- *Corresponding Author:
- Gehan Balata
Department of Pharmaceutics, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
E-mail: dawn.foster@yale.edu
| Date of Submission | 16-Feb-2011 |
| Date of Revision | 30-Sept-2011 |
| Date of Acceptance | 2-Oct-2011 |
| Indian J Pharm Sci, 2011, 73 (5): 517-526 |
Abstract
Solid dispersions of a slightly water-soluble drug, clotrimazole, were prepared in different weight ratios using polyethyleneglycol 4000 and different molecular weight polyvinyl pyrrolidones as carriers. Moreover, binary and ternary β-cyclodextrin complexes were prepared in different molar ratios. Both solid dispersions and β-cyclodextrin complexes were prepared by solvent evaporation technique. A phase solubility method was used to evaluate the effect of the tested carriers on the aqueous solubility of clotrimazole. The dissolution of all the preparations was tested using the USP paddle method. The selected solid dispersions and inclusion complexes were characterized by differential scanning calorimetry and X-ray powder diffractometry studies, and results clarified the role of the tested carriers in decreasing the crystallinity of clotrimazole and complexing abilities. Based on physical characters and in vitro drug release pattern, polyvinylpyrrolidone solid dispersions (1:1 weight ratio) and ternary cyclodextrin complexes (clotrimazole-β-cyclodextrin complexes with either polymer, 1:1 molar ratio) were selected as ideal batches for suppositories. Suppocire AM/50 mg carbopol 940, was chosen as a suppository base and the suppositories were prepared by molding technique. The prepared suppositories were characterized for weight variation, softening time and drug content. All these properties were found to be ideal. The in vitro drug release pattern was determined in citrate buffer (pH 4.5) containing 1% sodium lauryl sulfate. The in vitro release of clotrimazole from its solid dispersions and inclusion complexes incorporated suppositories was markedly improved when compared to the intact drug incorporated suppositories. Polyvinyl pyrrolidone solid dispersions incorporated suppositories were found to possess excellent antifungal activity.
Keywords
Clotrimazole, polyethyleneglycol, polyvinylpyrrolidone, suppository, β-cyclodextrin
Introduction
A vaginal clotrimazole-containing formulation may be of use for several reasons including the opportunity to generate high local tissue levels, more rapid drug delivery, and lower systemic exposure. This may be especially important for treating pregnant patients. In addition, clotrimazole (CLOT) is effective against several fungal strains such as Torulopsis glabrate and Candida topicalis, which are responsible for refractory vaginal candidiasis in more than 25% of patients suffering from this condition. In fact, Candida-related fungal vaginitis is a common gynecological disease affecting two thirds of all women at least once during their lifetime [1]. In studies of mechanisms underlying in vitro availability of different drugs from suppositories, the water solubility of drugs was found to be the fundamental factor influencing the release rate and extent [2]. Different approaches have been used to improve solubility and dissolution rate of poorly water-soluble drugs from suppositories using solid dispersion, crystallization of the drug with carriers such as urea, sodium salicylates, hydroxypropylmethylcellulose and β-cyclodextrin (β-CD) complexation [3-6]. Since CLOT is a lipophilic drug (Log K o/w = 4.1) with low aqueous solubility (solubility=0.49 mg/l) [7,8] and slow dissolution in water [7], its slow release from suppository formulations may be anticipated. Previous studies have been carried out to improve the solubility of CLOT by microcapsule [9], liposome [10], suspension with hydroxypropylmethylcellulose and nanosphere [11], cyclodextrin inclusion complex [7,8,12-14] and solid dispersion technique using mannitol as carrier [15]. Continuing that research, our study is to gain insight into the effect of water-soluble polymers such as polyethyleneglycol 4000 and different molecular weight polyvinyl pyrrolidones on the physicochemical properties of clotrimazole in the solution and the solid state. In addition, the effect of water-soluble polymers on the complexation of CLOT with β-CD was studied. Moreover, the influence of solid dispersion and inclusion complex formation on the in vitro drug release and antifungal activity from suppocire AM/50 mg carbopol 940 suppository base was investigated.
Materials and Methods
Clotrimazole (CLOT), polyvinyl pyrrolidone k-17 (PVPk-17), (PVP k-25), (PVP k-30), suppocire AM and carbopol 940 were kindly supplied as gift samples by Memphis Co. (Cairo, Egypt). β-Cyclodextrin (β-CD) (Mw 1135) and sabouraud dextrose agar were purchased from Sigma-Aldrich (St Louis, MO, USA). Methanol, ethanol, chloroform, citric acid, sodium hydroxide, hydrochloric acid and sodium lauryl sulphate were purchased from El-Gomhoria Co. (Cairo, Egypt). Polyethylene glycol 4000 (PEG4000) was purchased from Hoechest Chemikalien (Werk Gendort, Germany). A semipermeable cellophane membrane 30/32 was supplied by (Fischer Scientific Co., London, England).
Phase solubility studies
Solubility studies were performed according to the method described by Higuchi and Connors, 1965 [16]. An excess amount of drug (50 mg) was added to 10 ml of water or carrier aqueous solutions of different concentrations (1–20%) for PVP and PEG4000 polymers and (0.1-1.8%) for β-CD with and without the addition of 0.5% w/v hydrophilic polymers (PVP k-17, PEG4000) in 25-ml stopper conical flasks and shaken at 25° in a thermostatically controlled water bath (Julabo SW 20C, Osaka, Japan). At equilibrium after 4 days (for β-CD with and without polymers) or 2 days (for the other carriers), aliquots were withdrawn, filtered (0.22 µm pore size, Whatman UK), suitably diluted with methanol and spectrophotometrically (Shimadzu UV-160A Spectrophotometer, Shimadzu, Japan) assayed for drug content at 259 nm. The solubility experiment was conducted in triplicate and the average was reported.
The apparent 1:1 stability constant of the CLOTcyclodextrin complex was calculated from the phasesolubility diagram: Kc = slope / S0 (1 – slope), Where Kc is the stability constant (L mol-1), slope is obtained from the linear relationship between the concentration of CLOT and β-CD and S0 is the intrinsic solubility of CLOT (mol/l).
Preparation of solid inclusion complexes
Solid inclusion complexes of CLOT and β-CD with and without the addition of 10% w/w hydrophilic polymers (PVP k-17, PEG4000) were prepared in the molar ratios 1:1, 1:2.5 and 1:5 by co-evaporation method [17].
CLOT and β-CD with and without the addition of hydrophilic polymers were dissolved at 40° in the lowest volume of 50% ethanol (which is necessary to obtain solution) and stirred for 30 min. Then, the solvent was evaporated in a vacuum oven at 50° until complete drying was obtained as shown by constant weight. The dried mass was pulverized, passed through 60-mesh size sieve and stored in a desiccator until used for further studies.
Preparation of solid dispersions
Solid dispersions of CLOT and PEG4000 or PVP k-17 were prepared in drug to polymer ratios 1:1, 1:5 and 1:10 (w/w) by the solvent evaporation method [18]. CLOT and the polymer were dissolved in ethanol and the solvent was removed under vacuum in a rotavapor at 65° and 80 rpm. The dried mass was stored in desiccator until constant mass was obtained, pulverized and passed through 250-µm sieve (mesh size 60).
To study the effect of the PVP molecular weight, SDs with PVP k-25 and PVP k-30 were prepared as described above at the weight ratio that produced the best dissolution results.
Preparation of physical mixtures
Physical mixtures of CLOT (<250 µm) were prepared by mixing the appropriate amounts of CLOT and carriers in geometric proportions using a mortar and pestle, until a homogeneous mixture was obtained. The physical mixtures were subsequently stored at room temperature in screw capped glass vials until use.
Evaluation of clotrimazole content
About 20 mg of each preparation was placed in a 25-ml volumetric flask. Methanol (10 ml) was added, mixed thoroughly and sonicated for 30 min. The volume was made up to the mark with methanol and then filtered (0.22 μm pore size, Whatman UK). The solution was suitably diluted with the same solvent and the drug content spectrophotometrically assayed at 259 nm. The percentage drug content was calculated for all batches using the equation: Drug content % = [CLOTact / CLOTtheor] ×100, Where CLOTact is the actual CLOT content in 20 mg product and CLOTtheor is the theoretical amount of CLOT in 20 mg product.
Dissolution studies
Dissolution of CLOT powder, physical mixtures, solid dispersions and inclusion complexes equivalent to 20 mg of CLOT was carried out with the USP II Dissolution Test Apparatus (paddle) (Pharma Test SP6-400, Hamburg, Germany) at 37±0.5° and 100 rpm using 500 ml distilled water as dissolution medium (n=3). Samples of dissolution medium (5 ml) were withdrawn at predetermined time intervals and an equal amount of fresh dissolution medium was added. Test samples were filtered (0.22 µm pore size, Whatman UK), suitably diluted and assayed for CLOT at 259 nm using a blank solution as reference. The percentage of CLOT dissolved was calculated using a regression equation generated from standard data.
The dissolution efficiency (DE) was calculated from the area under the dissolution curve at time t (measured using the trapezoidal rule) and expressed as a percentage of the area of the rectangle described by 100% dissolution over the same time [19].
Differential scanning calorimetry (DSC)
DSC analysis was performed using a Model DT-60 DSC (Shimadzu). Samples weighing 1.5 mg were heated in hermetically sealed aluminum pans over a temperature range of 30-200° at a constant rate of 10°/min under a nitrogen steam.
X-ray powder diffractometry (XRPD)
X-ray powder diffraction patterns were recorded on a Siemens Kristallofex D-5000 powder diffractometer with CuKa radiation. The scanning rate employed was 8°/min over a 2θ range of 0–80°.
Preparation of clotrimazole suppositories
Suppositories weighing 2 g each, containing 100 mg of CLOT or its equivalent in either PVP solid dispersions (CLOT-PVP k-25 and CLOT-PVP k-30, 1:1 weight ratio) or ternary cyclodextrin complexes (CLOT–β-CD complexes with either 10% PVP or 10% PEG, 1:1 molar ratio) were prepared using a lipophilic suppository base (mixture of suppocire AM and 50 mg of carbopol 940) taking into account the displacement value of CLOT or its solid products in the base using metal moulds. Suppocire AM / 50 mg carbopol 940 base was chosen on the basis of its bioadhesive properties that guaranteed longer permanence in the target area and allowed less active ingredient to leave the target site [20].
Suppocire AM was melted at 40-45º, and then the mucoadhsive polymer and CLOT or its solid products were added to the melted mass. The product was stirred with a mechanical stirrer for 5 min at a constant temperature of 40º. Suppositories were then dosed into unlubricated moulds to obtain suppositories of 2.0±0.1 g each. Blank suppositories containing no active substance were prepared to determine the absorbance of the base. The prepared suppositories were wrapped in aluminum foil, kept in refrigerator and were used in the investigation.
Weight variation
Average weight of suppositories was calculated and then each suppository was individually weighed and the variation from the average was calculated. Not more than two of the individual weights should deviate from the average weight by more than 5%, and none deviates by more than 10%.
Content uniformity
Three randomly selected suppositories were stirred individually in a 150 ml citrate buffer pH 4.5 at 60° by a magnetic stirrer. After melting process was completed, the clear filtered solutions were measured spectrophotometrically at 259 nm against the blank prepared using suppositories without drug.
Determination of softening time
Three suppositories of each formulation were placed individually in a dialysis membrane and placed in a beaker containing 150 ml of citrate buffer pH 4.5 at 37° and shaken in a thermostatic shaker water bath at 100 rpm. The average time needed for formulated suppositories to soften was measured in minutes [21].
In vitro release studies
The in vitro release of CLOT from different suppository formulae was performed according to the method described by Samy et al [4]. A previously soaked cellophane membrane was fastened on the open end of a glass tube (20 cm in length) having a surface area of 4.53 cm2, using a rubber band. The tube was then immersed upside-down in a 250-ml beaker containing 150 ml of citrate buffer (pH 4.5) and 1% sodium lauryl sulfate. Sodium lauryl sulfate was included in the release medium to maintain sink conditions. A volume of 5 ml of citrate buffer was poured into the glass tube. The system was placed into a thermostatically-controlled water bath and the temperature was maintained at 37º and the stirring rate was kept constant at 100 rpm. Drug release from different suppository formulae was determined by introducing each suppository in a dialysis tube and three milliliter samples of the release fluid were withdrawn at specified intervals and each time replaced with equal volume of fresh release medium. The samples were analyzed spectrophotometerically at 259 nm (Shimadzu Corp, Kyoto, Japan) and percentage drug release was calculated using an equation obtained from a standard curve.
Interference experiments showed that the components of the base did not interfere with the spectrophotometric measurement of the drug at the specified wavelength. Each release experiment was performed in triplicate.
Data analysis
The extent of drug release was assessed from the total amount of drug present in the release medium at the end of the 360 min drug release experiment. The type of drug release kinetics applicable for the suppository bases was determined by evaluation of three models, viz: zero-order kinetic model (Q vs t), diffusion controlled model (Q vs square-root of t) and first-order model (log (Qo–Q) vs t), where Q is the amount of drug released at time ‘t’ and Qo is the initial amount of the drug. The model that consistently produced the highest correlation among the suppository preparations was used for the assessment of drug release rates, and a slope obtained from linear regression analysis of the plot was determined as the drug release rate constant.
Antifungal activity
The antifungal activity of CLOT from different suppository formulae (suppocire AM/50 mg carbopol 940 containing pure CLOT, ternary CLOT–β-CD– PVP/PEG complex or CLOT-PVP k-25/k-30 solid dispersions) was determined using Candida albicans [22]. The suspension of Candida albicans was streaked on triple plates of Sabouraud dextrose-agar. The discs of different suppository formulae (200 mg) which equivalent to 10 mg CLOT potency were prepared and placed on the inoculated plates and were incubated at 37° for 3 days. The diameters of the inhibition zones were measured and the inhibition distance was calculated. The discs for suppocire AM/50 mg carbopol 940 without CLOT were used as a control.
Results and Discussion
The solubility of CLOT in water at 25° was found to be 5.6 μg/ml, which confirms a previously reported value [23]. On the other hand, the water solubility of clotrimazole was reported as 0.49 mg/l in different test reports [24,25]. Table 1 depict the effect of different carriers on CLOT solubility in water at 25°. In general, the solubility enhancement of CLOT obtained with various carriers followed the rank order of PVP polymers>>PEG 4000>β-CD. Analogous results have been reported with several other drugs using different water-soluble carriers, attributable to the formation of weakly soluble complexes [26,27] and/or co-solvent effect of the carrier [28]. In addition, hydrophilic carriers are known to interact with drug molecules mainly by electrostatic forces and occasionally by other types of forces like hydrogen bonds [29]. In case of PVP and PEG4000 polymers, the drug solubility increased linearly as a function of carrier concentration resulting in AL- type phase solubility diagram. It can be observed that the influence of different molecular weights of PVP upon the solubility of CLOT did not follow a definite pattern (PVP k-25>PVP k-30>PVP k-17). On the other hand, the phase solubility diagrams of CLOT-β-CD complexes can be classified as type AM with a linear relationship between solubilized CLOT and β-CD concentration with a positive curvature. Because the initial linear ascending part of the solubility diagram had a slope <1 in each case, the increase in solubility was due to the formation of a 1:1 M complex in solution with β-CD in the presence and absence of hydrophilic polymers. β-CD alone yielded a 3-fold increase in the solubility of CLOT, whereas in the presence of hydrophilic polymers it yielded a 5.5- and 3.4-fold increase with PVP and PEG, respectively. Thus the addition of PVP polymer markedly enhanced the solubilizing efficiency of β-CD. The apparent stability constant (K1:1) for the CLOT: β-cyclodextrin complex was calculated from the solubility data and found to be 42.97 M-1, which is in agreement with a previously reported value [30]. The values of the stability constant were found to be higher in the presence of hydrophilic polymers, indicating higher complexation efficiency. A 2.43 and 1.14-fold increase in the K1:1 value was observed in the presence of PVP k-17 and PEG4000, respectively. Similar results were obtained by Mura et al. [17], Chowdary and Srinivas [31] and El-Maradny et al. [32].
| Carrier | Phase | Optimum carrier | Solubility | Solubilizing |
|---|---|---|---|---|
| solubility | concentration | (µg/ml) | efficiency* | |
| diagram | (%) | |||
| PEG4000 | AL | 20 | 16.6 | 3.01 |
| PVP k-17 | AL | 20 | 76.2±0.8 | 13.6 |
| PVP k-25 | AL | 20 | 136 | 24.3 |
| PVP k-30 | AL | 20 | 97.9 | 17.5 |
| β-CD | AM | 1.6 | 16.9±0.6 | 3 |
| β-CD + 10% | AM | 1.6 | 31±0.2 | 5.5 |
| PVP17 | ||||
| β-CD + 10% | AM | 1.6 | 19 | 3.4 |
| PEG4000 | ||||
| Solubilizing efficiency* = Total solubility / intrinsic solubility | ||||
Table 1: Solubility Enhancement Data Of Clotrimazole In Various Carrier Solutions At 25°C
Evaluation of drug content revealed that, the percentage of CLOT in the samples ranged between 96 and 99% of the theoretical values. The extent of dissolution after 30 and 120 min (Q30, Q120) and the dissolution efficiency after 120 min (DE%) are presented in Tables 2-4. It can be observed that the dissolution of pure CLOT in distilled water was very low with less than 14% of the drug was dissolved within 120 min. This is could be ascribed to poor wettability and/or agglomeration or particle size [33]. Co-habitation of carriers with CLOT in physical mixtures, clearly improved the dissolution rate of the drug and this could be attributed to wettability improvement of CLOT particles [34]. Formulation of solid dispersions improved the dissolution behavior of CLOT in comparison with its corresponding physical mixtures and pure drug and this may be due to reduction of drug particle size, formation of drug-carrier solid solutions, transformation of the drug into a faster dissolving amorphous state and by a more intimate contact between the carrier and the drug [35,36]. The effect of drug: carrier ratio did not follow a definite trend. In case of PVP k-17, best dissolution results were obtained at 1:1 (drug: carrier, weight ratio), while, in case of PEG4000, the best results were obtained at 1:5 (drug: carrier, weight ratio). The dissolution results of CLOT from solid dispersions (1:1, weight ratio) containing different PVP polymers were tested for significance by using analysis of variance (ANOVA: single factor). The calculated P-value was >0.05, indicating non-significant effect of PVP molecular weight on CLOT dissolution. The dissolution of CLOT was rapid and higher from all solid inclusion complexes when compared with drug alone. This enhancement can be attributed to the formation of an inclusion complex of the drug with β-CD and/or the conversion of the drug to an amorphous state or nearly amorphous state [37]. It is clear that the Q120 and DE120 values were increased as the proportion of β-CD in the complex was decreased. About 23.5% of drug was released at 1:1 (CLOT: β-CD, molar ratio) compared to only 15.8% of drug released at 1:5 (CLOT: β-CD, molar ratio). The addition of hydrophilic polymers further enhanced the dissolution rate and efficiency of CLOT from β-CD complexes. The CLOT-β-CD (1:1) complex yielded a 1.7-fold increase in the dissolution rate of CLOT, whereas in the presence of hydrophilic polymers, it yielded a 2.4- and 2.2-fold increase with PVP k-17 and PEG4000, respectively. This is in agreement with Mura et al. [17] and Hirlekar et al. [38]. This result could be ascribed to (1) the enhancement of solubilization efficiency of β-CD by the added hydrophilic polymers, and (2) the stronger drug amorphization and better inclusion caused by the combined action of β-CD and the hydrophilic polymers [31].
| System | Q30 (%) | Q120 (%) | DE (%) |
|---|---|---|---|
| CLOT | 6.5±0.2 | 13.7±0.6 | 7.5±0.2 |
| CLOT-PVPk-17 (PM): | |||
| 1:1 | 7.2±0.2 | 20.6±1.4 | 9.9±0.2 |
| 1:5 | 8.1±0.2 | 17.6±0.1 | 10.3±0.2 |
| 1:10 | 9.6±0.2 | 18.9±0.9 | 10.8±0.9 |
| CLOT-PVPk-17 (SD): | |||
| 1:1 | 9±0.4 | 25.2±0.6 | 12.1±0.4 |
| 1:5 | 9.5 | 19.9±0.9 | 11.8±1 |
| 1:10 | 9.9±0.3 | 20±0.7 | 11.8±0.3 |
| CLOT-PVPk-25 (SD): | |||
| 1:1 | 9.4±0.4 | 27.9±0.8 | 13.6±1.4 |
| CLOT-PVPk-30 (SD): | |||
| 1:1 | 7.8±0.3 | 24.6±1.4 | 12.2±0.8 |
| SEM; Standard error of mean; CLOT; Clotrimazole; PM; Physical mixture; SD; Solid dispersion Q30; Percentage of drug dissolved after 30 min; Q120; Percentage of drug dissolved after 120 min and DE; Dissolution efficiency |
|||
Table 2: Dissolution Parameters (± Sem) Of Clotrimazole From Different Clotrimazole - Polyvinypyrrolidone Physical Mixtures And Solid Dispersions In Distilled Water
| System | Q30 (%) | Q120 (%) | DE (%) |
|---|---|---|---|
| CLOT | 6.5±0.2 | 13.7±0.6 | 7.5±0.2 |
| CLOT-PEG4000 (PM): | |||
| 1:1 | 7.5±0.4 | 14.5 | 9.2 |
| 1:5 | 6.5 | 17.4±0.4 | 8.5±0.2 |
| 1:10 | 7.6 | 16.5±0.6 | 9.3±0.08 |
| CLOT - PEG4000 (SD): | |||
| 1:1 | 10.7± 0.3 | 23.2±2 | 13.1 |
| 1:5 | 11.9±0.5 | 27±0.5 | 14.2±0.1 |
| 1:10 | 7.5 | 17.5±0.9 | 10±0.1 |
| SEM; Standard error of mean; CLOT; Clotrimazole; PM; Physical mixture; SD; Solid dispersion. Q30; Percentage of drug dissolved after 30 min; Q120;Percentage of drug dissolved after 120 min and DE; Dissolution efficiency | |||
Table 3: Dissolution Parameters (±Sem) Of Clotrimazole From Different Clotrimazole– Polyethylene Glycol4000 Physical Mixtures And Solid Dispersions In Distilled Water
| System | Q30 (%) | Q120 (%) | DE (%) |
|---|---|---|---|
| CLOT | 6.5±0.2 | 13.7±0.6 | 7.5±0.2 |
| CLOT-β-CD (PM): | |||
| 1:1 | 7.2±0.3 | 17.5±0.2 | 8±0.3 |
| 1:2.5 | 8.6 | 15.5±0.8 | 10.3±0.7 |
| 1:5 | 6.2 | 15.2±1.3 | 7.8 |
| CLOT-β-CD (IC): | |||
| 1:1 | 8.9 | 23.5±1.3 | 12.2±1 |
| 1:1/10% PVP k-17 | 9.5±0.5 | 33.2 | 16.9±1.5 |
| 1:1 / 10% PEG 4000 | 9.7±0.3 | 30 | 13.5 |
| 1:2.5 | 10 | 18.2±0.5 | 10.6±0.2 |
| 1:2.5/ 10% PVP k-17 | 10.6±0.4 | 26.3±0.6 | 13.9±0.3 |
| 1:2.5 / 10% PEG 4000 | 7.6±0.2 | 17.8±0.6 | 10±0.7 |
| 1:5 | 7.7±0.2 | 15.8±1.3 | 9.9±0.1 |
| 1:5 / 10% PVP k-17 | 11.7±0.14 | 23.6±0.6 | 12.8±1.5 |
| 1:5/ 10% PEG4000 | 10.5 | 16.9±0.8 | 11 |
| SEM; Standard error of mean; CLOT; Clotrimazole; PM; Physical mixture; SD; Solid dispersion, Q30; Percentage of drug dissolved after 30 min; Q120; percentage of drug dissolved after 120 min and DE; Dissolution efficiency | |||
Table 4: Dissolution Parameters (± Sem) Of Clotrimazole From Different Clotrimazole-β-Cycodextrin Physical Mixtures And Inclusion Complexes In Distilled Water
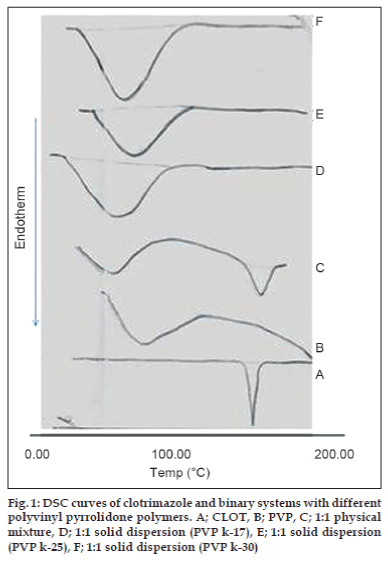
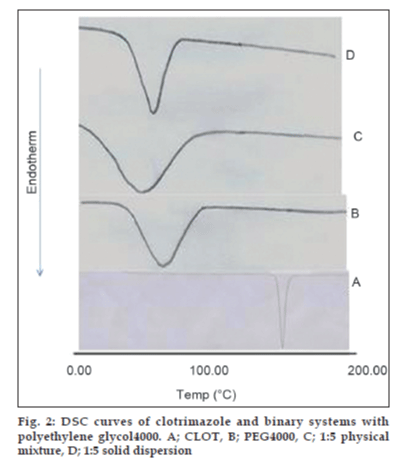
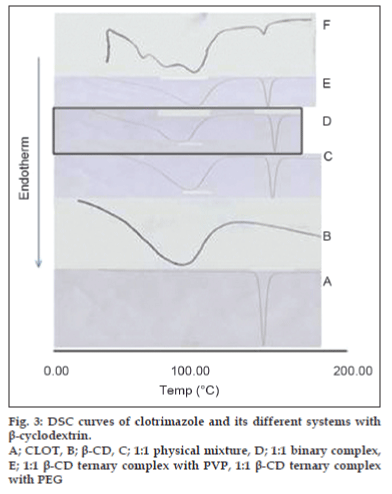
The thermal behavior of CLOT solid products demonstrated the presence of intense solid-state interactions between CLOT and the tested carriers (PVPs, PEG4000 and β-CD). DSC curves of the pure drug, PVP polymers, 1:1 weight ratio physical mixture and 1:1 weight ratio solid dispersion are shown in (fig. 1). DSC curve of CLOT shows one characteristic sharp endothermic peak at around 145° (ΔH = -77.31 J g-1) indicating the melting point of the drug. DSC curve of PVP k-17 shows a large endotherm over the temperature range 50 to 100° (ΔH = -164.57 J g-1) associated with water loss. The thermogram of 1:1 physical mixture shows superposition of its parent products endotherms with reduction in their intensity. This indicated a partial loss of crystallinity within the PVP matrix. DSC curves of CLOT solid dispersions with different polyvinyl-pyrrolidone polymers at 1:1 weight ratio show the absence of CLOT melting peak but one endothermic peak is present at 69.69° with ΔH of -170.34 to -204.83 J/g. The absence of a CLOT melting peak in SD suggested that CLOT was completely soluble in the liquid phase of the polymer or the absence of a crystalline form of CLOT [39]. DSC curves of the pure drug, PEG4000, 1:5 weight ratio physical mixture and 1:5 weight ratio solid dispersion are shown in (fig. 2). DSC curve of PEG4000 shows a single endothermic peak at 60.96° corresponding to its melting. Thermograms of both PM and SD show absence of CLOT melting peak but a single endothermic peak corresponding to fusion of the carrier. The absence of CLOT melting peak in SD suggested that CLOT was completely soluble in the liquid phase of the polymer or the absence of a crystalline form of CLOT. Absence of an endothermic peak of different drugs in polyethylene glycol SDs had also been reported by other researchers [40,41]. DSC curves of the pure drug; β-CD, 1:1 molar physical mixture and 1:1 molar inclusion complex prepared with and without hydrophilic polymers are shown in (fig. 3). DSC curve of β-CD shows a broad endothermic peak at 90.73° (ΔH = -287.2 J g-1), which is related to the exit of adsorbed water. DSC curve of the 1:1 molar ratio physical mixture shows two broad endothermic peaks, one broad peak at 144.8° (ΔH = -25.56 J g-1) which is due to fusion of clotrimazole and another broad peak at 89.4° corresponding to β-CD (ΔH = -210.4 J g-1). DSC curve of the 1:1 molar ratio inclusion complex shows two broad endothermic peaks, one broad peak at 144.9° (ΔH = -23.01 J g-1) corresponding to CLOT and another broad peak at 83.12° (ΔH = -80.7 J g-1) corresponding to β-CD. The reduction of clotrimazole ΔH values in both inclusion complex and physical mixture compared to the pure drug could be attributed to solid–solid transition from the crystalline form to the amorphous form of the drug. With the CLOT-β-CD ternary systems, the endothermic peak at 144.8° was markedly reduced, indicating the absence of crystalline drug and its complete complexation with β-CD. Similar results were reported by Loftsson [42] and Ribeiro et al. [43].
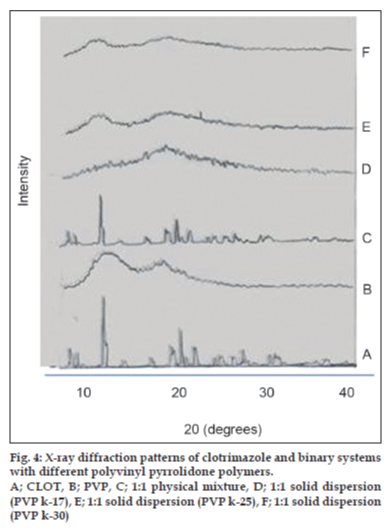
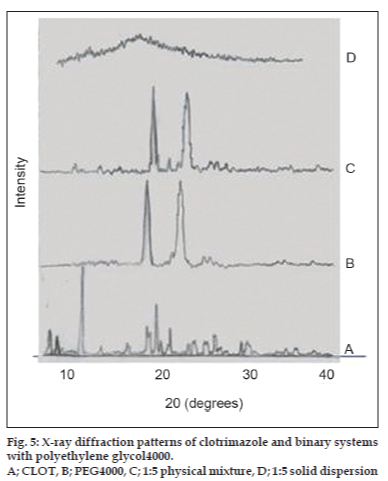
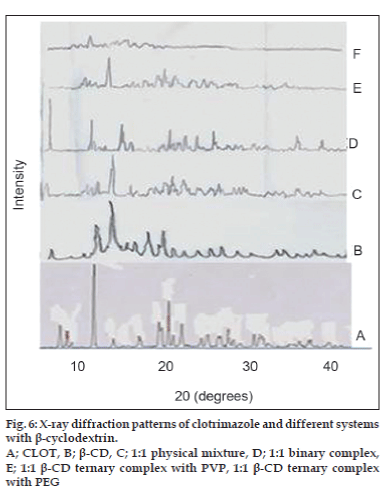
X-ray powder diffractometry patterns of CLOT, PVP k-17; 1:1 weight ratio physical mixture and 1:1 weight ratio solid dispersions with different PVP polymers are shown in (fig. 4). The diffraction spectrum of pure CLOT shows that the drug was crystalline in nature as demonstrated by numerous peaks at 12°, 19°, 19.5° and 22°. The amorphizing power of PVP toward the drug was clear in its solid dispersions with different PVP polymers, since no crystalline CLOT was detected. However, the simple blending with PVP caused a partial decrease in CLOT crystallinity. Characteristic peaks of PEG 4000 (fig. 5) appeared at 2θ equal to 19.24° and 23.32°. The principle peaks from PEG4000 were present in 1:5 PM, while, there was a marked decrease in the intensity of CLOT peaks. On the other hand, the peaks of CLOT disappeared completely at 1:5 SD. It could be attributed to the destruction of its crystal lattice, because of melting of drug into carrier. The peaks associated to the carrier (PEG4000) were converted into one broad peak. This suggested the formation of an insertion-type solid where drug molecules found place inside the structure of the carrier with a deformation of the original crystal lattice. X-ray diffractometry patterns of CLOT and its various complexes with β-CD are shown in (fig. 6). The Diffraction pattern of 1:1 molar ratio physical mixture showed superposition of the spectra of each component with marked reduction in intensity compared to the pure drug. The diffraction pattern of 1:1 molar ratio inclusion complex showed superposition of the spectra of individual components with the appearance of new peaks at 5°, 18°, 32° and 35° reflecting CLOT-β-CD complex formation. The diffraction peaks were much reduced or completely disaapeared in ternary β-CD complexes. The broadening of some CLOT crystalline peaks and disappearance of others confirmed the stronger drug amorphization and entrapment in β-CD due to the combined action of β-CD and the hydrophilic polymers.
The physical parameters of the prepared CLOT suppositories are shown in Table 5. The weight variation and content uniformity of the prepared suppositories complied with British Pharmacopoeia (within ±5%). The softening times of suppocire/ carbopol940 suppositories were high (4.5–5.5 h) and this behavior could be due to the formation of three-dimensional net in the semisynthetic triglyceride matrix or to an increase of the viscosity [20]. The mean extent and rate of release of CLOT from different suppocire AM/carbopol suppositories are shown in Table 6. The in vitro release of CLOT from its solid dispersions or inclusion complexes incorporated suppositories was significantly improved when compared to the intact drug incorporated suppositories. The percentage of drug released after 6h was found to be 27% for CLOT- PVP k-25 incorporated suppositories compared to only 4.5% of drug released from suppositories containing drug alone (6-fold increase). This enhancement of drug release may be attributed to the change in CLOT crystallinity as illustrated by DSC and XRPD studies which increased its wettability and thereby increased its dissolution rate. Gowthamarajan et al. [3], found that meloxicam-β-cyclodextrin complexes showed enhanced release from the polyethylene glycol suppository base. The mathematical evaluation of the in vitro release of the drug was done by using zero, first and diffusion models. The highest values of the correlation coefficients were obtained with the zero-order kinetic. Table 6 shows the anticandida activity of CLOT solid dispersions with different PVP polymers or β-cyclodextrin complexes incorporated in suppocire AM/carbopol940 suppository base. It was clear that the tested discs containing clotrimazole solid dispersions with different PVPs showed the largest inhibition zone size (43–45 mm) among all the discs tested. Comparing the inhibition zone size revealed that the effect of clotrimazole solid dispersions with different PVPs incorporated in suppocire AM/carbopol940 was about two times the size exhibited by the pure drug in the same base. This can be attributed to the enhanced dissolution rate of these solid dispersions as compared to the drug alone. The anticandida activity results coincide with the in vitro release results shown in Table 6.
| Formulation | Characteristic | ||
|---|---|---|---|
| Weight (g) | Content | softening | |
| uniformity (%) | time (min) | ||
| Suppocire/50 mg | |||
| carbopol base | |||
| containing: | |||
| CLOT | 1.94±0.018 | 98.08±0.45 | 261.2±1.8 |
| CLOT-PVP k-25 | 2.107±0.019 | 98.4±0.8 | 338.3±2.4 |
| CLOT-PVP k-30 | 2.119±0.015 | 98.2±2.3 | 337.4±2.8 |
| CLOT-β-CD/PEG | 2.065±0.007 | 100.42±0.62 | 318.9±2.3 |
| CLOT-&beta-CD/PVP | 2.111±0.010 | 101.9±0.51 | 302.6±1.6 |
| Valuses are express as Mean±SD, SD; Standard deviation | |||
Table 5: Weight Variation, Softening Time And Content Uniformity Of Clotrimazole Suppositories (N = 20)
| Formulation | Release rate | Extent of | Size of |
|---|---|---|---|
| technique | constant | release | inhibition |
| (mg/min) | (%) | zone (mm) | |
| CLOT alone | 0.0126 | 4.5 | 20 |
| Drug/PVP k-25 | 0.0569±0.07 | 27±1.8 | 45 |
| (1:1) | |||
| Drug/PVP k-30 | 0.0526±0.062 | 25.4±0.6 | 43 |
| (1:1) | |||
| Drug/β- | 0.049±0.053 | 22.6±0.9 | 33 |
| CD/10%PEG (1:1) | |||
| Drug/β- | 0.0396±0.051 | 16.4±0.9 | 25 |
| CD/10%PVP (1:1) | |||
| SEM; Standard error of mean | |||
Table 6: Effect Of Clotrimazole Solid Dispersions And Inclusion Complexes On Its Mean Extent And Rate Of Release (± Sem) And Anticandidal Activity From Suppocire Am/ Crabopol940 Suppository Base
References
- Ross RA, Lee MT, Onderdonk AB. Effect of Candida albicansinfection and clotrimazole treatment on vaginal microflorain vitro.ObstetGynecol 1995;86:925-30.
- Realdon N, Ragazzi E, Ragazzi E. Effect of drug solubility on in vitro availability rate from suppositories with polyethylene glycol excipients.Pharmazie 2001;56:163-7.
- Gowthamarajan K, Girirajkulkarni T, Venkateswaran G, Samanta MK, Suresh B. Formulation and dissolution properties of meloxicam solid dispersion incorporated suppositories. Indian J Pharm Sci 2002;64:525-8.
- Samy EM, Hassan MA, Tous SS, Rhodes CT. Improvement of availability of allopurinol from pharmaceutical dosage forms I – suppositories. Eur J Pharm Biopharm 2000;49:119-27.
- Usayapant A, Iyer BR. The effect of 2-hydropropyl-betacyclodextrin on in vitro drug release of steroids from suppositories. Drug Develop Ind Pharm 1999;25:390-7.
- Oribe T, Yamada M, Takeuchi K, Tsunemi S, Imasaka K, Shirakura O, et al. Formulation and in vivo-in vitro correlation of the dissolution property of lemildipine solid dispersions-incorporated suppositories. Int J Pharm 1995;124:27-35.
- Pedersen M. Effect of hydrotropic substances on the complexation of clotrimazole with b-cyclodextrin. Drug Develop Ind Pharm 1993;19:439-48.
- Pedersen M, Bjerregaard S, Jacobsen J, Sørensen AM. A genuine clotrimazole β-cyclodextrin inclusion complex -isolation, antimycotic activity, toxicity and an unusual dissolution rate. Int J Pharm 1998;176:121-31.
- Abdel-Moety EM, Khattab FI, Kelani KM, Abu Al-Alamein AM. Chromatographic determination of clotrimazole, ketoconazole and fluconazole in pharmaceutical formulations. Farmaco 2002;57:931-8.
- Ning M, Gu Z, Pan H, Yu H, Xiao K. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antifungal drugclotrimazole. Indian J ExpBiol 2005;43:150-57.
- Memisoglu E, Bochot A, Ozalp M, Sen M, Duchene D, Hincal AA. Direct formation of nanospheres from amphiphilic beta-cyclodextrin inclusion complexes. Pharm Res 2003;20:117-25.
- Bilensoy E, Rouf MA, Vural I, Sen M, Hincal AA. Mucoadhesive, thermosensitive, prolonged-release vaginal gel for clotrimazole:β-cyclodextrin complex. AAPS PharmSciTech 2006;7:Article 38.
- Ahmed MO, El-Gibaly I, Ahmed SM. Effect of cyclodextrins on the physicochemical properties and antimycotic activity of clotrimazole. Int J Pharm 1998;171:111-21.
- Taneri F, Guneri T, Aigner Z, Erös I, Kata M. Improvement of the physicochemical properties of clotrimazole by cyclodextrincomplexation. J InclPhenom Macro Chem 2003;46:1-13.
- Madhusudhan B, Rambhan D, Gudsoorkar VR, Shete JS, Apte SS. Development and Evaluation of antifungal activity of O/W type Creams Containing solid dispersion of Clotrimazole. Indian J Pharm Sci 1999;61:346-9.
- Higuchi T, Connors KA. Phase solubility techniques. Adv Anal ChemInstrum 1965;4:207-12.
- Mura P, Faucci MT, Bettinetti GP. The influence of polyvinylpyrrolidone on naproxen complexation with hydroxypropylbeta-cyclodextrin. Eur J Pharm Sci 2001;13:187-94.
- Suhagia B, Patel HM, Shah SA, Rathod I, Parmar VK. Preparation and characterization of etoricoxib-polyethylene glycol 4000 plus polyvinylpyrrolidone K30 solid dispersions. Acta Pharm 2006;56:19.
- Khan KA, Rhodes CD. The concept of dissolution efficiency. J Pharm Pharmacol 1975;27:48-9.
- Ceschel GC, Maffei P, Lombardi Borgia S, Ronchi C, Rossi S. Development of a mucoadhesive dosage form for vaginal administration. Drug Develop Ind Pharm 2001;27:541-7.
- Babar A, Bellete T, Plakogiannis FM. Ketoprofen suppository dosage forms: in vitro release and in vivo absorption studies in rabbits. Drug Develop Ind Pharm 1999;25:241-5.
- National Committee for Clinical Laboratory standards (Approved standard M2-A3). Performance standards for anti-microbial disk susceptibility tests. NCCLS, Villanova; 1984.
- Coshh Safety Data Sheet: Vétoquinol Co., UK; 2002.
- Bayer AG. Bruns. Clotrimazol: Acute fish toxicity. Leverkusen. Internal report; 2003, 1253A/03F.
- Bayer AG. Bruns. Clotrimazol: Fish, Juvenile Growth Test. Leverkusen, Germany. Internal report. 2003, 1253 A/03 FF.
- Seedher N, Bhatia S. Solubility enhancement of COX-2 inhibitors using various solvent systems. AAPS PharmSciTech 2003;4:E33.
- Cirri M, Mura P, Rabasco AM, Ginés JM, Moyano JR, Gònzalez-Rodrìguez ML. Characterization of ibuproxam binary and ternary dispersions with hydrophilic carriers. Drug Develop Ind Pharm 2004;30:65-74.
- Chiou WL. Pharmaceutical applications of solid dispersions systems: X-ray diffraction and aqueous solubility studies on griseofulvin polyethylene glycol 6000 systems. J Pharm Sci 1977;66:989-91.
- Rácz I. Physicochemical interactions encountered in the course of drug product preparation, in: Drug Formulation, New York: Wiley J and Sons; 1989. p. 212-42.
- Prabagar B, Yoo B, Woo J, Kim J, Rhee J, Piao MG, et al. Enhanced bioavailability of poorly water-soluble clotrimazole by inclusion with β-cyclodextrin. Arch Pharm Res 2007;30:249-54.
- Chowdary KP, Srinivas SV. Influence of hydrophilic polymers on celecoxibcomplexation with hydroxypropyl-beta-cyclodextrin. AAPS PharmSciTech 2006;7:79.
- El-maradny HA, Mortada SA, Kamel OA, Hika AH. Characterization of ternary complexes of meloxicam-HP_CD and PVP or L-arginine prepared by the spray drying technique. Acta Pharm 2008;58:455-66.
- Prajapati S, Gohel MC, Patel LD. Studies to enhance dissolution properties of carbamazepine. Indian J Pharm Sci 2007;69:427-30.
- Ahuja N, Katare OP, Singh B. Studies on dissolution enhancement and mathematical modelling of drug release of a poorly water-soluble drug using water soluble carriers. Eur J Pharm Biopharm 2007;65:26-38.
- Nokhodchi A, Talari R, Valizadeh H, BarzegarMJ . An investigation on the solid dispersions of chlordiazepoxide. Int J Biomed Sci 2007;3:211-7.
- Rupal J, Kaushal J, Mallikarjuna SC, Dipti P. Preparation and evaluation of solid dispersions of aceclofenac. Int J Pharm Sci Drug Res 2009;1:32-5.
- Jadhav GS, Vavia PR, Nandedkar TD. Danazol-beta-cyclodextrin binary system: A potential application in emergency contraception by the oral route. AAPS PharmSciTech 2007;8:Article 35.
- Hirlekar RS, Sonawane SN, Kadam VJ. Studies on the effect of watersoluble polymers on drug–cyclodextrin complex solubility. AAPS PharmSciTech 2009;10:858-63.
- Biswal S, Sahoo J, Murthy PN. Characterisation of gliclazide-PEG 8000 solid dispersions. Trop J Pharm Res 2009;8:417-24.
- Damian F, Blaton N, Naesens L, Balzarini J, Kinget R, Augustinjns P, et al. Physicochemical characterization of solid dispersions of the antiviral agent UC-781 with polyethylene glycol 6000 and Gelucire 44/14. Eur J Pharm Sci 2000;10:311-22.
- Yamashita K, Nakate T, Okimoto K, Ohike A, Tokunaga Y, Ibuki R, et al. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int J Pharm 2003;267:79-91.
- Loftsson T. Increasing the cyclodextrincomplexation of drugs and drug bioavailability through addition of water soluble polymers. Pharmazie 1998;53:733-40.
- Ribeiro L, Loftsson T, Ferreira D, Veiga F. Investigation and physicochemical characterization of vinpocetine-sulfobutyl ether beta cyclodextrin binary and ternary complexes. Chem Pharm Bull 2003;51:914-22.