- *Corresponding Author:
- Songhua Guo
First Affiliated Hospital of Huzhou University, Huzhou, Zhejiang Province 310003, China
E-mail: xueqiang2002@126.com
| Date of Received | 09 October 2021 |
| Date of Revision | 08 November 2022 |
| Date of Acceptance | 14 July 2023 |
| Indian J Pharm Sci 2023;85(4):936-943 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the impact of anthocyanin on apoptosis of spinal cord ischemia-reperfusion injury rats and its regulation on Toll-like receptor 4/cyclooxygenase 2/nuclear factor-kappa B signaling pathway. Sixty healthy Sprague–Dawley rats were randomly separated into sham operation group, model group, anthocyanin low (50 mg/kg), medium (100 mg/kg), high (150 mg/kg) concentration groups, anthocyanin (150 mg/kg)+TAK-242 (10 mg/kg) group, 10 per group, administered at 72 h before surgery. After the experiment, the behavioral score after spinal cord injury was performed; blood was collected to separate serum, and the contents of interleukin-6, interleukin-1 beta and tumor necrosis factor alpha were measured; the rats were sacrificed to separate spinal cord tissue, and terminal deoxynucleotidyl transferase dUTP nick-end labeling staining was used to detect cell apoptosis, the expression of B-cell lymphoma protein 2-associated X was measured by immunohistochemistry, the expression of toll-like receptor 4, cyclooxygenase 2, p-nuclear factor-kappa B and nuclear factor-kappa B proteins was measured by Western blot. Compared with the sham operation group, the behavioral score and B-cell lymphoma protein 2-associated X expression in the model group were obviously decreased (p<0.05); the apoptosis index, serum interleukin-6, interleukin-1 beta, tumor necrosis factor alpha contents, B-cell lymphoma protein expression, toll-like receptor 4, cyclooxygenase 2 protein expression and p-nuclear factor-kappa B/nuclear factor-kappa B in spinal cord tissue were obviously increased (p<0.05). Compared with the model group, the behavioral scores and B-cell lymphoma protein 2-associated X expression in the anthocyanin low, medium and high concentration groups were obviously increased (p<0.05); the apoptosis index, serum interleukin-6, interleukin-1 beta, tumor necrosis factor alpha contents, B-cell lymphoma protein 2 expression, toll-like receptor 4, cyclooxygenase 2 protein expression and p-nuclear factor-kappa B/nuclear factor-kappa B in spinal cord tissue were gradually decreased (p<0.05). Compared with the anthocyanin high concentration group, the behavioral score and B-cell lymphoma protein 2-associated X expression in the anthocyanin+TAK-242 group were obviously increased (p<0.05); the apoptosis index, serum interleukin-6, interleukin-1 beta, tumor necrosis factor alpha contents, B-cell lymphoma protein 2 expression, toll-like receptor 4, cyclooxygenase 2 protein expression and p-nuclear factor-kappa B/nuclear factor-kappa B in spinal cord tissue were obviously decreased (p<0.05). Anthocyanin can ameliorate cell apoptosis in spinal cord ischemia-reperfusion injury rats by inhibiting the expression of toll-like receptor 4/cyclooxygenase 2/nuclear factor-kappa B signaling pathway.
Keywords
Anthocyanin, spinal cord ischemia-reperfusion injury, apoptosis, toll-like receptor 4, cyclooxygenase 2, nuclear factor-kappa B
Spinal Cord Ischemia-Reperfusion Injury (SCII) is one of the common and severe complications in thoracoabdominal aortic surgery clinically[1], and the occurrence of SCII has unpredictable and irreversible characteristics, causing neuronal death in the spinal cord and leading to extreme paralysis lethality[2]. The SCII pathological mechanism is complex, and previous studies have confirmed that inflammatory response[3,4], calcium ion overload as well as apoptosis are the main pathological mechanisms involved in the development of SCII. Toll-Like Receptor 4 (TLR4)/Cyclooxygenase 2 (COX-2)/ Nuclear Factor Kappa B (NF-κB) signaling pathway is an important proinflammatory pathway in a variety of inflammatory diseases in the clinic, the aberrant activation of signaling pathways of TLR4/COX-2/ NF-κB mediates physio pathological processes such as inflammatory responses, oxidative stress as well as apoptosis and proliferation, leading to disease development[5,6]. Anthocyanin is a water-soluble flavonoid present in a variety of plants and known as a natural antioxidant[7]. Anthocyanin is clinically used in the treatment of several inflammatory diseases as well as neurological disorders because of their antimicrobial, anti-inflammatory, antioxidant as well as anti-apoptotic properties[8,9]. However, there are no studies on the therapeutic effects of anthocyanin on SCII. In this study, we investigated the effects of anthocyanin on apoptosis and the regulatory role of anthocyanin on TLR4/COX-2/NF-κB signaling pathway in SCII rats by establishing a SCII rat model.
Materials and Methods
Experimental animal:
Sixty Specific-Pathogen Free (SPF) grade healthy Sprague Dawley (SD) rats, 6 w old, half male and half female, with a body mass of 180-200 g, were provided by the Henan Experimental Animal Center with the Permit No. SCXK (Y) 2017-0001. 1 w of adaptive feeding before the experiment, housed in the SPF grade animal laboratory, maintained air circulation, room temperature at 20°-22°, and fed with standard rat chow and clean water, during which time they had free access to water and feeding. The experimental process and methods were in accordance with the requirements of Experimental Animal Ethics Committee of Luoyang Orthopedic- Traumatological Hospital of Henan Province.
Experimental drugs and reagents:
Anthocyanin (T19558, Spec. 25 g/nos., Shanghai Yuanye Bio-Technology Co., Ltd.); TLR4 inhibitor TAK-242 (GC16148, GlpBio, United Statas of America (USA)); Interleukin (IL)-6 (SEKR-0005), IL-1 beta (β) (SEKR-0002), Tumor Necrosis Factor Alpha (TNF-α) (SEKR-0009) Enzyme-linked Immunosorbent Assay (ELISA) kit and Terminal Deoxynucleotidyl Transferase dUTP Nick-end Labeling (TUNEL) staining kit (T2190) (Beijing Solarbio Science and Technology Co., Ltd.); B-Cell Lymphoma 2 (Bcl-2) antibody (26593-1-AP), BCL2 Associated X (BAX) antibody (60267-1-Ig), TLR4 antibody (19811-1-AP), COX-2 antibody (27308-1-AP) and goat anti-mouse Immunoglobulin G (IgG) (Horseradish Peroxidase (HRP), 15015) (Wuhan Proteintech Biotechnology Co., Ltd.); phosphorylated-NF-κB (p-NF-κB) antibodies (3039S), NF-κB antibodies (8242S), β-actin antibodies (4970S), goat anti rabbit IgG (HRP, 7074S) (Central Standard Time (CST), USA).
Experimental instrumentation:
Olympus model BX53 bio microscope (Olympus Corporation, Japan) and E-gel Imager gel imaging system (Thermo Fisher, USA).
Method:
Model building and group administration: Sixty healthy SD rats were randomly divided into sham operation group, model group, anthocyanin low, medium and high concentration groups, and anthocyanin+TAK-242 group (n=10 each). Administration 72 h prior to surgery, and 50 mg/ kg, 100 mg/kg, 150 mg/kg anthocyanin were administered by gavage in the anthocyanin low, medium and high concentration groups, respectively[10]; anthocyanin+TAK-242 group was treated with 150 mg/kg cyanidin by gavage and 10 mg/kg TAK-242 by tail vein injection[11], twice daily, fasting 8 h before operation and water restriction 4 h after operation.
In this experiment, a modified Zivin method[12] was used to establish the SCII model; after the abdominal skin preparation and routine iodophor disinfection, an incision of about 3 cm small mouth was made in the middle of the abdomen, after entering the abdominal cavity, the intestinal tube was pushed to the right side, and after confirming the left renal artery and abdominal aorta, the abdominal aorta was exposed by blunt separation; at 0.5 cm-1 below the origin of the left renal artery, the abdominal aorta was completely clamped and 40 min later reopened to restore perfusion. During surgery, care was taken to protect other organs. Sodium heparin 150 U/kg via the ear edge vein 5 min before clipping. After the surgery, check the peritoneal cavity without oozing blood after the intraperitoneal injection of penicillin, use metronidazole to flush the abdominal cavity, and suture the abdominal incision layer by layer.
Behavioral scoring after SCII in rats: Refer to modified Tarlov scoring criteria after the experiment; as shown in Table 1[13]. Behavioral scoring after SCI was performed by three fixed observers who were completely blinded to the grouping, and the average of the three scores was taken as the final scoring result, from which the motor function was judged.
| Item | Standard scores (points) |
|---|---|
| The hindlimbs were completely paralyzed with no perceptible hindlimb activity. | 0 |
| The hindlimbs moved slightly but were unable to move against gravity. | 1 |
| The hindlimbs were movable but unable to move against gravity. | 2 |
| Able to walk or jump, but with significant ataxia. | 3 |
| Hindlimb gait was normal and able to jump normally. | 4 |
Table 1: Modified Tarlov Scoring Criteria
Inflammatory cytokines IL-6, IL-1 β and TNF-α content in serum by ELISA: At the end of scoring, approximately 2 ml of jugular venous blood was obtained from anesthetized rats, and after centrifugation, the serum was isolated and the serum was assayed for IL-6, IL-1β and TNF-α contents according to the kit instructions.
Apoptosis of spinal cord tissue was assessed by TUNEL assay: After anesthetized rats for blood collection, five rats from each group were sacrificed, and L2-5 segments of bone marrow were isolated for routine fixation, embedded in paraffin and sectioned in reserve. Three random sections from each group were deparaffinized in xylene, rehydrated using graded alcohol, washed and then stained using a TUNEL staining kit, with neutral gum mounting, photographed under a light microscope, and apoptotic cells were counted from five fields per section and averaged as the final result.
Apoptosis index (%)=number of apoptotic cells/ number of total cells×100 %
Apoptosis related proteins Bcl-2 and BAX were detected by immunohistochemistry: The prepared spare sections of 2.4 were deparaffinized and then rehydrated using graded alcohol, incubated in Hydrogen peroxide (H2O2) for 30 min, heat retrieval of antigen was performed by adding citrate solution, after blocking in 4 % non-fat milk for 20 min, rinsed, primary antibodies for Bcl-2 and BAX were added, incubated overnight at 4°, rinsed again, secondary antibodies were added, incubated for 20 min at room temperature, developed using 3,3' Diaminobenzidine (DAB) color development kit after rinsing, counterstained with hematoxylin, rinsed, dehydrated in graded alcohol, cleared in xylene. Pictures were taken under a light microscope, five fields were taken per section, and the Integrated Optical Density (IOD) value was analyzed by ImageJ analysis software.
TLR4/COX-2/NF-κB signaling pathway related protein expression by Western Blot: The remaining rats from each group were sacrificed, and L2-5 segments of bone marrow were separated, high-performance protein lysate was added, the supernatant was centrifuged and the total protein concentration was determined by the BCA method. 30 μl loading buffer was used for electrophoresis, transmembrane, blocking solution was added for 1 h at room temperature, and TLR4, COX-2, p-NF-κB and NF-κB primary antibodies were added, incubated overnight at 4°, secondary antibody was added, incubated for 2 h at room temperature, Enhanced Chemiluminescence (ECL) solution was added, and pictures were acquired with a gel imaging analysis system to take β-actin as the internal reference, and gray values were analyzed to calculate TLR4 and COX-2 protein expression and p-NF-κB/NF-κB.
Statistical methods:
Statistical Package for the Social Sciences (SPSS) 24.0 software was used to analyze the data. The metrology data were expressed as (x? ±s), and the t-test was used for comparison between two groups, one-way Analysis of Variance (ANOVA) was used for comparison among multiple groups, and the Student–Newman–Keuls (SNK)-q test was used for comparison between two groups. Results were considered statistically significant at p<0.05.
Results and Discussion
Compared with the sham operation group, the behavioral scores of rats in the model group were significantly lower (p<0.05); compared with the model group, the behavioral scores of each concentration of anthocyanin increased gradually as the concentration increased (p<0.05); the behavioral scores of the anthocyanin+TAK-242 group were significantly higher than those of the high anthocyanin concentration group (p<0.05). The results were presented in Table 2.
| Group | Behavioral scoring (points) |
|---|---|
| Sham operation group | 3.96±0.31 |
| Model group | 1.58±0.13a |
| Anthocyanin low concentration group | 1.91±0.18b |
| Anthocyanin medium concentration group | 2.33±0.22bc |
| Anthocyanin high concentration group | 3.05±0.33bcd |
| Anthocyanin+TAK-242 group | 3.42±0.31e |
Notes: Compared with sham operation group, ap<0.05; compared with the model group, bp<0.05; compared with the anthocyanin low concentration group, cp<0.05; compared with the anthocyanin medium concentration group, dp<0.05 and compared with the high anthocyanin dose group, ep<0.05
Table 2: Comparison of Behavioral Scores of Rats in Each Group (x?±s, n=10)
The IL-6, IL-1β and TNF-α contents in the serum of model rats were significantly increased compared with the sham operation group (p<0.05); compared with the model group, each concentration group of anthocyanin increased with concentration, and IL-6, IL-1β and TNF-α content in serum gradually decreased (p<0.05); and IL-6, IL-1β and TNF-α contents in the serum of rats in anthocyanin+TAK-242 group was significantly reduced compared with anthocyanin high concentration group (p<0.05). The results are presented in Table 3.
| Group | IL-6 (pg/ml) | IL-1β (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|
| Sham operation | 103.46±10.12 | 22.46±2.03 | 6.32±0.54 |
| Model | 362.77±30.49a | 282.55±12.34a | 29.47±2.61a |
| Anthocyanin low concentration | 291.84±30.06b | 217.48±12.09b | 20.96±2.03b |
| Anthocyanin medium concentration | 232.55±26.47bc | 165.42±10.33bc | 16.75±1.24bc |
| Anthocyanin high concentration | 182.74±16.51bcd | 104.91±10.16bcd | 13.27±1.09bcd |
| Anthocyanin+TAK-242 | 139.29±10.94e | 51.49±4.87e | 9.52±0.83e |
Notes: Compared with sham operation group, ap<0.05; compared with the model group, bp<0.05; compared with the anthocyanin low concentration group, cp<0.05; compared with the anthocyanin medium concentration group, dp<0.05 and compared with the high anthocyanin dose group, ep<0.05
Table 3: Comparison of Serum IL-6, IL-1β and TNF-α in Serum of Each Group (x?±s, n=10)
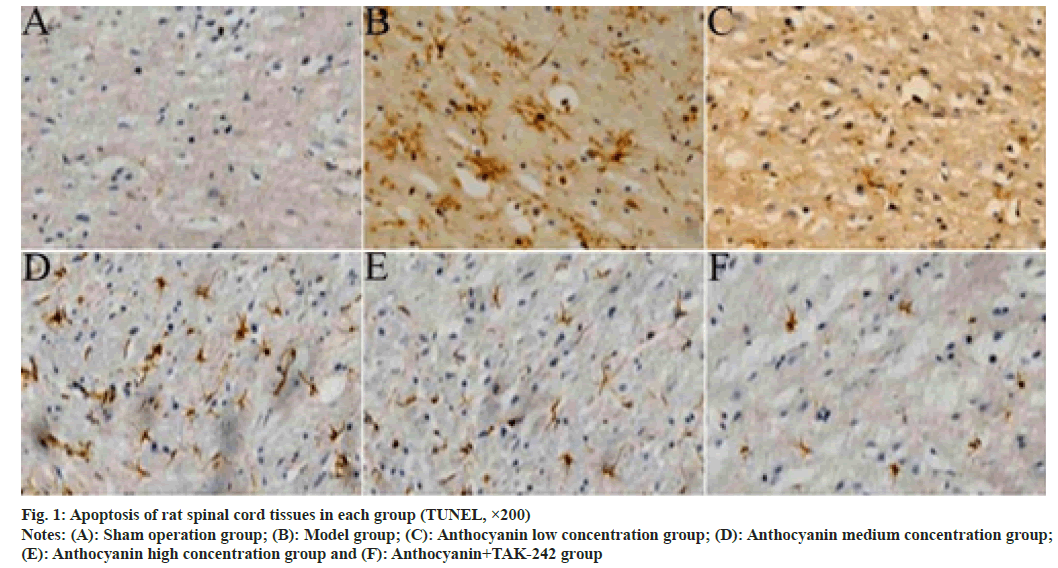
From the results of TUNEL staining, only a few apoptotic cells were found in the spinal cord tissue of rats in the sham operation group, a large number of apoptotic cells were seen in the spinal cord tissue of rats in the model group, and the number of apoptotic cells gradually decreased in the spinal cord tissue of rats in each concentration group of anthocyanin and anthocyanin+TAK-242 group. The results of quantitative analysis showed that the apoptotic index in the spinal cord tissue of model rats was significantly higher than that in the sham operation group (p<0.05); compared with the model group, the anthocyanin low, medium and high concentration groups showed a gradual decrease in the apoptotic index (p<0.05); the apoptotic index was significantly reduced in the anthocyanin+TAK-242 group compared with the anthocyanin high concentration group (p<0.05). The results are shown in fig. 1 and Table 4.
| Group | Apoptosis index (%) |
|---|---|
| Sham operation | 8.02±0.62 |
| Model | 39.65±3.56a |
| Anthocyanin low concentration | 32.38±3.15b |
| Anthocyanin medium concentration | 26.81±2.53bc |
| Anthocyanin high concentration | 18.12±1.78bcd |
| Anthocyanin+TAK-242 | 13.75±1.06e |
Notes: Compared with sham operation group, ap<0.05; compared with the model group, bp<0.05; compared with the anthocyanin low concentration group, cp<0.05; compared with the anthocyanin medium concentration group, dp<0.05 and compared with the high anthocyanin dose group, ep<0.05
Table 4: Comparison of Apoptotic Index in Spinal Cord Tissue of Rats in Each Group (x?±s, n=5)
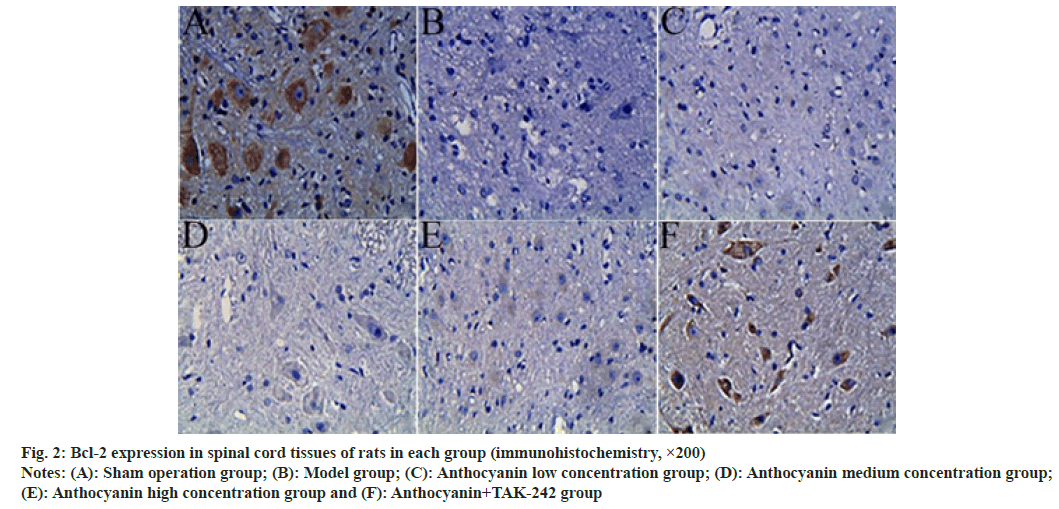
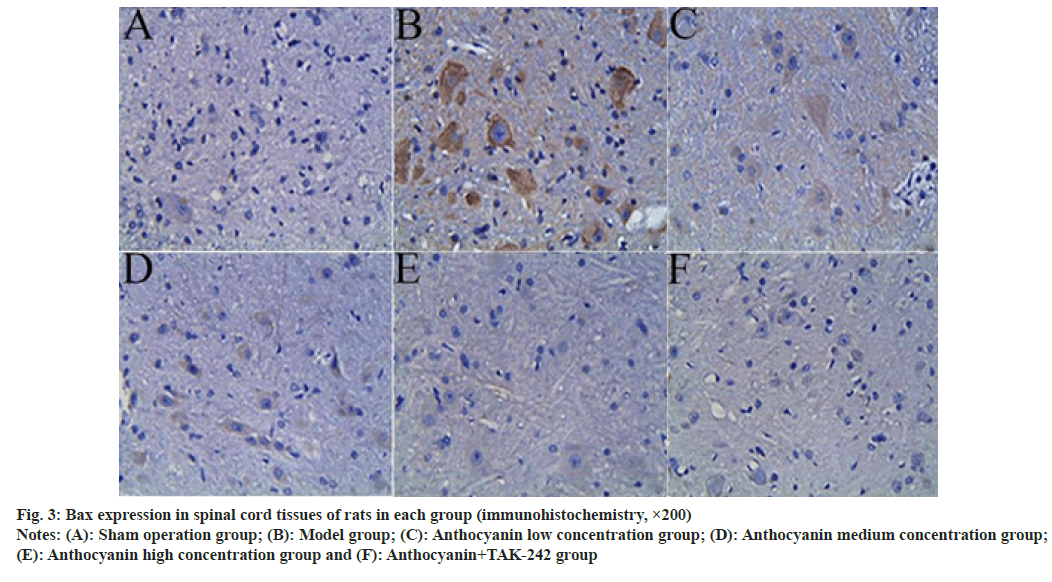
Compared with the sham operation group, the expression of Bcl-2 in the spinal cord tissue of model rats was significantly decreased, and the expression of BAX was significantly increased (p<0.05); compared with the model group, the expression of Bcl-2 in the spinal cord tissue of the anthocyanin low, medium and high concentration groups increased sequentially, while the expression of BAX decreased sequentially (p<0.05); compared with the anthocyanin high concentration group, the expression of Bcl-2 in the spinal cord tissue increased significantly in the anthocyanin+TAK-242 group, while the expression of BAX decreased significantly (p<0.05). The results are presented in fig. 2, fig. 3 and Table 5.
| Group | Bcl-2 | BAX |
|---|---|---|
| Sham operation | 8.13±0.59 | 2.32±0.23 |
| Model | 1.65±0.14a | 7.91±0.68a |
| Anthocyanin low concentration | 2.58±0.17b | 5.76±0.36b |
| Anthocyanin medium concentration | 3.74±0.41bc | 4.82±0.35bc |
| Anthocyanin high concentration | 4.68±0.34bcd | 3.53±0.23bcd |
| Anthocyanin+TAK-242 | 6.85±0.36e | 2.84±0.25e |
Notes: Compared with sham operation group, ap<0.05; compared with the model group, bp<0.05; compared with the anthocyanin low concentration group, cp<0.05; compared with the anthocyanin medium concentration group, dp<0.05 and compared with the high anthocyanin dose group, ep<0.05
Table 5: Comparison of BCL-2 and BAX Expression in Spinal Cord Tissue of Rats In Each Group (x?±s, n=5, IOD Value)
Fig. 2: Bcl-2 expression in spinal cord tissues of rats in each group (immunohistochemistry, ×200)
Notes: (A): Sham operation group; (B): Model group; (C): Anthocyanin low concentration group; (D): Anthocyanin medium concentration group; (E): Anthocyanin high concentration group and (F): Anthocyanin+TAK-242 group
Fig. 3: Bax expression in spinal cord tissues of rats in each group (immunohistochemistry, ×200)
Notes: (A): Sham operation group; (B): Model group; (C): Anthocyanin low concentration group; (D): Anthocyanin medium concentration group; (E): Anthocyanin high concentration group and (F): Anthocyanin+TAK-242 group
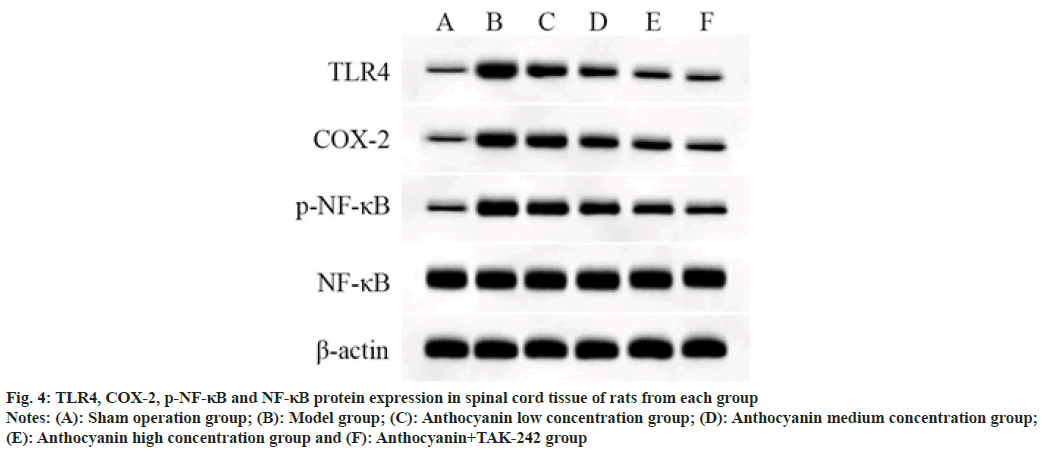
TLR4 and COX-2 protein expression and p-NF- κB/NF-κB in spinal cord tissue of model rats were significantly increased compared with sham operation group rats (p<0.05); TLR4 and COX-2 protein expression and p-NF-κB/NF-κB were decreased significantly in the spinal cord of rats in each concentration group of anthocyanin compared with the model group, with a concentration effect (p<0.05); compared with the high anthocyanin concentration group, TLR4 and COX-2 protein expression, and p-NF-κB/NF-κB in the spinal cord of anthocyanin+TAK-242 group rats were decreased significantly (p<0.05). The results are shown in fig. 4 and Table 6.
| Group | TLR4/β-actin | COX-2/β-actin | p-NF-κB/NF-κB |
|---|---|---|---|
| Sham operation | 0.13±0.01 | 0.16±0.02 | 0.23±0.02 |
| Model | 0.96±0.08a | 0.87±0.09a | 0.91±0.10a |
| Anthocyanin low concentration | 0.79±0.07b | 0.72±0.07b | 0.75±0.06b |
| Anthocyanin medium concentration | 0.56±0.04bc | 0.58±0.06bc | 0.61±0.05bc |
| Anthocyanin high concentration | 0.38±0.04bcd | 0.43±0.03bcd | 0.48±0.05bcd |
| Anthocyanin + TAK-242 | 0.27±0.02e | 0.31±0.03e | 0.37±0.02e |
Notes: Compared with sham operation group, ap<0.05; compared with the model group, bp<0.05; compared with the anthocyanin low concentration group, cp<0.05; compared with the anthocyanin medium concentration group, dp<0.05; compared with the high anthocyanin dose group, ep<0.05
Table 6: Comparison of TLR4 and COX-2 Protein Expression and P-NF-κB/NF-κB in Spinal Cord Tissue of Rats from Each Group (x?±s, n=5)
Fig. 4: TLR4, COX-2, p-NF-κB and NF-κB protein expression in spinal cord tissue of rats from each group
Notes: (A): Sham operation group; (B): Model group; (C): Anthocyanin low concentration group; (D): Anthocyanin medium concentration group; (E): Anthocyanin high concentration group and (F): Anthocyanin+TAK-242 group
Due to the complex mechanism of SCII occurrence and the existence of irreversible pathological processes in the occurrence and development of SCII, no effective treatment has been found so far. SCII mostly causes neurological damage to the spinal cord, and the rate of paralytic lethality is still high, in which inflammatory response and cell death are the main mechanisms responsible for neurological damage by SCII[14,2]. Bcl-2 has been confirmed to be a key factor in the regulation of apoptosis after nerve injury[15]. BAX is a pro-apoptotic factor in cells, and it is able to bind to Bcl-2, thereby inhibiting Bcl-2 activity[16]. When SCII occurs, the dynamic balance between BAX and Bcl-2 is disrupted, Bcl-2 secretion is reduced, BAX secretion is increased and apoptosis is induced[17]. In this study, we found that SCII rats exhibited decreased behavioral scores, increased apoptotic index in spinal cord tissue, and increased inflammatory factors IL-6 and IL-1β, and TNF-α in serum, and the immunohistochemically results showed that Bcl-2 expression was significantly decreased and BAX expression was significantly increased in the spinal cord tissue, which indicated that the inflammatory reaction as well as cell apoptosis occurred in the body after SCII, leading to motor dysfunction.
Anthocyanin is natural flavonoid pigments that can be extracted in a variety of plants and have strong antioxidant, anti-inflammatory, anti-apoptotic as well as prevention against cardiovascular diseases[18,19]. In this study, SCII rats after intervention of different concentrations of anthocyanin showed IL-6, IL-1β, TNF-α content in serum decreased, the apoptotic index of spinal cord tissue decreased, Bcl-2 expression increased, Bax expression decreased, and the behavioral score of rats increased, suggesting that anthocyanin can effectively improve the inflammatory response, upregulate Bcl-2 expression and reduce apoptosis in SCII rats.
TLR4 is an important pathogen pattern recognition receptor in the cell that is able to deliver exogenous stimuli to the intracellular, causing the body inflammatory response[20]. TLR4 signaling is stimulated by pathogens and binds to its receptor protein Myeloid Differentiation Factor 88 (MyD88) to activate a MyD88 dependent signaling pathway that induces NF-κB downstream to undergo phosphorylation and mediates the expression of a variety of pro-inflammatory proteins such as COX-2, causing a large secretion of inflammatory factors[21]. In this study, we show that TLR4 and COX-2 protein expression as well as p-NF-κB/NF-κB are upregulated in the spinal cord of SCII rats, indicating that TLR4/COX-2/NF-κB signaling pathway in spinal cord tissue of SCII rats is activated, causing an inflammatory response that causes tissue damage in the spinal cord after SCII. Compared with the model group, TLR4 and COX-2 protein expression as well as p-NF-κB/NF-κB in the spinal cord tissue of SCII rats in anthocyanin low, medium and high concentration groups decreased gradually, and TLR4 and COX-2 protein expression as well as p-NF-κB/ NF-κB in the anthocyanin+TAK-242 group was further decreased, suggesting that the expression of TLR4/COX-2/NF-κB signaling pathway can be downregulated to some extent by anthocyanin to improve inflammatory response, reduce apoptosis and alleviate motor dysfunction after SCII.
In conclusion, anthocyanin can reduce apoptosis and improve motor function after SCII to some extent, which may be related to the inhibition of TLR4/ COX-2/NF-κB signaling pathway expression and improvement of inflammatory responses. These results provide a theoretical basis for the use of flavonoids in clinical prevention and treatment of the occurrence of SCII, but the specific mechanism still needs further exploration.
Conflict of interests:
The authors declared no conflict of interests.
References
- Ryu JH, Park JW, Hwang JY, Park SJ, Kim JH, Sohn HM, et al. The attenuation of neurological injury from the use of simvastatin after spinal cord ischemia-reperfusion injury in rats. BMC Anesthesiol 2018;18(1):31.
[Crossref] [Google Scholar] [PubMed]
- Liu ZG, Li Y, Jiao JH, Long H, Xin ZY, Yang XY. MicroRNA regulatory pattern in spinal cord ischemia-reperfusion injury. Neural Regen Res 2020;15(11):2123.
[Crossref] [Google Scholar] [PubMed]
- Zhu J, Yang LK, Chen WL, Lin W, Wang YH, Chen T. Activation of SK/KCa channel attenuates spinal cord ischemia-reperfusion injury via anti-oxidative activity and inhibition of mitochondrial dysfunction in rabbits. Front Pharmacol 2019;10:325.
[Crossref] [Google Scholar] [PubMed]
- Tana HS, An M, Zhang T, Deni W, Hou L, Jin K. Dexmedetomidine inhibits microglial activation through SNHG14/HMGB1 pathway in spinal cord ischemia-reperfusion injury mice. Int J Neurosci 2021;132(1):77-88.
[Crossref] [Google Scholar] [PubMed]
- Wang M, Niu J, Ou L, Deng B, Wang Y, Li S. Zerumbone protects against carbon tetrachloride (CCl4)-induced acute liver injury in mice via inhibiting oxidative stress and the inflammatory response: Involving the TLR4/NF-κB/COX-2 pathway. Molecules 2019;24(10):1964.
[Crossref] [Google Scholar] [PubMed]
- Quach DT, Hiyama T, Gotoda T. Do subjects with mild or moderate atrophic gastritis or intestinal metaplasia confined to the antrum benefit from gastric cancer surveillance? Gut 2020;69(5):968-9.
[Crossref] [Google Scholar] [PubMed]
- Li Xu, Bai X, Liu C. Review of antioxidant mechanisms and functional activities of natural anthocyanin. J Food Safety Quality 2021;12(20):8163-71.
- Liu J, Zhou H, Song L, Yang Z, Qiu M, Wang J, et al. Anthocyanins: Promising natural products with diverse pharmacological activities. Molecules 2021;26(13):3807.
[Crossref] [Google Scholar] [PubMed]
- Liu He, He Y, Gu Ji. Ameliorative effects of Vitis amurensis anthocyanin on cognitive impairment in mice with chronic alcoholism. Food Machinery 2021;37(8):163-200.
- Hu Ge, Cao J, Qin F. Mechanism of modulation of TLR4/p38MAPK/NF-κB signaling pathway related protein expression on protecting spleen of over trained rats by oligomeric proanthocyanidins. Acta Nutrimenta Sin 2020;42(5):479-84.
- Fujiwara M, Matoba T, Koga JI, Okahara A, Funamoto D, Nakano K, et al. Nanoparticle incorporating Toll-like receptor 4 inhibitor attenuates myocardial ischaemia–reperfusion injury by inhibiting monocyte-mediated inflammation in mice. Cardiovasc Res 2019;115(7):1244-55.
[Crossref] [Google Scholar] [PubMed]
- Liu XZ, Sun X, Shen KP, Jin WJ, Fu ZY, Tao HR, et al. Aldehyde dehydrogenase 2 overexpression inhibits neuronal apoptosis after spinal cord ischemia/reperfusion injury. Neural Regen Res 2017;12(7):1166.
[Crossref] [Google Scholar] [PubMed]
- Yang Yi, Huai Li, Fang B. Electroacupuncture promoting endogenous neural stem cell proliferation after spinal cord ischemia-reperfusion injury in rats. Prog Anatomical Sci 2021;27(4):433-6.
- Jia H, Ma H, Li Z, Chen F, Fang B, Cao X, et al. Downregulation of lncRNA TUG1 inhibited TLR4 signaling pathway-mediated inflammatory damage after spinal cord ischemia reperfusion in rats via suppressing TRIL expression. J Neuropathol Exp Neurol 2019;78(3):268-82.
[Crossref] [Google Scholar] [PubMed]
- Sater AP, Rael LT, Tanner AH, Lieser MJ, Acuna DL, Mains CW, et al. Cell death after traumatic brain injury: Detrimental role of anoikis in healing. Clin Chim Acta 2018;482:149-54.
[Crossref] [Google Scholar] [PubMed]
- Dong L, Ding J, Shi X. Correlation between endogenous NSCs proliferation and Bcl-2 after SCI. Guizhou Med J 2018;42(3):259-62.
- Ru T, Chang Y, Wei N. Neuroprotection of lipoxin A4 in a rat model of spinal cord ischemia-reperfusion injury. Chin J Tissue Eng Res 2021;25(26):4175-9.
- Brandenburg JP, Giles LV. Four days of blueberry powder supplementation lowers the blood lactate response to running but has no effect on time-trial performance. Int J Sport Nutr Exercise Metab 2019;29(6):636-42.
[Crossref] [Google Scholar] [PubMed]
- Huang W, Hutabarat RP, Chai Z, Zheng T, Zhang W, Li D. Antioxidant blueberry anthocyanins induce vasodilation via PI3K/Akt signaling pathway in high-glucose-induced human umbilical vein endothelial cells. Int J Mol Sci 2020;21(5):1575.
[Crossref] [Google Scholar] [PubMed]
- Bao H, Liao F, Fang L. PCKS9 regulating monocyte endothelial adhesion by TLR4/NF-κB/COX-2 pathway. Chin J Arterioscl 2019;27(5):395-400.
- Li H, Chen L, Tang C. Effects and mechanism of TLR4/NF-κB/COX-2 proinflammatory signaling pathway on the development and progression of Helicobacter pylori associated chronic atrophic gastritis. Chin J Gastroenterol Hepatol 2020;29(7):773-7.