 ± 1.37 | 49.43 ± 0.98 |
| Ethanol | 55.03 ± 0.42 | 48.32 ± 0.27 |
| Ethyl acetate | 42.86 ± 1.75 | 32.11 ± 0.53 |
| Chloroform | 37.54 ± 2.30 | 29.51 ± 1.72 |
Table 3: Total phenolic and total flavonoid Content of C. Cajan leaf extracts
The concentration of flavonoids in various leaf extracts of C. cajan ranged from 29.51 to 49.43 mg RE/g (Table 3). The highest concentration of flavonoid was found in aqueous and ethanol leaf extracts while in ethyl acetate and chloroform leaf extracts lowest flavonoid concentration was assessed. The concentration of flavonoid in aqueous leaf extract was 49.43 mg RE/g which is very similar to the value of ethanol leaf extract. These results are supportive with the Marrubium peregrinum extracts [31]. The concentration of the flavonoids depends on the polarity of the solvents used in the preparation of extract [34].
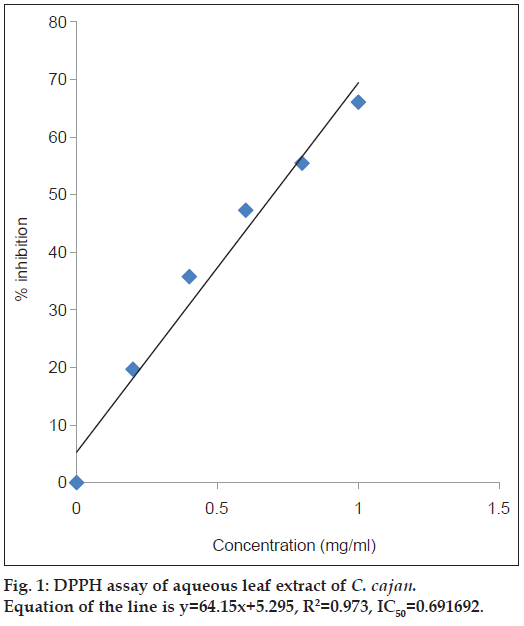
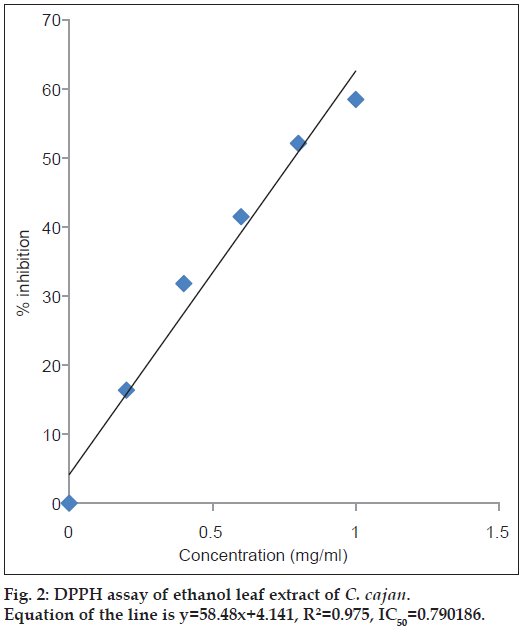
To evaluate the free radical scavenging activity, DPPH assay is widely used. At room temperature, DPPH is stable free radical which produces violet solution in methanol. At 517 nm DPPH shows strong absorption band in visible spectrum (deep violet colour) [35]. In the DPPH free radical scavenging activity, aqueous and ethanol leaf extracts were evaluated for their free radical scavenging activity with ascorbic acid as standard. At different concentrations tested 0.2, 0.4, 0.6, 0.8, 1 mg/ml, the scavenging effect of aqueous and ethanol leaf extracts of C. cajan were found to be 19.69, 35.75, 47.27, 55.45 and 66.06% in aqueous and 16.36, 31.81, 41.51, 52.12 and 58.48% in ethanol extracts, respectively. The IC50 value was calculated for each extract and shown in figs. 1 and 2. The IC50 value for aqueous extract is 0.69 mg/ml and ethanol extract is 0.79 mg/ml, respectively. With the increase in the concentration of extract the increase in the scavenging effect was observed. According to our observations, we opine that the strong activity of the extracts is due to the available hydroxyl group present in the substance [35]. From the results of DPPH, the aqueous extract showed highest antioxidant activity compared to the ethanolic extract. In the DPPH system, our results are in contradiction with Wu et al [8]. who reported that the antioxidant activity is superior to that of aqueous leaf extract in C. cajan. Similarly, 70% methanol extract of C. cajan leaves reported that they possess antioxidant and free radical scavenging properties [36]. Whereas highest antioxidant activity examined by DPPH assay was reported in ethanol seed extracts of C. cajan by Maneechai et al. [37].
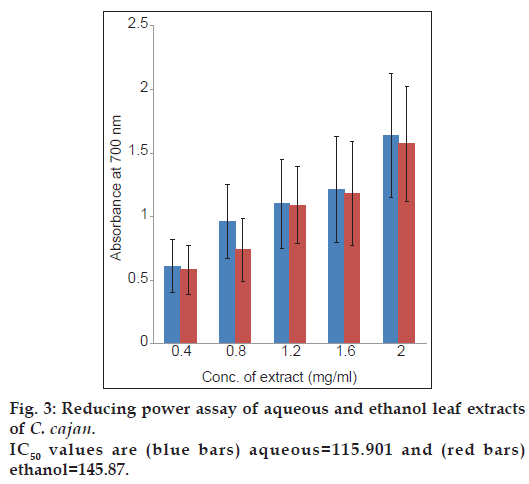
The reducing capabilities of aqueous and ethanol extracts of C. cajan are shown in fig. 3. Absorbance at 700 nm showed greater reducing power. At different concentrations 0.4, 0.8, 1.2, 1.6 and 2.0 mg/ml, the reducing power of aqueous and ethanol leaf extracts of C. cajan were found to be 0.61, 0.96, 1.30, 1.21 and 1.64 for aqueous and 0.58, 0.73, 1.29, 1.18 and 1.57 for ethanol leaf extracts, respectively. The IC50 values of aqueous and ethanol extracts were found to be 115.9 mg/ml and 145.8 mg/ml, respectively. Reducing power of the extract was found to be concentration dependent. The reducing power is based on the hydrogen donating ability. Reducing power of the compound may serve as a significant indicator of its potential antioxidant activity [38]. The reducing power of the extracts increased linearly with concentration. Similar results have been reported by Gosh et al. in ethanol leaf extracts of C. cajan [39].
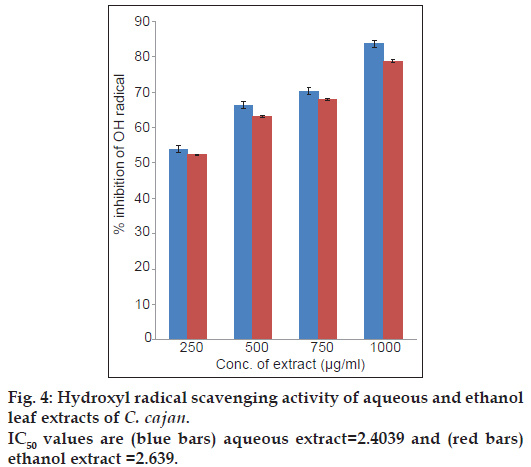
The hydroxyl radical scavenging activity of aqueous and ethanol leaf extracts of C. cajan in various concentrations tested 250, 500, 750 and 1000 μg/ml the scavenging activity were found to be 53.88, 66.32, 70.20 and 83.67% in aqueous and 52.27, 63.05, 67.87 and 78.75% in ethanol leaf extracts, respectively. The IC50 values of aqueous and ethanol extracts were found to be 2.4 and 2.6 μg/ml, respectively. In this activity aqueous and ethanol extracts of C. cajan increased in dose dependent manner (fig. 4). Similarly, good hydroxyl radical scavenging activity was also reported in legume seed extracts [40]. Oxidative damage to DNA, proteins and lipids is caused by hydroxyl radical [41]. The hydroxyl radical induces severe damage in adjacent biomolecules and is the most reactive of the reactive oxygen species [42]. In the present study, the aqueous and ethanol leaf extract at 250 μg/ml could reach more than 50%, while the best effect (up to 83% and 78% in aqueous and ethanol extracts, respectively) was observed at higher concentration (1000 μg/ml). Hence C. cajan leaf extracts can be considered as good scavengers of hydroxyl radicals.
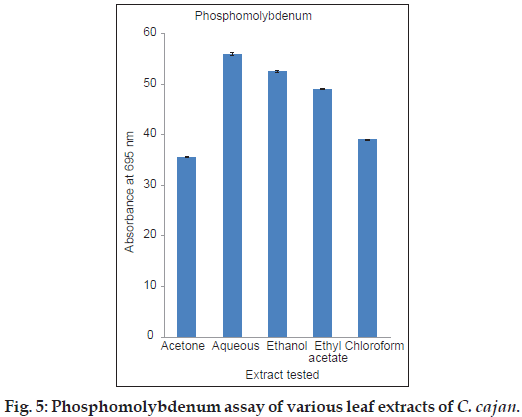
To assess the antioxidant capacity by phosphomolybdenum assay the basic principle implied is the reduction of molybdenum forms a green molybdenum complex by the antioxidant compounds present in the plant extracts which has absorption at 695 nm [43]. The data on total antioxidant capacity observed in acetone, aqueous, ethanol, ethyl acetate and chloroform leaf extracts of C. cajan are shown in fig. 5. The antioxidant activity of phosphomolybdenum assay was found in this order: aqueous>ethanol>ethyl acetate>chloroform>acetone. Among the extracts tested, aqueous extract showed highest antioxidant activity in comparison to others.
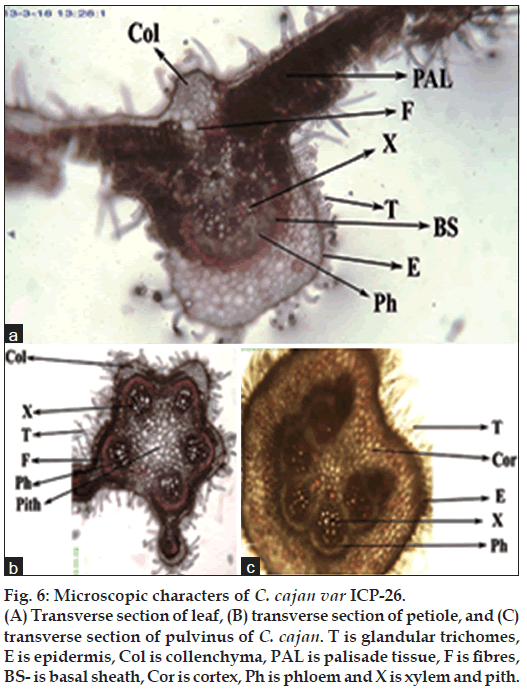
Leaves are trifoliate. The veins within the lamina are visible to the naked eye. In the midrib region of leaf, the vascular tissue occurs in the ventral half with xylem towards inside and phloem outside. The dorsal part of the midrib consists of fibres, above which a cap of collenchymatous cells are present. A distinct palisade layer is present in the leaf lamina. The simple and glandular hairs are found on leaves (fig. 6a). Similar glandular and simple hairs are found on all aerial parts of the plant [15]. The petiole contains number of xylem vessels outside which lies the fibres. The upper surface of the petiole contains two flanges in which vascular bundles are present. Pith is present towards the centre. Trichomes are present surrounding the petiole (fig. 6b). Pulvinus is a swollen region present at the proximal end of the petiole. At the junction of leaflet and petiole pulvinus is found. In the centre of pulvinus, vascular tissue is arranged in a horse shoe shape at the centre, xylem on inside and phloem surrounded by fibres. Pulvinus consists of cortical tissue (fig. 6c). The changes in state on different sides of pulvinus is responsible for the movements of petioles and leaves. The movements of leaves are influenced by intensity of light falling on pulvini [44]. Our results suggest that C. cajan leaf extract can be used as an effective and safe source of natural antioxidant that might be helpful in preventing various oxidative stress related diseases, as ethanomedicine and also for production of green drugs commercially.
Figure 6: Microscopic characters of C. cajan var ICP-26.
(A) Transverse section of leaf, (B) transverse section of petiole, and (C) transverse section of pulvinus of C. cajan. T is glandular trichomes, E is epidermis, Col is collenchyma, PAL is palisade tissue, F is fibres, BS- is basal sheath, Cor is cortex, Ph is phloem and X is xylem and pith.
In the present study, a set of pharmacognostical parameters were conducted on C. cajan leaves. These studies revealed the presence of various important bioactive compounds and proved that the plant leaves can also be of medicinal importantance. These results may help in standardization, identification and in carrying out further research in C. cajan leaf based drugs which are used in traditional and modern pharmacopoeia.
Acknowledgements
Ms. Mahitha Banala is greatly thankful to University Grants Commission, New Delhi for financial assistance as UGC‑BSR Research Fellow (UGC Sanction Lr. No. F.4‑1/ 2006(BSR)/7‑211/2009(BSR); dt. 26‑02‑2013).
References
- Singh V, Pande PC, Jain DK. Economic Botany. 2nd ed. Meerut: Rastogi Publication; 1998.
- Ambasta SP. The useful plants of India. 4th ed. New Delhi: National Institute of Science Communication; 2004. p. 94-5.
- Tang Y, Wang B, Zhou XJ. Effect of external application of herbal cajanipreparation on the fibronection content during healing process of open wound. J Guangzhou U Tradit Chin Med 1999;16:302-4.
- Zu YG, Fu Y J, Liu W, Hou CL, Kong Y. Simultaneous determination of four flavonoids in pigeon pea [Cajanuscajan L.] leaves using RP-LC-DAD.Chromatographia 2006;63:499-505.
- Zheng YY, Yong J, Chen DH, Sun L. Effect of the stilbene extracts from CajanuscajanL. on ovariectomy-induced bone lose in rats. Acta Pharm Sin 2007;42:562-5.
- Upadhyay B, Parveen, Dhaker AK, Kumar A. Ethnomedicinal and ethnopharmaco-statistical studies of Eastern Rajasthan. India J Ethnopharmacol2010;129:64-86.
- Ganeshan S. Traditional oral care medicinal plants survey of Tamil Nadu. Nat Prod Rad 2008;7:166-72.
- Wu N, Fu K, Fu Y, Zu Y, Chang F, Chen Y, et al. Antioxidant Activities of Extracts and Main Components of Pigeonpea [Cajanuscajan(L.) Millsp.] Leaves. Molecules 2009;14:1032-43.
- Lu LM, Zu Y, Fu Y, Zhang S, Yao L, Efferth T. Cajanol, a novel anticancer agent from Pigeonpea [Cajanuscajan (L.) Millsp.] Roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. ChemBiol Interact 2010;188:151-60.
- Zhang D, Zhang S, Zu Y, Fu Y, Kong Y, Gao Y, et al. Negative pressure cavitation extraction and antioxidant activity of genistein and genistin from the roots of pigeon pea [Cajanuscajan (L.) Millsp.]. Sep PurifTechnol 2010;74:261-70.
- Madsen HL, Bertelsen G. Spices as antioxidants. Trends Food Sci Tech 1995;6:271-7.
- Benzie IF. Evolution of dietary antioxidants. Comp BiochemPhysiol Part A 2003;136:113-26.
- Souri E, Amin G, Dehmobed-Sharifabadi A, Nazifi A, Farsam H. Antioxidative activity of sixty plants from Iran. Iranian J Pharm Res 2004;3:55-9.
- Purseglove JW. Tropical crops: Dicotyledons; London: Longmans; 1974.
- Bisen SS, Sheldrake AR. Res. Bull. No. 5. The Anatomy of the pigeon pea; Patancheru, India: ICRISAT; 1981. p. 1-24.
- Raghunathan K. Pharmacopoeial standards for Ayurvedic formulations. 25th ed. New Delhi: Central Council for Research in Indian Medicine and Homeopathy; 1976. p. 378.
- Usha S, Joshi P, Sharma HP. Pharmacognostic studies on AtemisiascopariaWaldst and Kit. Proc Indian AcadSci Plant Sci1984;93:151-64.
- Singleton VL, Rossie JA. Colorimetry of total phenolics with phosphotungstic acid reagents. Am J EnolVitic 1965;16:144-58.
- Jia Z, Tang M, Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxides radicals. Food Chem 1998;64:555-9.
- Patel RM, Patel NJ. In vitroantioxidant activity of coumarincompounds by DPPH, super oxide and nitric oxide free radical scavenging methods. J Adv Pharm Edu Res 2011;1:52-68.
- Oyaizu M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet 1986;44:307-15.
- Smirnoff N, Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochem 1989;28:1057-60.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantification of antioxidant capacity through the formation of a phosphomolybdenumcomplex: Specific application to the determination of vitamin E. Anal Biochem 1999;269:337-41.
- Bari SM, Prodhan AK. Anatomy of lignosus Bean (Dipongonlignosus) III. Stem. Pak J Bio Sci 2001;4:1063-9.
- Bari SM, Prodhan AK. Anatomy of lignosus bean (Dipongonlignosus) IV. Rachis of the inflorescence. Pak J Bio Sci 2001;4:1070-4.
- Johansen DA. Plant Microtechnique. New York: McGraw-Hill; 1940.
- Syed KM, Paramjyothi S. Preliminary Pharmacognostical and Phytochemical Evaluation of PortulacaquadrifidaLinn.Int J PharmTech Res 2010;2:1699-702.
- Herodez SS, Hadolin M, Skerget M, Knez Z. Solvent extraction study of antioxidants from balm (Melissa officinalis) Leaves. Food Chem 2003;80:275-82.
- Srikanth K, Vikram G, Archana P, Rajinikanth M, Rama Swamy N. Pharmacognostic and phytochemical investigations in StrychnospotatorumLinn. F. J PharmacogPhytochem 2013;2:46-51.
- Sumithra C. Importance of pharmacognostic study of medicinal plants: An overview. J PharmacogPhytochem 2014;2:69-73.
- Milan S. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubiumperegrinum L. Extracts. Kragujevac J Sci 2011;33:63-72.
- Canadanovic-Brunet J, Cetkovic G, Djilas S, Tumbas V, Bogdanovic G, Mandic A, et al. Radical scavenging, antibacterial, and antiproliferative activities of Melissa officinalis L. Extracts. J Med Food 2008;11:133-43.
- Tosun M, Ercisli S, Sengul M, Ozer H, Polat T, Ozturk E. Antioxidant properties and phenolic content of eight Saliva species from Turkey. Biol Res 2009;41:175-81.
- Min G, Chun-Zhao L. Comparision of techniques for extraction of flavonoids from cultured cells of Saussurea medusaMaxim. World JMicrobBiot 2005;21:1461-3.
- Subhashini N, Thangathirupathi A, Lavanya N. Antioxidant activity of TrigonellaFoenumGraecumusing variousin vitro and ex vivoo models. Int J Pharm PharmSci 2011;3:96-102.
- Sarkar R, Hazra B, Mandal S, Biswas S, Mandal N. Assessment of in vitro antioxidant and free radical scavenging activity ofCajanuscajan. J Comp Integ Med 2009;6:Article 24.
- Maneechai S, Rinthong P, Pumyen K, Koonkratok P, Sripirom C. Free radical scavenging activity of extracts from Cajanuscajan(L.) Millsp.Planta Med 2013;79:59-63.
- Heir S, Kanner J, Akiri B, Hadas SP. Determination and involvement of aqueous reducing compounds in oxidative difense syst. em of various senescining leaves. J Agri Food Chem 1995;43:1813.
- Gosh T, Maity TK, Bose A. In vitrofree radical scavenging activityof ethanolic extract of leaves of Cajanuscajan(L.) Millsp. J NatRemedies 2009;9:228-34.
- Loganayaki N, Siddhuraju P, Manian S. A comparative study on in vitro antioxidantactivity of the legumes Acacia auriculiformisand Acacia ferrugineawith a conventional legumeCajanuscajan. J Food2011;9:8-16.
- Spencer JP, Jenner A, Aruoma OI, Evans PJ, Kaur H, Dexter DT, et al. Intense oxidative DNA damage promoted by L-DOPA and itsmetabolites, implications for neurodegenerative disease. FEBS Lett 1994;353:246-50.
- Gutteridge JM. Reactivity of hydroxyl and hydroxyl-like radicals discriminated by release of thiobarbituric acid reactive material from deoxy sugars, nucleosides and benzoate. Biochem J 1984;224:761-7.
- Sudha G, SangeethaPriya M, Indhu Shree R, Vadivukkarasi S. Invitro free radical scavenging activity of raw pepino fruit (SolanumMuricatumAiton). Int J Curr Pharm Res 2011;3:137-40.
- Narayanan A, Sheldarke AR. Pigeon Pea Physiology In: Pulse Physiology. Annual report.Part I. Patancheru. AP: ICRISAT; 1974-5.