- *Corresponding Author:
- S. N. Hiremath
Department of Pharmaceutics, H. K. E. Society’s College of Pharmacy, Sedam Road, Gulbarga - 585 105, India.
E-mail: snhiremath@rediffmail.com
| Date of Submission | 8 February 2006 |
| Date of Revision | 8 November 2006 |
| Date of Acceptance | 3 June 2007 |
| Indian J. Pharm. Sci., 2007, 69 (3): 442-445 |
Abstract
Valdecoxib is a hydrophobic molecule that is practically insoluble in aqueous media and exhibits an exceedingly slow intrinsic dissolution rate. The present study was emphasized on improving the solubility and dissolution rate of drug by forming inclusion complex with hydroxypropyl-β -cyclodextrin. The inclusion complexes were prepared by physical mixture, kneading and common solvent methods. Phase solubility studies indicated the formation of a 1:1 M complex in solution. Drug excipient interactions were characterized using Fourier transformed infrared spectroscopy and differential scanning calorimetry studies. Differential scanning calorimetry studies indicated the formation of solid inclusion complex of valdecoxib hydroxypropyl-β -cyclodextrin at 1:2 M ratio in kneading and common solvent method. Solid inclusion complexes of valdecoxib hydroxypropyl-β -cyclodextrin (1:2 M) prepared by kneading and common solvent method exhibited higher rates of dissolution and dissolution efficiency values both in 0.1N HCl and 0.1N HCl with 0.25% sodiumlauryl sulphate.
Natural cyclodextrins (CDs) are cyclic oligosaccharides containing six (α-cyclodextrin), seven (β-cyclodextrins) or eight (γ-cyclodextrin), α-1,4-linked glycopyranose units with hydrophilic outer surface and a hydrophobic cavity. CDs are capable of forming inclusion complexes with many drugs by taking up a whole drug molecule (guest) or part of it, into the CDs cavity. Such molecular encapsulation will affect many of the physicochemical properties of drugs such as their aqueous solubility and rate of dissolution. Among the various approaches, preparation of inclusion complexes with cyclodextrin has proven to be successful in enhancing the solubility of poorly water soluble drugs [1,2]. The inclusion complexes have been shown to improve stability [3,4], solubility, dissolution rate [5,6], and bioavailability [7,8]. They also can reduce side effects associated with some drugs [9,10]. Hydroxypropyl- β-cyclodextrin (HPBCD) was specifically chosen because of its high aqueous solubility, which is nearly 30 times greater than that of the parent β-cyclodextrin. It is used for oral applications because it is very well tolerated [11,12].
Valdecoxib (Val) [13] is chemically a diaryl substituted isoxazole i.e., 4-(5-methyl-3-phenyl-4-isoxazolyl)- benzene sulfonamide, which is a relatively new nonsteroidal antiinflammatory analgesic drug. It is used in the treatment of rheumatoid arthritis, osteoarthritis, in the management of acute pain and dysmenorrhea. The drug like Val is practically insoluble in water. This limits several advantages of the drug with respect to its absorption, distribution and therapeutic efficacy. The aim of this study was to improve the solubility and dissolution rate of Val in aqueous solution and thereby improve its bioavailability. This was attained through the formation of inclusion complexes with HPBCD. The complexes were prepared by physical mixture, kneading and common solvent methods. The phase solubility studies, FTIR spectral studies, DSC and in vitro dissolution were used to characterize the complexes in the solid state.
Valdecoxib was a gift sample from Glenmark Pharmaceuticals, Mumbai and hydroxypropyl-β- cyclodextrin was procured from S. A. Pharma Chem. Pvt. Ltd., Mumbai. Methanol, sodiumlauryl sulphate and hydrochloric acid were all obtained from Qualigens.
Phase solubility studies were performed according to the method reported by Higuchi and Connors [14]. Excess Val (50 mg) was added to 25 ml portions of distilled water, each containing various concentrations of hydroxypropyl-β-cyclodextrins (HPBCD) (i.e., 0.0025-0.0150 M) taken in a series of 25 ml stoppered conical flasks and the mixtures were shaken for 72 h at room temperature (28º) on a rotary flask shaker. After 72 h of shaking to achieve equilibrium, 5 ml aliquots were withdrawn at 1 h interval and filtered immediately using a 0.45 µ nylon filter. The filtered samples were diluted suitably and assayed for Val by measuring absorbance at 243.5 nm against blank prepared in the same concentrations of HPBCD in water so as to cancel any absorbance that may be exhibited by the HPBCD molecules. Shaking was continued until three consecutive estimates are the same. The solubility experiments were conducted in triplicate.
The solid complexes of Val and HPBCD were prepared in 1:1 M and 1:2 M by three methods, physical mixture, kneading and common solvent methods [15,16]. Physical mixtures of Val and HPBCD in the different molar ratios (1:1 M and 1:2 M) were mixed in a mortar for about one hour with constant trituration, passed through sieve no. 100 and stored in dessicator over fused calcium chloride. In kneading method Val and HPBCD in different molar ratios (1:1 M and 1:2 M) were taken. HPBCD was added to the mortar, small quantity of 50% ethanol was added while triturating to get slurry like consistency. Then slowly drug was incorporated into the slurry and trituration was further continued for 1 h. Slurry was further air dried at 45º for 24 h, pulverized and passed through sieve No. 100 and stored in dessicator over fused calcium chloride. In common solvent method Val and HPBCD in different molar ratios (1:1 M and 1:2 M) were dissolved in ethanol to get a clear solution. The resulting solution was stirred at ambient temperature until complete evaporation of the solvent occurred. The resulting preparations were kept in a dessicator for the least 48 h and then grounded in glass mortar for size reduction and passed through sieve No. 100 and stored in a dessicator.
Characterization of prepared complexes was done by Fourier transformed infrared (FTIR), DSC and in vitro dissolution studies. The FTIR spectra of Val and inclusion complexes prepared by kneading and common solvent methods by different molar ratios were recorded in KBr pellets using a Jasco FT/IR- 5300 (Tokyo, Japan). Differential scanning calorimeter (DSC) model DSC-220 C, Seiko, Japan was used. The samples were sealed in aluminum pans and the DSC thermogram were recorded at a heating rate of 10º/min from 50º-300º. In vitro dissolution studies of pure drug, commercial formulations, physical mixtures, inclusion complexes prepared by kneading and common solvent methods were carried out in 900 ml of (i) 0.1N HCl and (ii) 0.1N HCl with 0.25% SLS using USP XXIII 6-station dissolution rate test apparatus (Electro-Lab) with a paddle stirrer. Sample equivalent to 20 mg of Val, a speed of 50 rpm and a temperature of 37±1º were used in each test. Samples were withdrawn at different time intervals, filtered using a 0.45 µ nylon disc filter and assayed for Val by measuring absorbance at 243.5 nm. The dissolution experiments were conducted in triplicate.
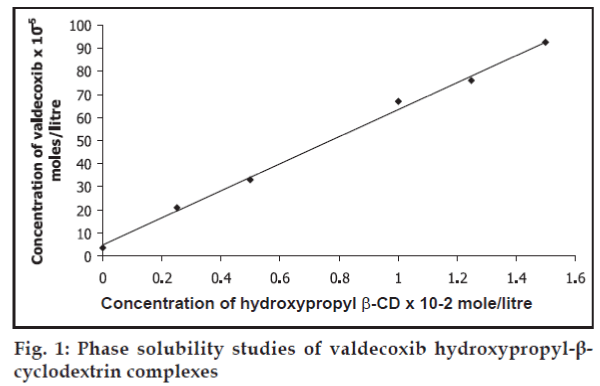
The phase solubility diagram for the complex formation between Val and HPBCD is shown in fig. 1. The aqueous solubility of Val increased linearly (r=0.9973) as a function of HPBCD concentration. The phase solubility diagram (fig. 1) can be classified as type AL according to Higuchi and Connors14. Because the straight line had a slope less than unity, it was due to the formation of a 1:1 M complex. The apparent 1:1 M stability constant, KC was calculated from the linear plot of the phase solubility diagram according to the equation, KC= slope/S0(1-slope), where S0 is the solubility of Val in the absence of HPBCD. The stability constant KC of Val-HPBCD 1:1 M complex was found to be 1.634219 M-1.
FTIR studies of Val exhibited peak at 3377.20 cm–1 and 3250.10 cm-1 due to N–H stretching of sulphonamide. Peaks at 1333.54 cm-1 and 1241.59 cm–1 are antisymmetric and symmetric bands due to S=O stretching which confirm the structure of Val. The IR spectrum of Val-HPBCD (1:2 M) complex prepared by kneading exhibited peak at 3378.00 cm-1 is due to N–H stretching of sulphonamide group and peak at 1334.12 cm-1 is due to S=O stretching confirm the undisturbed drug in the formulation. However, shift in peaks in IR spectrum of formulation indicates the interaction between drug and base. In case of Val-HPBCD complex 1:2 M prepared by co-solvent method exhibited peaks at 3410.11 cm-1 is due to N–H stretching of sulphonamide group instead of 3377.70 cm-1, which is observed in the IR spectrum of pure Val and shift in peaks due to S=O stretching at 1335.03 cm-1 and 1244.18 cm-1 (instead of 1333.84 cm-1and 1241.59 cm-1) indicates the interaction between drug and cyclodextrin.
The thermal behaviour of Val-HPBCD inclusion complexes was studied using DSC in order to confirm the formation of the solid complexes. The DSC thermogram of Val exhibited an endothermic peak at 175.571º corresponding to its melting point. HPBCD showed a broad endothermic peak at 169.136º which may be attributed to a dehydration process. The thermograms of Val-HPBCD (1:1 M) system showed the persistence of the endothermic peak of Val at 175º. The result indicated that the inclusion complex has not formed at 1:1 M ratio in solid state. In case of Val-HPBCD (1:2 M) physical mixture the endothermic peak of Val shifted to a low temperature, 163.3º indicating a weak interaction. The DSC thermograms of Val-HPBCD (1:2M) systems prepared by both kneading and common solvent method did not show the melting endotherm of Val. The disappearance of endotherm peak with these systems indicated the formation of a solid inclusion complexes of Val- HPBCD at 1:2 M ratio.
The dissolution characteristics of Val, Val-HPBCD systems and commercial formulations are given in Tables 1 and 2. The dissolution data were evaluated on the basis of (i) dissolution efficiency (DE) [17] and (ii) first order dissolution rate constant, K1 min-1. The correlation coefficient (r) between log percent undissolved and time was in the range of 0.9362 to 0.9983 for various products. In both fluids (i.e., 0.1 N HCl and 0.1 N HCl with 0.25% SLS), the dissolution rate (K1) and dissolution efficiency were higher for Val-HPBCD solid complexes prepared by kneading and common solvent methods of 1:2 M ratio when compared to pure drug, commercial formulations and corresponding physical mixtures. A marked improvement in the dissolution rates of Val was observed with Val-HPBCD complexes prepared by kneading and common solvent methods of 1:2 M ratio. A 21.6 and 28.43 fold increase in dissolution rate in 0.1 N HCl and 67.54 and 80.8 fold increase in 0.1 N HCl with 0.25% SLS was observed respectively with Val-HPBCD 1:2 M complex prepared by kneading and common solvent method. The higher dissolution rates observed with complex prepared by kneading and common solvent method of 1:2 M ratio may be due to the better interaction of drug and HPBCD. The significant improvement in dissolution characters of inclusion complexes may be due to formation of readily soluble inclusion complex in the dissolution medium, increased drug particle wettability and reduction of crystallinity degree of the product.
| Product | Mean percent drug dissolved at different time interval (min) | DE30 min(%) | K1×10–2 (min–1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | 150 | 180 | ||||
| Valdecoxib | 16.36 | 26.1 | 32.72 | 34 | 36.58 | 42.46 | 26.46 | 0.237 | |
| (0.43) | (0.49) | (0.76) |
(0.64) | (0.2) | (0.25) | ||||
| Val-HPBCD (1:1M) PM | 37.9 | 54.3 | 79.59 | 100.37 | 35.66 | 2.08 | |||
| (0.43) | (0.71) | (0.47) | (0.5) |
||||||
| Val-HPBCD (1:1M) KC | 47.1 | 78 | 100.47 | 42.31 | 3.82 | ||||
| (0.35) | (0.54) | (0.65) | |||||||
| Val-HPBCD (1:1M) CSM | 56.5 | 81.89 | 100.25 | 43.4 | 3.547 | ||||
| (0.74) | (0.57) |
(0.45) | |||||||
| Val-HPBCD (1:2M) PM | 47.84 | 69.85 | 94.75 | 61.5 | 2.53 | ||||
| (0.65) | (0.43) | (0.4) | |||||||
| Val-HPBCD (1:2M) KC | 70 | 100.95 | 64.37 | 4.97 | |||||
| (0.65) | (0.83) | ||||||||
| Val-HPBCD (1:2M) CSM | 85.95 | 100.85 | 66.45 | 6.536 | |||||
| (0.75) | (0.65) | ||||||||
| Marketed | 87.3 | 100.15 | 34.65 | 2.51 | |||||
| product1 | (0.52) | (0.94) | 100.25 | 30.46 | 2.23 | ||||
| Marketed | 29.65 | 68.8 | |||||||
| products2 | (0.46) | (0.75) | (0.5) | ||||||
Table 1: Dissolution characteristics of valdecoxib and its various inclusion complexes with hpbcd in 0.1n hcl
| Product | Mean percent valdecoxib dissolved at different time intervals (min) | DE30 min (%) | K1×10-2 (min-1) | ||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | |||
| Valdecoxib | 45.2 | 56.18 | 64.70 | 66.51 | 69.20 | 35.83 | 2.85 |
| (0.65) | (0.35) | (0.51) | (0.85) | (0.77) | |||
| Val-HPBCD (1:1M) PM | 70.8 | 79.15 | 94.30 | 100.25 | 74.50 | 64.0 | |
| (0.35) | (0.49) | (0.68) | (0.35) | ||||
| Val-HPBCD (1:1M) KC | 83.45 | 91.64 | 100.35 | 76.25 | 97.45 | ||
| (0.42) | (0.25) | (0.64) | |||||
| Val-HPBCD (1:1M) CSM | 84.10 | 93.44 | 101.10 | 77.42 | 103.85 | ||
| (0.42) | (0.57) | (0.66) | |||||
| Val-HPBCD (1:2M) PM | 81.48 | 95.37 | 100.45 | 80.56 | 154.70 | ||
| (0.56) | (0.54) | (0.58) | |||||
| Val-HPBCD (1:2M) KC | 94.17 | 100.65 | 82.97 | 192.15 | |||
| (0.85) | (0.38) | ||||||
| Val-HPBCD (1:2M) CSM | 100.64 | 84.25 | 230.3 | ||||
| (0.47) | |||||||
| Marketed | 36.7 | 40.72 | 45.0 | 69.31 | 87.65 | 57.66 | 20.24 |
| product1 | (0.45) | (0.87) | (0.36) | (0.45) | (0.66) | ||
| Marketed | 27.45 | 30.74 | 33.05 | 39.84 | 45.80 | 49.50 | 12.32 |
| products2 | (0.72) | (0.40) | (0.64) | (0.75) | (0.42) |
Table 2: Dissolution characteristics of valdecoxib and its various inclusion complexes with hpbcd in 0.1n hcl with 0.25% sls
Thus, the results of the study indicated the formation of Val and HPBCD inclusion complexes at 1:1 M ratio in solution with a stability constant of 1.634219 M-1, whereas solid inclusion complexes of Val- HPBCD (1:2 M) prepared by kneading and common solvent method exhibited higher rates of dissolution efficiency values than the corresponding physical mixture, commercial formulations and pure drug.
Acknowledgements
The authors are grateful to Glenmark Private Limited, Mumbai for providing the gift sample of valdecoxib and S. A. Pharma Chem. Pvt. Limited, Mumbai for providing the gift sample of hydroxypropyl-β- cyclodextrin. We are also thankful to the Principal, H. K. E. Society’s College of Pharmacy for providing necessary facilities to carry out the work.
References
- Loftssan, T. and Brewster, M.E. J. Control. Release , 1996, 85, 1017. Back to cited text no. 1
- Rajewski, R.A. and Stella, V.J. J. Pharm. Sci ., 1996, 85, 1142. Back to cited text no. 2
- Andersen, F.A. and Bundgaard, H. Int. J. Pharm ., 1984, 19, 189. Back to cited text no. 3
- Loftsson, T., Bjornsdottir, S., Palsdottir, and Bodor, N. Int. J. Pharm ., 1989, 57, 63. Back to cited text no. 4
- Pitha, J., Milecki, J., Fales, H., Pannell, L. and Uekama, K . Int. J. Pharm ., 1986, 29, 73. Back to cited text no. 5
- Blanco, J., Vila-Jato, J.L., Otero, F. and Angiuana, S. Drug Develop. Ind. Pharm ., 1991, 17, 943. Back to cited text no. 6
- Chow, D. and Karara, A. Int. J. Pharma ., 1986, 28, 95. Back to cited text no. 7
- Szetti, J. and Szente, L. Pharmazie , 1981, 36, 694. Back to cited text no. 8
- Otero-Espinar, F.J., Anguiano-Igea, S., Blanco-Mendex, J. and Vila Jato, J.L. Int. J. Pharm ., 1986, 29, 73. Back to cited text no. 9
- Lin, S., Wouessidjewe, D., Pollman, M. and Duchene, D. Int. J. Pharm ., 1994, 106, 63. Back to cited text no. 10
- Brewster, M.E., Estes, K.S. and Bodor, N. Int. J. Pharm ., 1990, 59, 231. Back to cited text no. 11
- Veiga, F., Teixeira-Dias, J.J.C., Kedzierewicz, F., Sousa, A. and Maincent, P. Int. J. Pharm ., 1996, 129, 63. Back to cited text no. 12
- Sweetman, S.C., Eds., In; Martindale. The Complete Drug Reference, 33rd Edn., The Pharmaceutical Press, London, 2002, 90. Back to cited text no. 13
- Higuchi, T. and Connors, K.A. Adv. Anal. Chem. Instr ., 1965, 4, 117. Back to cited text no. 14
- Chowdhary, K.P.R. and Rao, S.S. Indian J. Pharm. Sci ., 2001, 63, 438. Back to cited text no. 15
- Sherif, I., Badawy, F., Mahmoud, M., Ghorab and ChristianahAdeyeye, C.M. Int. J. Pharm ., 1996,128, 45. Back to cited text no. 16
- Khan, K.A.J. Pharm. Pharmacol , 1975, 27, 48. Back to cited text no. 17