- Corresponding Author:
- A. Shirwaikar
Department of Pharmacognosy, Department of Pharmaceutics, Manipal College of Pharmaceutical Sciences, Manipal - 576 104, India.
E-mail: arunshirwaikar@yahoo.co.in
| Date of Submission | 24 December 2005 |
| Date of Revision | 6 December 2006 |
| Date of Acceptance | 15 May 2007 |
| Indian J. Pharm. Sci., 2007, 69 (3): 365-369 |
Abstract
Vasicine is a pyrralazoquinazoline monobasic alkaloid obtained from the plant Adhatoda zeylanica. In the current experiment, we have made an attempt to determine the sites of absorption for the bioactive component vasicine from the various segments of small intestine (duodenum, jejunum and ileum) and colon. Everted intestinal sac method was used to assess the in vitro absorption of vasicine. The absorption of standard vasicine, vasicine from methanol and ethanol extracts of vasaka from the small intestine of rats was studied using the Doluisio technique. Maximum absorption of 87.3±5.256% of vasicine was observed in the duodenal region, whilst the colon showed the minimum absorption of 42.6±7.314%. The jejunum and ileum showed 77.2±3.415% and 46.9±3.271% absorption of vasicine respectively. The luminal disappearance of vasicine by Doluisio technique was determined from the standard curve. Absorption of vasicine was found to be better from the ethanol extract than from the methanol extract. Standard vasicine has revealed steady absorption as evidenced by a typical sigmoidal curve. The absorption rate was found to be of the first order for the tested samples.
Keywords
Absorption, Doluisio technique, everted sac, in situ, in vitro, vasicine
Vasicine, a bioactive pyrralazoquinazoline alkaloid isolated from the medicinal herb Adhatoda zeylanica also known as Justacia adhatoda (Family-Acanthaceae) is reported to possess bronchodilatory and expectorant properties [1-3]. Biological in vitro methods are employed to assess the permeability of gastrointestinal mucosa to the drugs. These techniques are applied so as to assess or predict the bioavailability after oral administration of drugs and are based on methods ranging from the purely mathematical, in vitro permeation/uptake studies to perfusions [4]. There are no reports of studies on the absorption sites for vasicine or on the extracts of vasaka prepared by using various solvents. The current experiment describes the in vitro intestinal absorption of vasicine for the assessment of permeability and also the absorption of vasicine from methanol and ethanol extracts of vasaka by the Doluisio technique. Absorption kinetics of standard vasicine (V1), vasicine of the methanol extract (V1M) and vasicine of the ethanol extract (V1E) were compared.
Materials and Methods
Vasicine (>97% pure by HPLC) was purchased from SPIC Pharmaceuticals Division, Marai Malai Nagar, Chennai, India. Methanol (HPLC grade) and water (HPLC grade) were obtained from Merck Limited, India and Nice Chemicals Pvt. Ltd., respectively, while all other chemicals used in the experiment were of analytical grade.
Plant material and extracts
The plant Adhatoda zeylanica (family-Acanthaceae) procured from the local market was authenticated in the Department of Botany, Poorna Pragna College, Udupi, India and a voucher specimen has been deposited in the Department of Pharmacognosy, MCOPS, Manipal (PP-537 A). The non-infected leaves were shade dried, powdered and extracted with 80% ethanol by maceration for 36 h at room temperature. The resultant liquid extract was evaporated to dryness under vacuum to give a yield of 4.2% dry extract standardized to 1.5% w/w of vasicine. Standardized methanol extract containing 1.3% w/w vasicine was obtained as a gift sample from Sami Labs., Bangalore, India. IdentiÞ cation of vasicine in both the extracts was carried out by the TLC method as given in the Indian Herbal Pharmacopoeia [5].
Chromatographic conditions
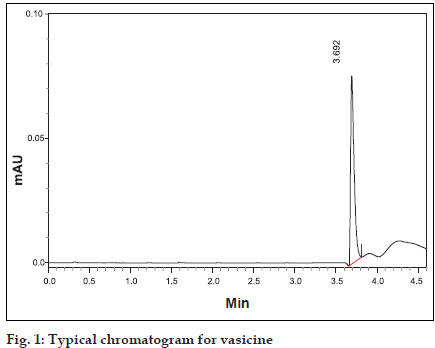
Shimadzu HPLC of SCL-10AVP equipped with LC- 10-ATVP pump and SPD-10-AVP UV detector was used (Shimadzu Corp., Japan). The column used was Luna 5µ-C18(2) of 250×4.6 mm id (Phenomenex, USA). Methanol and water in the ratio of 40:60 was used as a mobile phase. Flow rate was maintained at 0.7 ml/min and detection carried out at λmax at 298 nm [5] was used. Shimadzu UV/Vis Spectrophotometer of UV-1601 PC model was used to measure the absorbance. Vasicine was dissolved in methanol in a ß ask to obtain standard solution of concentration 100 µg/ml. Twenty microlitres of this standard solution was injected into the HPLC system and the peak area value and retention time were recorded (Þ gure 1).
HPLC method
Percentage recovery was calculated by the addition of a known amount of vasicine into plain buffer solution to which 20 µl of ammonia was added. This was followed by extraction with three 5 ml volumes of chloroform. The pooled chloroform extract, dried over anhydrous sodium sulphate was evaporated to dryness in vacuo. The dried chloroform extract, reconstituted with 1 ml of methanol was injected into the HPLC system. A standard stock solution of vasicine in Krebs- Henseleit buffer6 (100 µg/ml) was prepared. Further dilutions were made with the buffer to get aliquot concentrations 2, 4, 6, 8, 10 and 12 µg/ml in different 10 ml volumetric flasks. Vasicine was extracted as described earlier, reconstituted in 1 ml methanol and then injected into the HPLC system. Peak area was noted for all the concentrations tested and the standard plot was plotted with concentration (µg/ml) on the abscissa and peak area on the ordinate.
UV method
One millilitre of the extract containing known amount of vasicine in saline solution was shaken well with 20 µl of ammonia solution and then gently heated on a water bath. After cooling, vasicine was extracted using chloroform (3×2.5 ml). The mixture was shaken well, centrifuged and to the separated chloroform extract a little anhydrous sodium sulphate was added to remove the traces of moisture. The extract was evaporated to dryness under vacuum. Vasicine was reconstituted with 1 ml of saline and the absorbance measured at λmax of 281 nm using an UV Spectrophotometer. A stock solution of vasicine 10 µg/ml in saline was prepared using an ultrasonic bath. From this stock solution, a series of dilutions (1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 µg/ml) were made using saline. Absorbance was measured using UV spectrophotometer at λmax 281 nm and the standard graph plotted with concentration (µg/ml) on the abscissa and absorbance on the ordinate.
Animal experimentation
Three normal healthy Wistar rats of either sex weighing between 150-200 g were selected for the experiment from an inbred colony maintained under the controlled conditions of temperature (23±2°), humidity (50±5%) and light (10 and 14 h of light and darkness, respectively). The animals were fasted overnight but had free access to water prior to the experiment. The study was approved by the Institutional Animal Ethical Committee (IAEC), Kasturba Medical College, Manipal (IAEC/ KMC/80/2001-2002).
In vitro assessment of permeability
The abdomen of the rat was dissected open under light ether anaesthesia. Various intestinal segments were harvested, washed with saline and placed in buffer immediately. The everted intestinal sac method7 was used to assess the absorption. Sections (2-4 cm) of various segments of the intestinal tract were tied at one end and everted using a glass rod so as to expose the mucosal layer to the permeant. The sacs filled with plain buffer were tied at the other end and put in a ß ask containing vasicine (10 µg/ml) in oxygenated (95% O2 5%CO2) Krebs-Henseleit buffer to which 10 mM glucose was added. After 30 min, the sacs were cut open at one end and the serosal ß uid collected [8] from sections of various segments placed in different tubes.
Analysis of samples by HPLC
Fig. 1: Typical chromatogram for vasicine Different tubes containing serosal fluid samples (1 ml each) collected from various regions of the small intestine and colon were vortexed for a few seconds in a cyclomixer with 20 µl of strong solution of ammonia. The samples were then extracted with chloroform (3×5 ml). The different chloroform extracts were dried over anhydrous sodium sulphate to remove the water soluble impurities and the separated chloroform extract evaporated to dryness in vacuo. The different extracts were reconstituted with one ml of methanol and then injected into the HPLC system. Retention time and peak area were noted and vasicine concentrations were determined from the standard plot.
Ex vivo studies
The absorption of vasicine (V1), vasicine in the methanol extract (V1M) and vasicine in the ethanol extract (V1E) from the small intestine of anaesthetized rats was studied using the Doluisio in situ technique. Nine inbred rats divided into three groups of three each were placed in individual cages. Ketamine hydrochloride equivalent to 100 mg/kg of ketamine was injected intramuscularly to anaesthetize the rats and the anaesthetic effect further maintained by using anesthetic ether for an hour. Midline abdominal incision was made to open the abdomen and the jejunum was carefully lifted out without distortion to the blood supply and with the animal intact. Two slits were made, one at the beginning of the jejunum and another approximately 10 cm from the Þ rst one. The contents of the intestine were ß ushed with saline and a drug solution (100 µg standard vasicine in 10 ml saline) was filled into the intestine using a plastic syringe connected with a three way stopper through the Þ rst slit at the beginning of the jejunum. To another slit made at the other end of the jejunum was connected a plastic syringe with a three way stopper for drawing sample solutions of 1 ml each periodically every ten min at a perfusion rate of 0.1 ml/min. Absorbance of V1, V1M and V1E were recorded using UV Spectrophotometer at λmax 281 nm and the drug concentrations were determined from the standard curve.
Data analysis of intestinal perfusion
The steady-state effective intestinal permeability (Peff cm/s) in rats was calculated using the parallel-tube model ie, perfusion from an entrance in one end of the intestinal segment to an exit at the other end of the intestinal segment [9-12] as given in Eqn. 1, Peff = - Qin ln (Cout/Cin)/A, where Qin is perfusion ß ow rate, Cin and Cout are the inlet and outlet fluid-transport corrected concentrations of vasicine. A is the mass transfer surface area within the intestinal segment assumed to be a cylinder with a length of 10 cm [13,14].
Results and Discussion
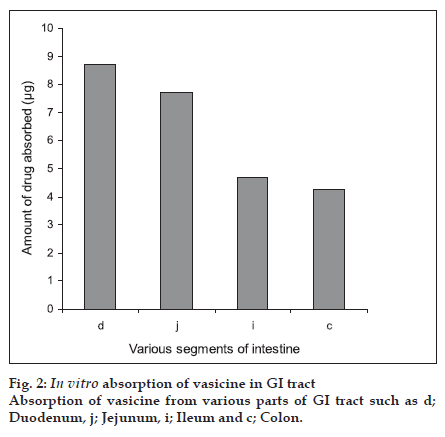
Eversion of the intestinal regions is performed to maximise the contact between the tissue at the uptake level and the solute. As the everted segments are claimed to be viable for a period of 30-60 min, they were maintained before use in a physiological buffer containing glucose [15]. Transport of the drug is known to decrease significantly within the first few min in animals killed by cervical dislocation before removal of the segment [16]. Hence in our study, intestines were isolated under anaesthesia so as to avoid any deterioration of the tissues [16]. Analysis of the samples were carried out by using the HPLC technique. The percentage recovery of vasicine was found to be 95.8± 3.26%. The standard plot was found to be linear for the concentrations studied with a linear regression coefÞ cient R2 of 0.999. Results are shown in Þ gure 2. The duodenum showed maximum absorption (87.3±5.256%) of vasicine whilst the least was observed from the colon (42.6±7.314%). The jejunum and ileum showed absorption of 77.2±3.415% and 46.9±3.271% vasicine, respectively.
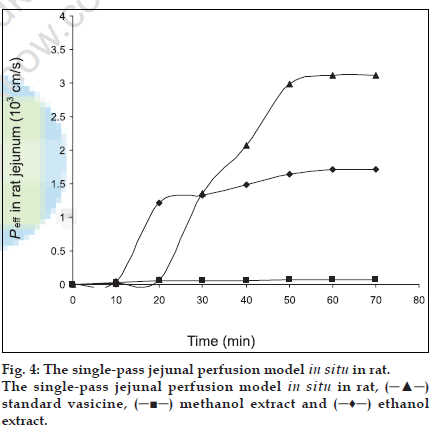
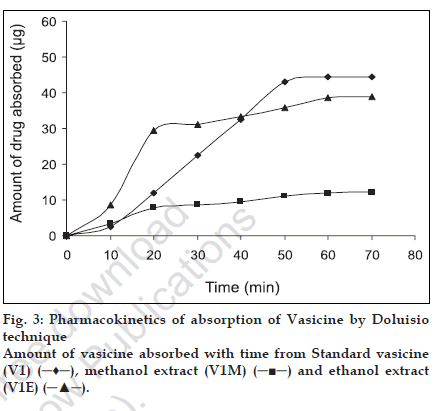
The extent of intestinal absorption in humans can be predicted from intestinal permeability values measured in situ in rats by single-pass perfusions and the values can be used to estimate the extent of oral drug absorption in humans, regardless of the transport mechanism [12]. For passively transported compounds, the effective permeability rank order has been found to be the same in perfused proximal jejunal segments of both humans and rats [11]. The absorption of steroidal hormones oestrone, oestrone glucuronide and oestrone sulphate from the small intestine of anaesthetized rats have been evaluated by the Doluisio in situ technique and the luminal disappearance determined in studies by Sim and Back [17]. Park and coworkers carried out studies on the absorption pattern of prostaglandins dinoprost in the rat jejunum using a modiÞ ed Doluisio technique [18]. In the present work, an attempt has been made to study the absorption pattern for vasicine from the methanol and ethanol extracts of vasaka using Doluisio technique. The regression coefÞ cient of the standard curve was found to be R2=0.999 and the mean percentage recovery of vasicine from both the extracts in saline was found to be 97±1.14. Luminal disappearance of the drug was determined from the standard curve. The pharmacokinetic graph was plotted by taking time (min) on the abscissa and the amount of drug (µg) absorbed on the ordinate to study the absorption pattern up to 70 min. While standard vasicine showed steady absorption and achieved a maximum concentration of 45 µg at 50 min, the ethanol extract achieved maximum absorption of vasicine (30 µg) in 20 min after which there was a decline in the rate of absorption. A further absorption of only 10 µg of vasicine was achieved between 20 to 60 min. The methanol extract revealed poor absorption as compared to the ethanol extract with a maximum concentration of only 10 µg achieved after 40 min. The absorption rates of all the tested samples were found to follow The First Order kinetics and showed a sigmoidal curve (fig. 3). As effective intestinal permeability (Peff) [19,20] is one of the key variables controlling the overall intestinal absorption rate, it may be used regardless of the transport mechanisms across the intestinal mucosa (Þ g. 4).
From the in vitro results, we can conclude that the absorption of vasicine a monobasic compound was found to be maximum in the intestinal pH rather than at the acidic pH thus suggesting that its availability is probably more in the un-ionised form. Though the absorption of vasicine takes place across various regions of the gastro-intestinal tract, our study using the Doluisio technique particularly reveals the jejunum as the best absorption site. The absorption of vasicine from the ethanol extract was better compared to that of the methanol extract and hence can be considered as the best extract for the preparation of formulations. Since 80% ethanol has been used for extraction, water soluble polar constituents are also extracted. These may not be present in the methanol extract and may be responsible for the enhanced absorption of vasicine from the standard ethanol extract. Further work envisages the preparation of suitable oral formulations of vasaka with maximum bioavailability.
Acknowledgements
The authors sincerely thank MAHE (a deemed university), Manipal for providing the necessary facilities to carry out this research work. The authors also thank Prof. G. K. Bhat for his support in authentication of the plant sample. The authors are also grateful to Sami Labs, Bangalore for providing the standardized methanol extract of Vasaka as a gift sample.
References
- The Wealth of India.Publication and Information Directorate, CSIR: New Delhi; 1948.Back to cited text no.1.
- Nadakarni KM.Indian Materia Medica, Mumbai; Popular Prakashan: 1976.Back to cited text no.2.
- Atal CK.Chemistry and Pharmacology of Vasicine.Director, RRL: Jammu Tawi; 1980.Back to cited text no.3.
- Tukker JJ.In vitro methods for the assessment of permeability.In: Dressman JB, Lenernas H, editors.Oral drug absorption, prediction and assessment.New York; Marcel Dekker Inc: 2000.p.51-72.Back to cited text no.4.
- Indian Herbal Pharmacopoeia.IDMA and RRL (CSIR): Jammu Tawi; 1998.Back to cited text no.5.
- Kulkarni SK.Hand book of Experimental Pharmacology.3 rd ed.Delhi; Vallabh Prakashan: 1999.Back to cited text no.6.
- Wilson TH, Wiseman G.The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface.J Physiol 1954;123:116-25.Back to cited text no.7.
- Yamamoto A, Kawaratani T, Kawashima K, Hashida M, Sezaki H.Intestinal transport of sulfanilic acid in rats immunised with protein-sulfanilic acid conjugate.Pharm Res 1990;7:767-71.Back to cited text no.8.
- Komiya I, Park JY, Kamani A, Ho NF, Higuchi WI.Quantitative mechanistic studies in simultaneous fluid flow and intestinal absorption using steroids as model solutes.Int J Pharmaceut 1980;4:249-62.Back to cited text no.9.
- Lennernas H.Human jejunal effective permeability and its correlation with preclinical drug absorption models.J Pharm Pharmacol 1997;49:627-38.Back to cited text no.10.
- Fagerholm U, Johansson M, Lennernas H.Comparison between permeability coefficients in rats and human jejunum.Pharm Res 1996;13:1335-41.Back to cited text no.11.
- Amidon GL, Sinko PJ, Fleisher D.Estimating human oral fraction dose absorbed: A correlation using rat intestinal membrane permeability for passive and carrier-mediated compounds.Pharm Res 1988;5:651-4.Back to cited text no.12.
- Lennernas H, Ahrenstedt O, Hallgren R, Knutson, L, Ryde M, Paalzow LK.Regional jejunal perfusion, a new in vivo approach to study oral drug absorption in man.Pharm Res 1992;9:1243-51.Back to cited text no.13.
- Lennernas H, Lee ID, Fagerholm U, Amidon GL.A RTD analysis of the hydrodynamics within the human intestine during a regional single-pass perfusion: In vivo permeability estimation.J Pharm Pharmacol 1997;49:682-6.Back to cited text no.14.
- Leppert PS, Fix JA.Use of everted intestinal rings for in vitro examination of oral absorption potential.J Pharm Sci 1994;83:976-81.Back to cited text no.15.
- Pento JT, Mousissian GK.Time-dependent deterioration of active transport in duodenal segments of rat intestine.J Pharmacol Met 1988;20:9-14.Back to cited text no.16.
- Sim SM, Back DJ.Intestinal absorption of oestrone, oestrone glucuronide and oestrone sulphate in the rat in situ-II.Studies with the Doluisio technique.J Steroid Biochem 1986;24:1085-9.Back to cited text no.17.
- Park JY, Ho NF, Morozowich W.Physical model approach to gastrointestinal absorption of prostaglandins II: In situ rat intestinal absorption of dinoprost.J Pharm Sci 1984;73:1588-94.Back to cited text no.18.
- Lennernas H.Human intestinal permeability: An overview.J Pharm Sci 1998;87:403-10.Back to cited text no.19.
- Amidon GL, Lennernas H, Shah VP, Crison JR.A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability.J Pharm Res 1995;12:413-20.Back to cited text no.20..
 ), methanol extract (V1M) (
), methanol extract (V1M) ( ) and ethanol extract (V1E) (
) and ethanol extract (V1E) ( ).
).