- *Corresponding Author:
- H. Deti
School of Pharmacy, College of Public Health and Medical Sciences, Jimma University, P. O. Box 378, Jimma, Ethiopia
E-mail: habtewold.deti@ju.edu.et
| Date of Submission | 10 August 2011 |
| Date of Revision | 06 January 2012 |
| Date of Acceptance | 16 January 2012 |
| Indian J Pharm Sci, 2012, 74 (1): 29-35 |
Abstract
The leaves extracts of two indigenous plants of Ethiopia: Clematis longicauda steud ex A. Rich. and Clematis burgensis Engl. are used in Southwestern Ethiopia to treat otorrhoea and eczema. Antimicrobial activity and MIC of crude extracts were determined by disk diffusion and broth dilution. Phytochemical screening was performed on the extracts. The methanol and petroleum ether extracts of both plants showed antibacterial and antifungal activity. Sensitivity of reference strains was concentration dependent. Methanol and petroleum ether extracts of C. burgensis leaves exerted greater inhibitory effects than C. longicauda extracts whereas aqueous extracts of both plants were inactive. The MIC study revealed a concentration of 0.78 mg/ml on bacteria and 3.125 mg/ml on fungi for methanol extract and 1.56 mg/ml on both fungi and bacteria for petroleum ether extract. Phytochemical screening results indicated the presence of proteins, fixed oils, carbohydrates, tannins, saponins, flavonoids, and steroids. Preliminary chromatographic investigation showed fluorescing spots with Rf values that ranged from 0.05 to 0.96 for phenolic compounds and saponins. As the study is one of the first reports on the two indigenous species of Clematis; isolation, purification and characterization of the different primary and secondary metabolites may further yield alternative options to the microbial chemotherapy.
Keywords
Clematis longicauda, Clematis burgensis, phytochemical screening, antimicrobial activity, ethiopia
Introduction
The use of medicinal plants as sources of medicine have been documented in all cultures [1]. The use of traditional medicine (TM) has expanded globally and is gaining popularity in the last decade. It has continued to be used not only for primary health care of the poor in developing countries, but also in countries where conventional medicine is predominant in the national health care system [2].
About 80% of the world’s population relies on herbal medicines to meet the health needs. This is quite significant for millions of people in the vast rural areas of developing countries [3]. In Ethiopia, traditional remedies represent not only part of the struggle of the people to fulfil their essential drug needs but also they are integral components of the cultural beliefs and attitudes [4].
Ethiopia has an enormous source of plant species, which are used in traditional medicine. More than 80% of the population has been relying on traditional medicine and greater than 95% of medicinal preparations are of plant origin [5]. It is also estimated that the flora of Ethiopia contain between 6,500 and 7,000 species of higher plants which have been used by traditional healers for the treatment of human as well as animal diseases [6,7] . Despite its significant contribution to society, TM has received very little attention in modern research and development and less effort has been paid to upgrade the traditional health practices in the country. But, the long history of use of medicinal plants in Ethiopia and its huge biotic riches can be of paramount importance in future research and drug discovery [8].
In this study, two indigenous species of plants, namely: Clematis longicauda steud ex A. Rich. and Clematis burgensis Engl. (Ranunculaceae), having traditional claims for the treatment of ear and skin disorders were investigated for their antimicrobial activities on bacterial and fungal strains. In addition, we conducted a phytochemcial screening on the methanol and petroleum ether extracts.
Plants in the genus Clematis are climbing or trailing shrubs. The leaves are opposite, pinnate or bipinnate with petioles, petiolules and rachis capable of twining round supports. Clematis longicauda steud ex A. Rich. grows in open montane forest and forest borders, along roads, streams and on fences and in woodland associations. It is endemic and widely distributed in different parts of Ethiopia at an altitude of 1300- 1500 m. Clematis burgensis Engl. is associated with shrubs near a dry river bed; and grows in some parts of Ethiopia up to an altitude of 1700 m [9]. Leaves of C. longicauda (Vernacular name: Fitti) and C. burgensis (Vernacular name: Fitti) are used by people in Southwestern Ethiopia, to treat Otorrhoea (Dhukuba Gurra) and Eczema (Chiifee, Abiyatoo).
As the plants are endemic and there is no previous studies on the plants as far as our knowledge is concerned, obtaining scientific data from literature is difficult. Therefore, this investigation was undertaken with the objective to provide scientific evidence on the traditional claim of antimicrobial effect of these plants and lay down basis for the search for new chemotherapeutic agents.
Materials and Methods
The leaves of C. longicauda and C. burgensis (fig. 1) were collected on November, 2010 from Unquuree and Caala (fig. 2), Sekoru district, Southwestern Ethiopia. The areas lie at an altitude range of 1693–1740 m above sea level and have a dry and hot climate with a mean annual temperature of 19.2° and annual rainfall that varies from 1300–1800 mm. Clay soil with a thin layer of humus top soil is the main soil type over the area and evergreen montane thickets and shrubs are characteristic vegetation types of the area. Botanical identity of collected plants leaves were confirmed in Jimma University Herbarium by Dr. M. Remesh (Taxonomy and Ethnobotany specialist); and voucher specimen was deposited as #00104 and #00103 respectively.
Extraction
The collected leaves were washed in running tap water, shed dried, powdered and used for extraction. 70 g of powder was soxhelet extracted with petroleum ether (BDH® chemicals ltd., Pool, England) and methanol (analytical reagent grade, Applichem GmbH, Germany); and it was macerated with distilled water, in order of increasing polarity. Each extract was collected and concentrated under reduced pressure using rotary vacuum evaporator (Bibly Sterilin ltd., Staffordshire, England) at 40°and the semi-solid mass was dried in an oven (Memmer GmbH co.KG, Germany) at 40° for 10 days; and then physical properties were recorded and the percentage yield was calculated.
Microbial reference strains and culture preparation
Staphylococcus aureus ATCC® 25923, Pseudomonas aeruginosa ATCC® 27853, and Candida albicans (clinical isolate) were used for the investigation of antimicrobial activity. All reference strains were obtained from Ethiopian Health and Nutrition Research Institute (EHNRI). Nutrient broth, Mueller Hinton agar and Sabouraud dextrose agar (Hi-media laboratories Pvt. ltd, Mumbai, India) were prepared according to manufacturer’s instruction. Inoculates were prepared using the direct colony suspension method [11]. Well isolated colonies of bacteria (from overnight growth) or fungi (from 72 h old culture) were transferred into test tube containing 5 ml of nutrient broth. The amount of bacteria or fungi needed to undertake the study was determined using UV/Vis spectrophotometer (CECIL instruments, Cambridge, England) at 625 nm so that the absorbance of the suspension was held between 0.08 and 0.1 which was assumed to contain 1-2×108 CFU/ml.
Controls and standard disks
Solvent impregnated disks; standard disks of gentamicin and vancomycin (Oxoid Ltd, Hampshire, England) and ketoconazole (prepared in the laboratory from ketoconazole powder, working standard, Cipla ltd, Mumbai, India) were used in the course of the study.
Antimicrobial activity test
Paper disk diffusion method described by Choudhury et al was used [12]. From 100 mg/ml stock solutions of extracts, different concentrations (250, 500 and 1000 μg) were loaded onto sterilized paper disks (Whatman filter paper, 6 mm in diameter). The crude extract loaded disks and solvent impregnated disks were dried in an oven (Memmer GmbH co. KG, Germany) at 37º for 6 h. The crude extract impregnated disks, solvent impregnated disks (negative controls), and standard disks (positive controls) were placed inverted by their bioactive face on the seeded plates inside microbiological safety cabinet (Labcaire systems ltd., England). Each disk was pressed down to achieve utter contact with the agar surface. The centers of the disks put on the agar surface were at least 24 mm apart [13]. The media were incubated at 37º (for bacteria) for 24 h and at 25º (for fungi) for 48 h and the test was carried out in triplicates. Evaluation of antimicrobial activity was done by observing the clear zone around disks and measuring the diameter of the zone of inhibition around disks in mm by sliding digital vernier calliper.
Determination of minimum inhibitory concentration (MIC)
The Vollekova et al. method modified by Usman et al. was employed [14,15]. Each inoculum was prepared in nutrient broth and density of bacteria/fungi was adjusted as described above. In this method, the broth dilution technique was utilized where the plant extract was prepared to concentration of 100 mg/ml (stock solution) in respective extracting solvent and serially diluted (two-fold) to a working concentration ranging from 0.195 to 25 mg/ml using nutrient broth and later inoculated with 0.2 ml suspension of reference strain. After incubation, the test tubes were observed for turbidity and the least concentration where no turbidity was observed was noted as MIC value.
Preliminary phytochemical screening
Extracts were tested for the presence of primary metabolites and secondary metabolites using different color tests by Oguyemi and Debela [16,17].
Thin layer chromatography
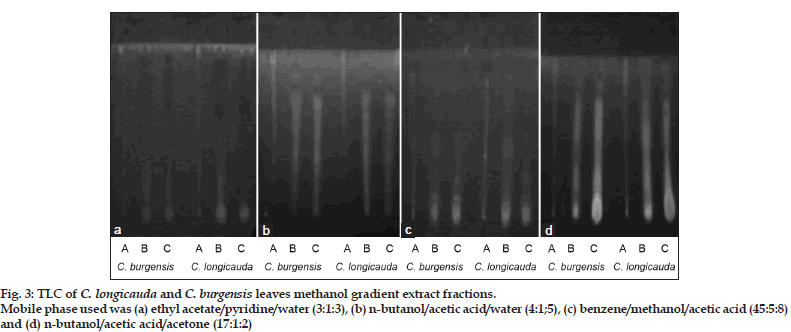
Separation of phenolic compounds and saponins from methanol extracts of both plants was carried out by TLC using pre-coated silica gel 60 F254 plates Whatman ltd., Kent, England after the methanol extracts were fractionated into Solution A (lipophilic substances fractionated with chloroform/acetic acid; 99:1), Solution B (lipophilic and slightly hydrophilic substances fractionated with methanol/chloroform/ acetic acid; 49.5:49.5:1), and Solution C (hydrophilic substances fractionated with methanol/water; 1:1). Solvent system for saponins [petroleum ether/ chloroform/acetic acid (7:2:1), ethyl acetate/pyridine/ water (3:1:3), and n-butanol/acetic acid/water (4:1:5)] and solvent system for phenolic compounds [benzene/ methanol/acetic acid (45:5:8), n-butanol/acetic acid/ acetone (17:1:2), and hexane/ethyl acetate (3:1)] were used to resolve components as described by Debela [17]. The detection of spots was performed in light-tight UV cabinet with UV lamp (St. John’s innovation center, Cambridge, England) under UV light (365 nm). Rf value of each spot was calculated as Rf=Distance travelled by the solute/Distance travelled by the solvent on the TLC.
Results and Discussion
The physical characteristics and percentage yield of the gradient extracts are summarized in Table 1. As indicated in the table, there were differences in the yield of extraction products which might be due to polarity difference of solvents used for extraction, solubility of various ingredients, and type of extraction method used [18]. The highest yield by methanol gradient is indicative of its extracting ability and the polarity of compounds found in the plants and furthermore, these methanol gradient extracts have prominent antimicrobial activity.
| Plant species | Type of extract | Texture | Color | Odor | Percentage yield (w/w) |
|---|---|---|---|---|---|
| C. longicauda | Methanol | Non sticky | Blackish green | Peculiar | 24.71 |
| Water | Sticky | Brownish | Peculiar | 22.71 | |
| Petroleum ether | Oily | Dark green | Peculiar | 2.14 | |
| C. burgensis | Methanol | Non sticky | Blackish green | Peculiar | 27.85 |
| Water | Sticky | Brownish | Peculiar | 24.43 | |
| Petroleum ether | Oily | Dark green | Peculiar | 2.57 |
Table 1: Physical characteristics and percentage yield of extracts from the leaves of C. Longicauda and c. Burgensis
The concentration of the extracts and inhibitory effects is indicated in Table 2. The study revealed that the inhibitory effects appeared at concentrations >250 μg for methanol and petroleum ether gradient extracts of both plants. The aqueous gradient extract of both plants were found to be inactive.
| Type of paper disk | Amount (µg/disk) | C. longicaudaASteud | C. burgensis | ||||
|---|---|---|---|---|---|---|---|
| Zone of inhibition (Mean±SD) | Zone of inhibition (Mean±SD) | ||||||
| Sa | Pa | Ca | Sa | Pa | Ca | ||
| Methanol extract impregnated | 250 | 2.30±0.07 | 1.74±0.07 | 6.74±0.08 | 3.18±0.07 | 2.47±0.05 | 7.45±0.05 |
| disks | 500 | 3.13±0.07 | 3.00±0.05 | 8.42±0.06 | 5.42±0.06 | 4.82±0.07 | 12.90±0.08 |
| 1000 | 3.98±0.06 | 3.74±0.06 | 13.01±0.07 | 7.25±0.08 | 5.93±0.07 | 15.32±0.06 | |
| Petroleum ether extract | 250 | 5.86±0.05 | 4.89±0.05 | 3.45±0.06 | 5.91±0.05 | 4.94±0.07 | 4.44±0.05 |
| impregnated disks | 500 | 7.14±0.08 | 6.69±0.06 | 6.14±0.07 | 7.23±0.06 | 7.11±0.05 | 7.81±0.08 |
| 1000 | 9.11±0.07 | 9.49±0.07 | 8.53±0.05 | 9.15±0.05 | 9.65±0.05 | 10.55±0.07 | |
| Methanol impregnated disks | 30 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 |
| Petroleum ether impregnated disks | 30 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 |
| Gentamicin impregnated disks | 10 | 16.46±0.08 | 10.67±0.06 | 16.46±0.08 | 10.67±0.06 | ||
| Vancomycin impregnated disks | 30 | 11.08±0.07 | 8.72±0.05 | Not Done | 11.08±0.07 | 8.72±0.05 | Not Done |
| Ketoconazole impregnated disks | 20 | Not Done | 22.17±0.08 | Not Done | 22.17±0.08 | ||
Ca -Candida albicans; Pa- Pseudomonas aeruginosa; Sa- Staphylococcus aureus
Table 2: Antimicrobial activity of the gradient extracts of c. Longicauda staud and C. Burgensis leaves
The petroleum ether gradient extracts of both plants were more effective against bacterial strains while methanol gradient extracts were more active against fungus. This indicates presence of more than one active principle. It is observed that a single plant is known to contain several active principles of biological significance [19]. The Gram-positive bacterium (S. aureus) was more sensitive to methanol and petroleum extracts of both plants than the Gram negative (P. aeruginosa). The higher sensitivity of Gram-positive bacteria could be attributed to their outer peptidoglycan layer which is not an effective permeability barrier. Gram-negative bacteria having an outer phospholipid membrane carrying the structural lipopolysaccharide components make the cell wall impermeable to lipophilic solutes while porins constitute a selective barrier to hydrophilic solutes with an exclusion limit of 600 Da [20]. The sensitivity of reference strains to both plants for their methanol and petroleum ether gradient extracts was concentration dependent. Both methanol and petroleum ether gradient extracts of C. burgensis leaves exerted greater inhibitory effects than that of C. longicauda. This might be due to higher yields from C. burgensis.
The gradient extracts which showed antibacterial and antifungal activity were selected further to determine MIC. The MIC of the methanol and petroleum ether extracts of C. longicauda and C. burgensis leaves against S. aureus, P. aeruginosa, and C. albicans is presented in (Table 3).
| Microorganisms | Type of extract | Concentration (mg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.195 | 0.39 | 0.78 | 1.56 | 3.125 | 6.25 | 12.5 | 25 | |||
| C. longicauda | Sa | Methanol | − | − | − | − | + | + | + | + |
| Petroleum ether | − | − | − | + | + | + | + | + | ||
| Pa | Methanol | − | − | − | − | + | + | + | + | |
| Petroleum ether | − | − | − | + | + | + | + | + | ||
| Ca | Methanol | − | − | + | + | + | + | + | + | |
| Petroleum ether | − | − | − | + | + | + | + | + | ||
| C. burgensis | Sa | Methanol | − | − | − | − | + | + | + | + |
| Petroleum ether | − | − | − | + | + | + | + | + | ||
| Pa | Methanol | − | − | − | − | + | + | + | + | |
| Petroleum ether | − | − | − | + | + | + | + | + | ||
| Ca | Methanol | − | − | + | + | + | + | + | + | |
| Petroleum ether | − | − | − | + | + | + | + | + | ||
[−] Resistance (growth of bacteria/fungi or turbidity) [+] Concentrations show no turbidity (inhibition of bacterial/fungal growth)
Table 3: Minimum inhibitory concentration of different extracts of c. Longicauda and C. Burgensis
Qualitative phytochemical screening showed presence of primary metabolites and secondary metabolites (Table 4). Steroids and fixed oils were only present in the petroleum ether gradient extracts while proteins, carbohydrates, and flavonoids were present in all extracts of both plants. Saponins and tannins were present only in the methanol and water gradient extracts of both plants. The secondary metabolites detected in methanol and petroleum ether gradient extracts could be responsible for the observed antimicrobial activity though the exact mode of action is not clearly understood. Polyphenolic compounds like flavonoids exhibit various biological activities. Polyphenols are strong antioxidants and free radical scavengers [21]. Flavonoids have also been reported to possess antibacterial activity, which could be attributed to their ability to form complex with extracellular, soluble proteins and bacterial cell wall [22]. Tannins, another class of polyphenolic compounds which are believed to have an astringent property, play a great role in healing of microbial associated inflamed surface of mouth and throat [21].
| Plant species | Phytochemicals screened | Plant extracts | ||
|---|---|---|---|---|
| Methanol | Water | Petroleum ether | ||
| C. longicauda | Proteins | + | + | + |
| Fixed oils | − | − | + | |
| Carbohydrates | + | + | + | |
| Tannins | + | + | − | |
| Saponins | + | + | − | |
| Alkaloids | − | − | − | |
| Flavonoids | + | + | + | |
| Cardiac glycosides | − | − | − | |
| Steroids | − | − | + | |
| C. burgensis | Proteins | + | + | + |
| Fixed oils | − | − | + | |
| Carbohydrates | + | + | + | |
| Tannins | + | + | − | |
| Saponins | + | + | − | |
| Alkaloids | − | − | − | |
| Flavonoids | + | + | + | |
| Cardiac glycosides | − | − | − | |
| Steroids | − | − | + | |
[+] Indicates Presence of phytochemicals [−] Indicates absence of phytochemicals
Table 4: Results Of The Preliminary Phytochemical Screening Of The Gradient Extracts
They result antimicrobial action by precipitating microbial protein [23]. Saponins, a special class of glycosides; have showed antibacterial activity against S. aureus [24]. Phytosterols, natural plant estrogens, are believed to have an abortifacient property and are expected to be new research areas in the development of safe and effective chemicals for legal medical abortion. Similar to previous phytochemical investigations done on Clematis species [25-33], the preliminary phytochemical screening of the gradient extracts of C. longicauda and C. burgensis leaves revealed the presence of saponins, and flavonoids. But unlike some studies done on the genus Clematis [33] alkaloids and cardiac glycosides were not present.
The separation of saponins and phenolic compounds in methanol gradient extract fractions of both plants was performed on TLC plates; the numbers of spots along with their travelled distance are shown in (fig. 3 and Table 5). Saponins were best resolved in ethyl acetate/pyridine/water (3:1:3) and n-butanol/ acetic acid/water (4:1:5) solvent systems, while phenolic compounds were resolved better in benzene/ methanol/acetic acid (45:5:8) and n-butanol/acetic acid/acetone (17:1:2). The spots fluoresced blue, purple and red when observed under UV light (365 nm). The components could not be resolved in hexane/ethyl acetate (3:1) and petroleum ether/ chloroform/acetic acid (7:2:1).
| Plants | Solution A | Solution B | Solution C |
|---|---|---|---|
| C. longicauda | 0.961, 0.932, 0.953, 0.773, 0.453, 0.934 | 0.961, 0.631, 0.211, 0.952, 0.892, 0.692, 0.392, 0.453, 0.173, 0.083, 0.934, 0.694, 0.534, 0.254 | 0.891, 0.631, 0.682, 0.62, 0.372, 0.453, 0.173, 0.083, 0.714, 0.644, 0.514, 0.234 |
| C. burgensis | 0.961, 0.932, 0.933, 0.83, 0.453, 0.934 | 0.961, 0.631, 0.952, 0.892, 0.692, 0.392, 0.453,0.123, 0.934, 0.684, 0.44, 0.084 | 0.891, 0.631 , 0.682, 0.292, 0.433, 0.123, 0.053, 0.934, 0.724, 0.634, 0.374, 0.084 |
Table 5: Rf Values of saponins and phenolic compounds in methanol gradient extract fractions Of c. Longicauda and c. Burgensis
As the study is one of the first reports on the two indigenous species of Clematis; isolation, purification and characterization of the different primary and secondary metabolites may further yield alternative options to the microbial chemotherapy.
References
- Baquar SR. The role of traditional medicine in rural environment. In: Issaq S, editor. Traditional Medicine in Africa. Nairobi: East Africa Educational Publishers Ltd.; 1995. p. 141?2.
- Lanfranco G. Invited review article on traditional medicine. E-J Biotechnol 1999;2:3.
- World Health Organization. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. Geneva, Switzerland: WHO; 2001. p. 1.
- Abebe D. The role of herbal remedies and the approaches towards their development. In: Proceedings of the Workshop on Development and Utilization of Herbal Remedies in Ethiopia. Addis Ababa: EHNRI; 1996. p. 29.
- Gebre Egziabher TB. Diversity of Ethiopian Floras. In: Eagles JM, Hawks JG, Worda M, editors. Plant Genetic Resource of Ethiopia. Cambridge: Cambridge University Press; 1991. p. 75-81.
- Gidey M. An ethnobotanical study of medicinal plants used by the Zay people in Ethiopia. Skriftserie 2001;3:81?99.
- Demissew S, Dagne E. Basic and applied research on medicinal plants of Ethiopia. In: Proceedings of the National Workshop on Conservation and Sustainable Use of Medicinal Plants in Ethiopia. Addis Ababa: EHNRI; 2001. p. 29.
- Tadeg H, Mohammed E, Asres K, Gebre-Mariama T. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J Ethnopharmacol 2005;100:168?75.
- Teketay D. Ranunculaceae. In: Sue Edwards, Tadesse M, Demissew S, Hedberg I, editors. Flora of Ethiopia and Eritrea: Magnoliaceae to Flacourtiaceae. Addis Ababa: The National Herbarium, Addis Ababa University; 2000, p. 18-21.
- Yineger H, Yewhalaw D. Traditional medicinal plant knowledge and use by local healers in Sekoru District, Jimma Zone, and Southwestern Ethiopia. J Ethnobiol Ethnomed 2009;3:24.
- NCCLS. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard, 8th Ed. NCCLS document M2-A8, Wayne, USA: National Committee for Clinical Laboratory Standards; 2003. p. 6-10
- Choudhury S, Sree A, Murherjee SC, Pattanik P, Baduji M. In vitro Antimicrobial activity of extracts of selected Marine Algae andMangroves against Fish pathogens. Asian Fish Sci 2005;8:285-94.
- Quinn PJ, Carter ME, Markey B, Carter GR. Clinical Veterinary Microbiology. London: Mosby publishing; 2004. p. 43-55, 327-344.
- Vollekova AD, Kostalova, Sochorova R. Isoquinoline Alkaloids from Mahonia aquifolium stem bark is active against Malassezia Sp. Folia Microbiol 2001;46:107-11.
- Usman H, Abdulrahman FI, Ladan AH. Phytochemical and Antimicrobial Evaluation of Tribulus terrestris L. (Zygophylaceae) growing in Nigeria. Res J Bio Sci 2007;2:244-7.
- Oguyemi AO. In: Sofowora A. editor. Proceedings of a conference on African Medicinal plants. Ife-Ife: Univ Ife; 1979. p. 20-22.
- Debela A. Manual for Phytochemical Screening of Medicinal Plants. Addis Ababa: Ethiopian Health and Nutrition Research Institute; 2002. p. 35-47.
- Mahour K, Mishra A, Kumar A, Vihan VS. Preliminary Pharmacognostical and phytochemical investigation on Feronia elephantum corr. Fruit. J Pharm Res 2008;1:45-8.
- Ming DS, Hillhouse BJ, Guns ES, Eberding A, Xie S, Vimalanathan S, et al. Bioactive compounds from Rhodiola rosea (Crassulaceae). Phytother Res 2005;19:740-3.
- Nostro A, Germano MP, Angelo VD, Marino A, Cannatelli MA. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol 2000;30:379-84.
- Evans WC. Trease and Evans Pharmacognosy. 15th ed. London: Saunders Company Ltd; 2002. p. 33-129.
- Tsuchiya H, Sato M, Miyazaki T, Fuziwara S, Tanigaki S, Ohyama M, et al. Comparative study on the antibacterial activity of phytochemical flavonones against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol 1996;50:27-34.
- Scalbert A. Antimicrobial properties of tannins. Phyotochem 1991;30:3875-83.
- Soetan KO, Oyekunle MA, Aiyelaagbe OO, Fafunso MA. Evaluation of the antimicrobial activity of saponins extract of Sorghum bicolor L. Moench. Afr J Biotechnol 2006;5:2405-7.
- Dennis WM, Bierner MW. Distribution of flavonoids and their systemic significance in Clematis subsection viornae. Biochem Syst Ecol 1980;8:65-7.
- Chen Y, Liu J, Davidson RS, Howarth OW. Isolation and structure of clematine. A new flavanone glycoside from Clematis armandii Franch. Tetrahedron 1993;49:5169-76.
- Southwell IA, Tucker DJ. Protoanemonin in Australian Clematis. Phytochem 1993;33:1099-102.
- Kizu H, Shimana H, Tomimori T. Studies on the constituents of Clematis species VI. The constituents of Clematis stans Sieb. et Zucc. Chem Pharm Bull 1995;43:2187-94.
- Slavik J, Slavikova L. Quaternary isoquinoline alkaloids and some diterpenoid alkaloids in plants of the Czech Republic. Collect Czech Chem Comm 1995;60:1034-41.
- Kawata Y, Kizu H, Tomimori T. Studies on the constituents of Clematis species. VII. Triterpenoid saponins from the roots of Clematis terniflora DC. Var. robusta Tamura. Chem Pharm Bull 1998;46:1891- 900.
- Kawata Y, Kizu H, Miyaichi Y, Tomimori T. Studies on the constituents of Clematis species VIII. Triterpenoid saponins from the aerial part of Clematis tibetana Kuntz. Chem Pharm Bull 2001;49:635-8.
- Yesilada E, Küpeli E. Clematis vitalba L. aerial part exhibits potent antiinflammatory, antinociceptive and antipyretic effects. J Ethnopharmacol 2007;110:504-15.
- Yan LH, Xu LZ, Lin J, Yang SL, Feng YL. Triterpenoid saponins from the stems of Clematis parviloba. J Asian Nat Prod Res 2009;11:332?8.