- *Corresponding Author:
- Fatma Fathi Hussein
Department of Oral Medicine,
Oral Diagnosis and Periodontology,
Faculty of Dentistry,
Minia University,
Damaris,
Minya,
Egypt
E-mail: fattma2003@yahoo.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “255-265” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Vitamin D is a well-known secosteroid hormone, which exerts multiple essential roles in bone physiology, cell growth and differentiation, the neuromuscular system, immunomodulatory functions and autoimmune diseases. Deficiency of vitamin D has been linked to many oral disorders including tooth decay, periodontitis, oral cancer, oral candidiasis, oral lichen planus and recurrent aphthous ulcers. Many studies have revealed a significant lower serum vitamin D level in recurrent aphthous ulcer patients compared with healthy volunteers. This proposes that vitamin D supplementation may reduce the severity of the lesion. To evaluate the efficacy of vitamin D3 replacement on recurrent aphthous ulcer severity is the objective of the study. This study involved 65 patients with idiopathic minor recurrent aphthous ulcer and vitamin D deficiency/insufficiency. These patients received vitamin D3 supplement over 1 y. Severity of the disease was delineated by the duration of episodes, the number of ulcers per attack and the frequency of recurrence. Severity parameters were compared before and after vitamin D3 intake. In addition, 25-hydroxy vitamin D3 concentrations were measured before and after administration of vitamin D3 by enzyme-linked immunosorbent assay technique. We found a significant decrease in the frequency of attacks and the number of ulcers in each attack with p-value<0.05 following vitamin D replacement. Moreover, a highly significant lowering in the duration of episodes was detected with p-value<0.001. Gender specific analysis showed no statistically significance regarding severity parameters at any point of the study stages. Correlation studies revealed the only detectable correlation was between serum 25-hydroxy vitamin D3 level at baseline and the number of ulcers before and after vitamin D correction. Vitamin D3 supplementation has a safe and positive impact on improving the severity of recurrent aphthous ulcer with regards to the number of lesions, the frequency of recurrence and the duration of episodes, in patients who have vitamin D deficiency/insufficiency.
Keywords
25-hydroxy vitamin D3, recurrent aphthous ulcer, minor recurrent aphthous ulcerations, vitamin D, vitamin D3 supplementation
Vitamin D (VD) (Calcitriol) is a multifunctional lipid soluble ecosteroid hormone, besides its classical endocrine activity in the regulation of serum calcium and phosphate concentration, bone biology and growth[1,2]. VD also has a novel autocrine and paracrine actions by regulating cell proliferation, maturation and apoptosis, nervous system, cardiovascular system, immune system, antimicrobial peptides, glucose metabolism and even acts as antiaging factor[3-9]. Bioactivities of calcitriol are mediated through intracellular Vitamin D Receptor (VDR). Nearly all human tissues and cells express VDR[10].
The major endogenous source of calcitriol (>80 %) is obtained through the conversion of cutaneous 7-dehydrocholesterol to previtamin D3 following solar ultraviolet B rays exposure. Previtamin D3 is transformed to Vitamin D3 (VD3) (cholecalciferol) under the effect of heat-mediated process in the skin[11], the remaining 20 % of 25-Hydroxy (25-OH) VD3 is acquired exogenously from diet and supplements. In the liver, VD3 undergoes hydroxylation into calcifediol (25(OH)D3), which is usually estimated to determine vitamin D status[11-13]. Further hydroxylation is carried out in the kidney where the biologically active VD (1,25-hydroxycholecalciferol) is formed[11]. Also, hydroxylation of VD3 takes place in the skin and lymph nodes[14].
Vitamin D Deficiency (VDD)/insufficiency are now more pervasive than ever and have become a widespread public health issue. Almost one billion people across the world are believed to have VDD (25(OH)D3 level <20 ng/ml). On the other hand, about 50 % of population have VD insufficiency (25(OH) D3 level≥20 and <30 ng/ml)[15,16]. Based on the crucial pathophysiological role of calcitriol, inadequate VD level has been reported in various musculoskeletal disorders, autoimmune diseases, hypertension, diabetes, respiratory and neurological diseases[16-18]. A low VD concentration has also increased the risk of any type of malignant lesions[19]. Regarding oral health, VDD has a negative effect including demineralization of enamel and dentine with increasing susceptibility to fracture and caries[20]. In addition, there is a reverse association with an increased severity of periodontitis, higher tooth loss and VD concentrations[21-24]. Furthermore, VDD is markedly more prevalent in patients with oral cancer, oral lichen planus and Recurrent Aphthous Ulcer (RAU)[25-32].
RAU (canker sore) is one of the most frequent oral ulceration which characterized by recurrent painful episodes and unclear multifactorial etiopathogenesis[33]. The lesion usually manifests as a self-limiting single or multiple oval, round or elliptical ulcers with central necrosis and bordered by well-circumscribed erythematous margin, mainly involving nonkeratinized oral mucosa[34]. The onset of RAU is usually in childhood and adolescence with a slight female predominance[35]. Unlike herpetiform and Major Aphthous Ulcer (MaAU), Minor Aphthous Ulcer (MiAU) represents the most prevalent form and resolves in 7-10 d without leaving any scar[36]. The exact cause of RAU is undefined and may be attributed to an impressive array of multiple factors. The common triggering factors include genetic predisposition, local factors, infection, trauma, psychological stress, systemic diseases, allergy, hematological diseases, immunological defect, endocrine disorders, hormonal fluctuations and micronutrient deficiencies[37,38]. RAU is believed to be caused by a cell mediated immune defect characterized by alteration of Cluster of Differentiation, (CD) 4+:CD8+ T lymphocyte ratio and subsequent mucosal cytokines cascade dysfunction[39].
Micronutrients are defined as these magic substances that enhance the production of hormones, enzymes and other substances necessary for proper development and growth. A reduction in the concentration of these substances induce severe consequences[40]. The role of various micronutrients in RAU, such as (i.e., Vitamin B12, VD, folic acid, iron and zinc), and their deficiencies have been subjected to several studies since the 1960s[29,30,41-47]. Recently, the association of RAU and VDD has been considered in several studies. These have revealed the presence of significantly reduced serum levels of VD in patients with RAU compared to healthy controls[29-32,48,49]. Consequently, VD replacement therapy may be beneficial in management of this lesion for patients who have inadequate 25(OH) D3 level. We set out to assess the effectiveness of VD3 supplementation on RAU severity with regards to the duration of episodes, the frequency of recurrence and the number of lesions in each attack.
Materials and Methods
The study was implemented over a period of 1 y. 65 patients had idiopathic Minor Recurrent Aphthous Ulcer (MiRAU) and VD deficiency/insufficiency who participated in our previous study to detect the relationship between RAU and serum VD level, were recruited in this current study[32]. All former patients were chosen from the out-patient clinic of College of Dental Medicine, Umm Al-Qura University in Mecca. All participants were given their written consent form after being informed about the objectives of the study, procedure and possible benefits and risks of participating in this clinical trial. The study protocol was approved by the Biomedical Research Ethics Committee of Umm Al-Qura University (Approval No. HAPO-02-K-012).
Patient selection:
Inclusion criteria: Selected patients were of both sexes with 18-60 y age range; patients were diagnosed with at least three idiopathic MiRAUs/year; severity parameters of the disease were assessed at baseline and after 12 mo of receiving VD3 by the most protracted episode duration, the number of ulcers/attack and the frequency of recurrence, reliant on the patient’s report using standardized chart.
Exclusion criteria: Patient who had sufficient serum VD concentration (25(OH)D3≥30 ng/ml); uses of any medical treatment for RAU in the last 3 mo; previous history of allergic reaction to any kind of VD preparations; history of taking any drugs that interact with VD3 supplement including: Drugs that raise the serum level of VD (e.g., Estrogen Replacement Therapy (ERT), Isoniazid (INH), thiazide, multivitamin, calcium and VD supplements), drugs that reduce serum VD level such antacids, sun blockers or sunscreen cream and antiseizure medications (e.g., phenobarbital, phenytoin, primidone and valproic acid), bile acid sequestrants that reduce VD absorption (e.g., Cholestyramine, cholestipol, rifampin, mineral oil and orlistat), VD may interfere or reduce the action of atorvastatin and calcium channel blockers, VD may potentiate the action of digoxin, herbal medicines mainly Kava kava, St. John’s wort (Hyperforin) which interfere VD metabolism; history of taking any medication which influences bone metabolism in the past 6 mo like glucocorticoids, Highly Active Antiretroviral Treatment (HAART), cytotoxic drugs, chemotherapy, bisphosphonates, anticoagulant (heparin, warfarin); patients with history of chronic liver, chronic renal, bone metabolic diseases, hyperparathyroidism, fibromyalgia, Behcet’s syndrome, malabsorption diseases, short bowel, Human Immunodeficiency Virus (HIV), Diabetes Mellitus (DM) type 1, malignancies, thyroid disease, pregnancy as well as any blood disorders.
VD3 replacement strategies:
For patients who had VDD (25(OH)D3 of <20 ng/ml) initially received loading dose of 50 000 International Units (IU) of cholecalciferol (Biodal)* weekly for 8 w[50], followed by VD3 dose reduction to a maintenance regimen generally 50 000 IU of cholecalciferol monthly for 10 mo.
For patients with VD insufficiency (25(OH)D3 between 21-29 ng/ml) were replaced with 50 000 IU of cholecalciferol monthly for 12 mo[51].
Blood samples collection and serum VD levels measurement:
Blood specimens were collected before the administration of VD3, after 2 mo following VD3 replacement and at the end of the study to assess VD status. 25(OH) D3 concentrations were measured by commercially available Enzyme-Linked Immunosorbent Assay (ELISA) kits.
Statistical analysis:
Data from 65 subjects were recorded before and after treatment on excel spreadsheet and statistically analyzed using Statistical Package for the Social Sciences (SPSS) v. 21.0 for Windows Software. Descriptive data were illustrated as mean±Standard Deviation (SD) and range (minimum, maximum). While, analytical data were performed using paired t test, Analysis of Variance (ANOVA), student’s test, correlation matrix and coefficient of correlation (Pearson’s method). The probability values were significant at p<0.05.
Results and Discussion
65 VD deficient/insufficient patients who had MiRAU, 36 female (55 %) and 29 male (45 %) were enrolled in this study. The mean patient’s age was 28.923±10.638 with non-significant difference between female and male. Serum levels of 25(OH)D3 were analyzed at baseline, 2 and 12 mo, while the clinical outcomes were evaluated at baseline and 12 mo following VD3 replacement. Descriptive statistics of all recruited subjects were recorded, tabulated and presented in Table 1.
| Minimum | Maximum | Mean±SD | |
|---|---|---|---|
| Age | 18 | 60 | 28.923±10.638 |
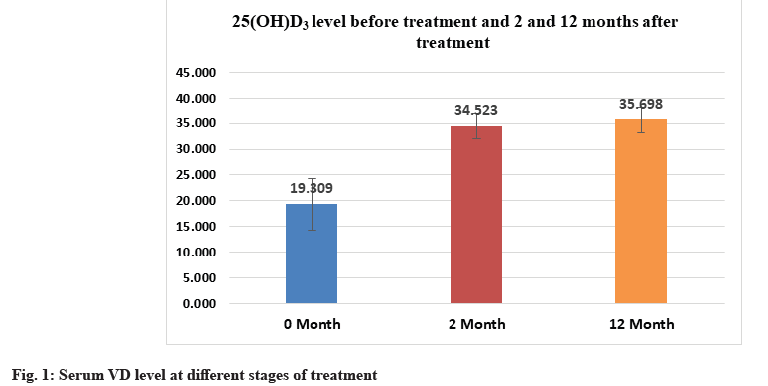
| 25(OH)D3 ng/ml-M0 | 5.3 | 28.1 | 19.309±5.061 |
| 25(OH)D3 ng/ml-M2 | 27.9 | 40.1 | 34.523±2.572 |
| 25(OH)D3 ng/ml-M12 | 31.7 | 41.3 | 35.698±2.425 |
| Number of ulcers-M0 | 1 | 4 | 1.462±0.752 |
| Number of ulcers-M12 | 1 | 3 | 1.338±0.538 |
| Frequency-M0 | 3 | 24 | 5.000±4.077 |
| Frequency-M12 | 3 | 22 | 4.646±3.356 |
| Duration-M0 | 7 | 10 | 7.354±0.623 |
| Duration-M12 | 6 | 8 | 7.077±0.407 |
Table 1: Clinical Parameters Before and After VD3 Supplementation
Serum 25(OH)D3 level:
Response of total serum 25(OH)D3 values to VD3 replacement over time, showed highly significant elevation of the mean serum 25(OH)D3 concentration when the 2 mo (34.523±2.572) and 12 mo (35.698±2.425) measurements were compared to the starting (baseline 19.309±5.061) value (p<0.001). The same level of significance was observed between the 2 mo and 12 mo data. There was an 84.9 % improvement in mean serum VD level from the baseline interval till the end of the study (Table 2-Table 4 and fig. 1).
| 0 mo | 2 mo | T-test | |
|---|---|---|---|
| Mean±SD | Mean±SD | p value | |
| 25(OH)D3 ng/ml | 19.309±5.061 | 34.523±2.572 | 0.000000 |
Table 2: Comparison Between Serum 25(OH)D3 Level At Baseline and 2 mo After Treatment
| 2 mo | 12 mo | T-test | |
|---|---|---|---|
| Mean±SD | Mean±SD | p value | |
| 25(OH)D3 ng/ml | 34.523±2.572 | 35.698±2.425 | 0.000000 |
Table 3: Comparison Between Serum 25(OH)D3 Level At 2 and 12 Mo After Treatment
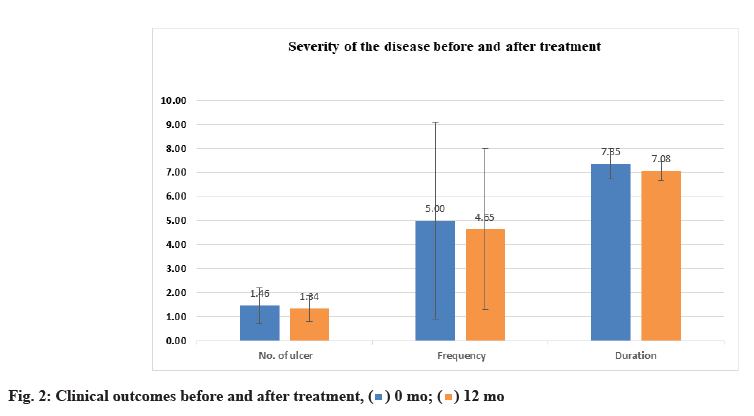
Clinical findings before and after treatment were shown below. Regarding the number of ulcers, the clinical findings indicated a significant decrease in number of ulcers/attack with p-value<0.05 following VD replacement. Exactly, the same statistically significant value was observed for the frequency (number of attacks/year). Moreover, a highly significant lowering in duration of episodes was detected with p-value<0.001 (Table 4 and fig. 2).
| 0 mo | 12 mo | T-test | |
|---|---|---|---|
| Mean±SD | Mean±SD | p value | |
| 25(OH)D3 | 19.309±5.061 | 35.698±2.425 | 0.000000 |
| Number of ulcers | 1.462±0.752 | 1.338±0.538 | 0.005149 |
| Frequency | 5.000±4.077 | 4.646±3.356 | 0.003073 |
| Duration | 7.354±0.623 | 7.077±0.407 | 0.000060 |
Table 4: Comparison of Different Clinical Parameters Before and After VD3 Supplementation
Upon comparing the results between female and male, female showed a significant low mean serum VD value (17.664±5.355) more than male (21.352±3.860) at baseline p-value<0.05. Furthermore, we observed a high significant elevation in the mean serum 25(OH)D3 level in male more than female at 2 mo following VD3 administration with p-value<0.001. Notwithstanding that the VD3 supplement was more efficient in rising 25(OH)D3 concentration in female by 89.8 % in comparison to male (67.50 %) after 2 mo. On the contrary, 25(OH)D3 mean value displayed no significant difference between them at the end of the study. As for the severity parameters, there was no detectable significant difference along the whole studied period with p-value>0.05 (Table 5).
| Female | Male | T-test | |
|---|---|---|---|
| Mean±SD | Mean±SD | p value | |
| 25(OH)D3-M0 | 17.664±5.355 | 21.352±3.860 | 0.001394604 |
| 25(OH)D3-M2 | 33.522±2.491 | 35.766±2.117 | 0.000136865 |
| 25(OH)D3-M12 | 34.700±2.108 | 36.938±2.239 | 5.28451E-05 |
| Number of ulcers-M0 | 1.583±0.806 | 1.310±0.660 | 0.07344099 |
| Number of ulcers-M12 | 1.417±0.554 | 1.241±0.511 | 0.097131469 |
| Frequency-M0 | 4.833±3.745 | 5.207±4.515 | 0.358284828 |
| Frequency-M12 | 4.528±2.893 | 4.793±3.904 | 0.377052646 |
| Duration-M0 | 7.333±0.535 | 7.379±0.728 | 0.385044835 |
| Duration-M12 | 7.056±0.410 | 7.103±0.409 | 0.320556246 |
Table 5: Comparison of Different Clinical Parameters Between Female and Male Before and After VD3 Supplementation
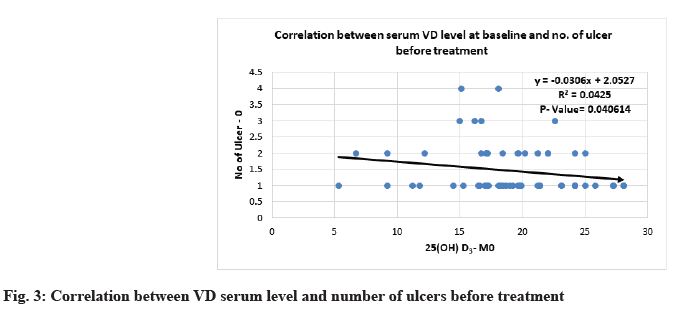
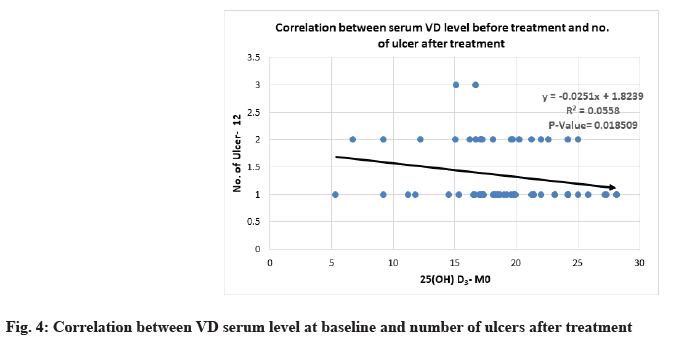
Correlation between 25(OH)D3 serum level and severity parameters of RAU is shown below. Severity of MiRAU was set by the number of ulcers per attack, frequency of recurrence and duration of episodes. The only mild to moderate inverse correlation was noticed between VD serum level at baseline and the number of ulcers before and after treatment with p-value<0.05 (Table 6 and Table 7, fig. 3 and fig. 4).
| 25(OH)D3-M0 | ||
|---|---|---|
| R | p value | |
| Number of ulcers-M0 | -0.206125 | 0.040614 |
| Number of ulcers-M12 | -0.236274 | 0.018509 |
| Frequency-M0 | -0.085416 | 0.400515 |
| Frequency-M12 | -0.091342 | 0.368509 |
| Duration-M0 | -0.107063 | 0.291477 |
| Duration-M12 | 0.152031 | 0.132973 |
Table 6: Correlation Between 25(OH)D3 Serum Level At Baseline And Severity Parameters of Rau At Different Stages of The Study
| 25(OH)D3-M12 | ||
|---|---|---|
| R | p-value | |
| Number of ulcers-M0 | -0.087905 | 0.386879 |
| Number of ulcers -M12 | -0.107298 | 0.290412 |
| Frequency-M0 | -0.005847 | 0.954193 |
| Frequency-M12 | -0.018883 | 0.852819 |
| Duration-M0 | 0.031380 | 0.757812 |
| Duration-M12 | 0.011196 | 0.912411 |
Table 7: Correlation Between 25(OH)D3 Serum Level At 12 Mo and Severity Parameters of Rau At Different Stages of The Study
VD is the principle organic lipophilic calcitriol precursor that regulates intestinal calcium absorption[52] and renal reabsorption, to maintain musculoskeletal health[53,54]. Activated VD has a fundamental immunomodulatory role in different inflammatory and T-cell mediated autoimmune diseases[55] through regulation and modulation of innate and acquired immune response[56]. VDD has been linked with many oral diseases and carries a high risk of oral treatment failure[57]. The association between the RAU incidence and VD status has been studied numerous times[58]. Whilst several studies have found a significant relationship between recurrent aphthous stomatitis and low serum level of VD[29-32,48,49,59,60], others have failed to reveal a significance[61,62].
RAU is the most common painful oral lesion that can impair speech, daily nutrition, oral hygiene and life quality[63,64], hence the main objectives of RAU treatment are to relief pain, reduce the duration of episodes, decrease the number of ulcers and increase lesion-free periods. Eradication of etiological predisposing factors is the prime concern. So far, the etiopathogenesis of RAU is indefinite; however, both innate and acquired immune dysregulation and inflammatory processes caused by several triggers may enhance the development of RAU. This concept has been confirmed in large-scale bioinformatics analysis[65,66]. Altered immunoregulatory balances involve T-lymphocytes infiltration that mediates local epithelial cells destruction[67], higher serum immunoglobulins levels, boost in antibodydependent cell-mediated cytotoxicity and defects in lymphocyte subpopulations with decreased numbers of T-suppressor/inducer cells, increased T-helper/inducer cells and suppressed response to mitogens[68,69]. T helper type 1 (Th1) hyperimmune response stimulates the production of Interleukin 2 (IL-2), tumour necrosis factor alpha (α) and interferon-gamma (γ). These proinflammatory mediators promote inflammatory process that proceed ulceration[70,71]. In view of the foregoing, low VD level correction may be beneficial for RAU, due to the VD immunomodulatory role on both innate and adaptive immune response and suppression of the inflammatory response by inhibiting Th1 proinflammatory cytokines and increasing Th2 antiinflammatory cytokines like IL-4, IL-5, IL-10 and IL- 17[55,72].
Nowadays, the most common commercial preparations and fortified food of VD are VD2 (ergocalciferol) and VD3 (cholecalciferol)[73]. Meta-analysis and Tripkovic et al.[74,75] demonstrated that VD3 is more proficient in restoring serum 25(OH)D3 than equivalent VD2. This observation may be attributed to a higher rate of VD3 hydroxylation[76] and a higher affinity of VD3 metabolites for VD-Binding Protein (VDBP)[77] which lead to longer half-life and decreased rate of circulatory clearance[78] suggesting the fact that cholecalciferol remain biologically active and maintain serum 25(OH) D3 status[79,80]. For these reasons, we recommended VD3 administration in our study.
In this study, we followed the current strategies for VD3 replacement in adult patients. Patients with a serum 25(OH)D3 concentration >20 ng/ml were initially treated with 50 000 IU of VD3/week for 8 w. VD status was tested 2 mo after completion the course to ensure serum 25(OH)D3 repletion. Once replete, VD3 was administered at a maintenance dose of 50 000 IU/mo, as the majority of these patients were more susceptible to VDD and would likely require life-long therapy. Patients with a 25(OH)D3 serum level between 21- 29 ng/ml were administered only a maintenance dose of 50 000 IU/mo[81]. de Niet et al. proved that higher dose of VD3 (50 000 IU) administered monthly speed up serum 25(OH)D3 repletion than daily dose of 2000 IU[82]. Monitoring the success of VD3 replacement is the key to ensure sufficiency and avoid toxicity. The first follow-up of VD status should not be earlier than 8-12 w following the start of treatment[83].
The response of total 25(OH)D3 concentration to VD3 administration was the primary endpoint used to determine VD repletion. VD status was measured at different intervals through this study to fulfill this point. We observed a significant rise in the 25(OH)D3 serum level throughout all study periods (p value<0.001) with an 84.9 % improvement from the baseline interval till the end of the study. This highly significant responsiveness may be owing to proper dose, type of VD (cholecalciferol) and low initial 25(OH)D3 level (19.309±5.061 ng/ml).
Mazahery et al. found that baseline 25(OH)D3 level, body mass index, duration, dose and type of VD are the most important factors affecting the response to the administered dose of VD[84]. Baseline 25(OH)D3 value markedly affects bioavailability of VD because VD hepatic hydroxylation may be a saturable process. VD status has a significant converse relation with initial 25(OH)D3 level with the greatest increase in response has been seen in subjects with the lowest baseline 25(OH)D3 value[85]. Our results are consistent with the study conducted by Bacon et al. who concluded that large bolus dose of VD3 rapidly and safely repletes 25(OH)D3 in deficient elderly subject [<50 nmol/l (<20 ng/ml)] at 1 mo while monthly dosing of 50 000 IU of VD3 takes about 3 mo to attain similar effect[86].
In the current study, gender-specific analysis revealed significant rise in the mean 25(OH)D3 level for male more than female, 2 mo after the onset of VD3 supplementation. The gender effect can be indirectly attributed to either more physical activity, increased sunlight exposure, more outdoor activities, less adipose tissue, clothing style in male[87] or sample size effect. However, VD3 supplement was more efficacious in boosting 25(OH)D3 concentration in female by 89.8 % in comparison to male (67.50 %) since female showed lower/suboptimal baseline serum 25(OH) VD levels (17.664±5.355) vs. male (21.352±3.860). Lack of this significance at the end of the study indicates both male and female attained near serum 25(OH)D3 sufficiency.
In our previous study[32], patients with MiRAU displayed deficient/insufficient serum level of 25(OH)D3, which correlated with the number of ulcers and frequency of episodes, indicating a possible benefit of using VD for these patients. As far as we know, the current clinical trial may be the first study to provide data showing the impact of VD3 replacement on severity parameters of RAU. The present findings show marked decrease in severity parameters, following low VD correction, shedding light on the possible immuno-modulatory therapeutic effect of VD3 on RAU patients.
It was hard to compare our results with those of other studies due to different study design, sample sizes, dose and type of VD and follow-up periods. In a controlled clinical trial, Bratel et al. found that LongoVital (herbal based multivitamins including VD) significantly decreased the number of ulcers as well as the duration of pain[88]. Similarly, Pederson et al. evinced the significant role of LongoVital on lessening the recurrence of RAU[89]. On the contrary, Lalla et al. revealed that the use of Recommended Daily Intake (RDI) vitamins containing vitamin B complex, A, C, D and E for RAU on a regular basis over 1 y had no effect on reducing the number of lesions and duration of episodes[63]. Noteworthy, the initial 25(OH)D3 level has not been addressed in the three aforementioned studies.
Moreover, in RAU related disorders where RAU is one of the main diagnostic criteria as Behcet’s Disease (BD) and Periodic Fever, Aphthous Stomatitis, Pharyngitis and Cervical Adenitis (PFAPA), few trials with respect to VD correction have been published. The findings of Adeeb et al. suggested that VD may potentially down regulate the inflammatory response in BD patients and recommended VD supplementation to assess whether it confers protective benefits or not[90]. However, in Aslan et al. study, who detected that VD has no efficacy on the activity and clinical manifestations of BD[91]. In the study by Stagi et al. the positive effect of PFAPA patients with deficient/insufficient serum level of 25(OH)D3 to 400 IU/d of VD for approximately 7 mo was reported. They observed a significant shortening of mean duration of episodes from 4.3 to 2.3 d in 36 % of cases and reduction in the number of febrile episodes[92]. Furthermore, a case of 32 mo old girl with PFAPA syndrome and insufficient VD serum level (23.7 ng/ml) was reported by Rodes et al. They noticed the outcomes of using 400 IU of cholecalciferol until achieving normal level (30 ng/ml), resulting in reduction in the severity and interval between the episodes from episode/6-8 w to only two mild attacks over the following 12 mo[93].
As regard to correlation, we detected the only significant inverse correlation was between the 25(OH)D3 serum level at baseline and the number of lesions before and after VD3 supplementation (p-value<0.05). Even the fact that, the correlation between VD concentration and the number of ulcers and frequency of recurrence were recorded in our previous study[32], we observed loss of this correlation with frequency before treatment in the current study. This observation may be explained by exclusion of patients who had MiRAU with sufficient VD level in the present study resulting in more smaller sample size.
VD3 supplementation seems markedly, improves the severity of RAU regarding the number of lesions, frequency of recurrence and duration of episodes, proving a safe promising intervention for the treatment of RAU patients who have low serum level of VD.
Conflict of interests:
The authors declared no conflict of interest.
References
- Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr 2008;87(4):1080S-6S.
[Crossref] [Google scholar] [Pubmed]
- Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: Form, function and metabolism. Endocr Rev 2013;34(1):33-83.
[Crossref] [Google scholar] [Pubmed]
- Morris HA, Anderson PH. Autocrine and paracrine actions of vitamin D. Clin Biochem Rev 2010;31(4):129-38.
[Google scholar] [Pubmed]
- Kim IM, Norris KC, Artaza JN. Vitamin D and cardiac differentiation. Vitam Horm 2016;100:299-320.
[Crossref] [Google scholar] [Pubmed]
- Bikle DD. Vitamin D and the immune system: Role in protection against bacterial infection. Curr Opin Nephrol Hypertens 2008;17(4):348-52.
[Crossref] [Google scholar] [Pubmed]
- Carlberg C. Molecular approaches for optimizing vitamin D supplementation. Vitam Horm 2016;100:255-71.
[Crossref] [Google scholar] [Pubmed]
- Bikle DD. Vitamin D metabolism, mechanism of action and clinical applications. Chem Biol 2014;21(3):319-29.
[Crossref] [Google scholar] [Pubmed]
- Jeon SM, Shin E. Exploring vitamin D metabolism and function in cancer. Exp Mol Med 2018;50(4):1-4.
[Crossref] [Google scholar] [Pubmed]
- Xu Y, Sun Z. Molecular basis of Klotho: From gene to function in aging. Endocr Rev 2015;36(2):174-93.
[Crossref] [Google scholar] [Pubmed]
- Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys 2012;523(1):123-33.
[Crossref] [Google scholar] [Pubmed]
- Razzaque MS. The dualistic role of vitamin D in vascular calcifications. Kidney Int 2011;79(7):708-14.
[Crossref] [Google scholar] [Pubmed]
- Razzaque MS. Sunlight exposure: Do health benefits outweigh harm? J Steroid Biochem Mol Biol 2018;175:44-8.
[Crossref] [Google scholar] [Pubmed]
- Brown RB, Haq A, Stanford CF, Razzaque MS. Vitamin D, phosphate and vasculotoxicity. Can J Physiol Pharmacol 2015;93(12):1077-82.
[Crossref] [Google scholar] [Pubmed]
- Hewison M, Zehnder D, Bland R, Stewart PM. 1alpha-hydroxylase and the action of vitamin D. J Mol Endocrinol 2000;25(2):141-8.
[Crossref] [Google scholar] [Pubmed]
- Nair R, Maseeh A. Vitamin D: The “sunshine” vitamin. J Pharmacol Pharmacother 2012;3(2):118-26.
[Crossref] [Google scholar] [Pubmed]
- Zmijewski MA. Vitamin D and human health. Int J Mol Sci 2019;20(1):145.
[Crossref] [Google scholar] [Pubmed]
- Jung HC, Seo MW, Lee S, Kim SW, Song JK. Vitamin D3 supplementation reduces the symptoms of upper respiratory tract infection during winter training in vitamin D-insufficient taekwondo athletes: A randomized controlled trial. Int J Environ Res Public Health 2018;15(9):2003.
[Crossref] [Google scholar] [Pubmed]
- Moretti R, Morelli ME, Caruso P. Vitamin D in neurological diseases: A rationale for a pathogenic impact. Int J Mol Sci 2018;19(8):2245.
[Crossref] [Google scholar] [Pubmed]
- Liu W, Zhang L, Xu HJ, Li Y, Hu CM, Yang JY, et al. The anti-inflammatory effects of vitamin D in tumorigenesis. Int J Mol Sci 2018;19(9):2736.
[Crossref] [Google scholar] [Pubmed]
- Schroth RJ, Levi JA, Sellers EA, Friel J, Kliewer E, Moffatt ME. Vitamin D status of children with severe early childhood caries: A case–control study. BMC Pediatr 2013;13(1):1-8.
[Crossref] [Google scholar] [Pubmed]
- Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr 2004;80(1):108-13.
[Crossref] [Google scholar] [Pubmed]
- Zhan Y, Samietz S, Holtfreter B, Hannemann A, Meisel P, Nauck M, et al. Prospective study of serum 25-hydroxy vitamin D and tooth loss. J Dent Res 2014;93(7):639-44.
[Crossref] [Google scholar] [Pubmed]
- Millen AE, Hovey KM, LaMonte MJ, Swanson M, Andrews CA, Kluczynski MA, et al. Plasma 25‐hydroxyvitamin D concentrations and periodontal disease in postmenopausal women. Journal Periodontol 2013;84(9):1243-56.
[Crossref] [Google scholar] [Pubmed]
- Antonoglou GN, Knuuttila M, Niemelä O, Raunio T, Karttunen R, Vainio O, et al. Low serum level of 1, 25 (OH) 2D is associated with chronic periodontitis. J Periodontal Res 2015;50(2):274-80.
[Crossref] [Google scholar] [Pubmed]
- Fathi N, Ahmadian E, Shahi S, Roshangar L, Khan H, Kouhsoltani M, et al. Role of vitamin D and vitamin D receptor (VDR) in oral cancer. Biomed Pharmacother 2019;109:391-401.
[Crossref] [Google scholar] [Pubmed]
- Tak MM, Chalkoo AH. Vitamin D deficiency-a possible contributing factor in the aetiopathogenesis of oral lichen planus. J Evol Med Dent Sci 2017;6(66):4769-73.
- Gupta A, Mohan RP, Kamarthi N, Malik S, Goel S, Gupta S. Serum vitamin D level in oral lichen planus patients of North India-a case-control study. J Dermatol Res Ther 2017;1(2):19.
- Seif S, Jafari-Ashkavandi Z, Mardani M, Hamidizadeh N. Evaluation of serum vitamin D level in oral lichen planus patients. J Mashhad Dent Sch 2018;42(1):58-49.
- Bahramian A, Falsafi P, Abbasi T, Ghanizadeh M, Abedini M, Kavoosi F, et al. Comparing serum and salivary levels of vitamin D in patients with recurrent aphthous stomatitis and healthy individuals. J Dent 2018;19(4):295.
[Crossref] [Google scholar] [Pubmed]
- Öztekin A, Öztekin C. Vitamin D levels in patients with recurrent aphthous stomatitis. BMC Oral Health 2018;18(1):1-5.
[Crossref] [Google scholar] [Pubmed]
- Khabbazi A, Ghorbanihaghjo A, Fanood F, Kolahi S, Hajialiloo M, Rashtchizadeh N. A comparative study of vitamin D serum levels in patients with recurrent aphthous stomatitis. Egypt Rheumatol 2015;37(3):133-7.
- Hussein FF, Elmarssafy LH, Sadek HS, Alqahtani M. Relationship between serum vitamin D level and recurrent aphthous ulcer. Egypt Dent J 2020;66(2):1015-23.
- Edgar NR, Saleh D, Miller RA. Recurrent aphthous stomatitis: A review. J Clin Aesthet Dermatol 2017;10(3):26-36.
[Google scholar] [Pubmed]
- Preeti L, Magesh KT, Rajkumar K, Karthik R. Recurrent aphthous stomatitis. J Oral Maxillofac Pathol 2011;15(3):252-6.
[Crossref] [Google scholar] [Pubmed]
- Majorana A, Bardellini E, Flocchini P, Amadori F, Conti G, Campus G. Oral mucosal lesions in children from 0 to 12 years old: Ten years' experience. Oral Surg Oral Med Oral Pathol Oral Radiol 2010;110(1):e13-8.
[Crossref] [Google scholar] [Pubmed]
- Peter T, Cherian D, Peter T. Recurrent aphtous stomatitis: Mystery unravelled. J Clin Exp Res 2014;2:141-5.
- Akintoye SO, Greenberg MS. Recurrent aphthous stomatitis. Dent Clin 2005;49(1):31-47.
[Crossref] [Google scholar] [Pubmed]
- Gallo CD, Mimura MA, Sugaya NN. Psychological stress and recurrent aphthous stomatitis. Clinics 2009;64:645-8.
[Crossref] [Google scholar] [Pubmed]
- Pekiner FN, Aytugar E, Demirel GY, Borahan MO. Interleukin‐2, interleukin‐6 and T regulatory cells in peripheral blood of patients with Behçet’s disease and recurrent aphthous ulcerations. J Oral Pathol Med 2012;41(1):73-9.
[Crossref] [Google scholar] [Pubmed]
- WHO. Micronutrients. World Health Organization; 2016.
- Ship II. Nutrition and food intake in aphthous ulcerations. Arch Environ Health 1962;5(2):158-66.
- Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: Scientific review. JAMA 2002;287(23):3116-26.
[Crossref] [Google scholar] [Pubmed]
- Ozturk P, Kurutas EB, Ataseven A. Copper/zinc and copper/selenium ratios, and oxidative stress as biochemical markers in recurrent aphthous stomatitis. J Trace Elem Med Biol 2013;27(4):312-6.
[Crossref] [Google scholar] [Pubmed]
- Lopez‐Jornet P, Camacho‐Alonso F, Martos N. Hematological study of patients with aphthous stomatitis. Int J Dermatol 2014;53(2):159-63.
[Crossref] [Google scholar] [Pubmed]
- Chen H, Sui Q, Chen Y, Ge L, Lin M. Impact of haematologic deficiencies on recurrent aphthous ulceration: A meta-analysis. Br Dent J 2015;218(4):E8.
[Crossref] [Google scholar] [Pubmed]
- Bao ZX, Yang XW, Shi J, Liu LX. Serum zinc levels in 368 patients with oral mucosal diseases: A preliminary study. Med Oral Patol Oral Cir Bucal 2016;21(3):e335.
[Crossref] [Google scholar] [Pubmed]
- Shah D, Sachdev HS, Gera T, De‐Regil LM, Peña‐Rosas JP. Fortification of staple foods with zinc for improving zinc status and other health outcomes in the general population. Cochrane Database Syst Rev 2016(6).
- Nalbantoğlu B, Nalbantoğlu A. Vitamin D levels in children with recurrent aphthous stomatitis. Ear Nose Throat J 2020;99(7):460-3.
[Crossref] [Google scholar] [Pubmed]
- Tamer F, Avci E. Decreased serum ferritin and vitamin D levels in patients with recurrent aphthous stomatitis. Our Dermatol Online 2019;10:229-33.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96(7):1911-30.
[Crossref] [Google scholar] [Pubmed]
- Patel RM, Bazydlo L, Brown SA, Dalkin AC. Vitamin D replacement in adults: Current strategies in clinical management. Pract Gastroenterol 2021.
- Reid IR, Bolland MJ. Controversies in medicine: The role of calcium and vitamin D supplements in adults. Med J Aust 2019;211(10):468-73.
[Crossref] [Google scholar] [Pubmed]
- Wacker M, Holick MF. Vitamin D-effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013;5(1):111-48.
[Crossref] [Google scholar] [Pubmed]
- Jarusriwanna A, Phusunti S, Chotiyarnwong P, Unnanuntana A. High-dose versus low-dose ergocalciferol for correcting hypovitaminosis D after fragility hip fracture: A randomized controlled trial. BMC Geriatr 2021;21(1):1-10.
[Crossref] [Google scholar] [Pubmed]
- Zhang Z, Chen F, Li J, Luo F, Hou T, Xu J, et al. 1,25(OH) 2D3 suppresses proinflammatory responses by inhibiting Th1 cell differentiation and cytokine production through the JAK/STAT pathway. Am J Transl Res 2018;10(8):2737-46.
[Google scholar] [Pubmed]
- Pulvirenti G, Parisi GF, Manti S, Licari A, Del Giudice MM, Salpietro C, et al. The immunomodulatory role of vitamin D in respiratory diseases. Curr Respir Med Rev 2019;15(3):238-45.
- Botelho J, Machado V, Proença L, Delgado AS, Mendes JJ. Vitamin D deficiency and oral health: A comprehensive review. Nutrients 2020;12(5):1471.
[Crossref] [Google scholar] [Pubmed]
- Diachkova E, Trifonova D, Morozova E, Runova G, Ashurko I, Ibadulaeva M, et al. Vitamin D and its role in oral diseases development. Scoping review. Dent J 2021;9(11):129.
[Crossref] [Google scholar] [Pubmed]
- Al-Amad SH, Hasan H. Vitamin D and hematinic deficiencies in patients with recurrent aphthous stomatitis. Clin Oral Investig 2020;24(7):2427-32.
[Crossref] [Google scholar] [Pubmed]
- Zakeri M, Parsian H, Bijani A, Shirzad A, Neamati N. Serum levels of vitamin D in patients with recurrent aphthous stomatitis. Dent Med Probl 2021;58(1):27-30.
[Crossref] [Google scholar] [Pubmed]
- Krawiecka E, Ślebioda Z, Szponar E, Kowalska A, Dorocka-Bobkowska B. Vitamin D status in recurrent aphthous stomatitis. Postepy Dermatol Alergol 2017;34(6):612.
[Crossref] [Google scholar] [Pubmed]
- Oner U, Oner F, Kurt O, Ozdemir S. The role of hematologic parameters in recurrent aphthous stomatitis. Ann Med Res 2021;28(2):0290-5.
- Lalla RV, Choquette LE, Feinn RS, Zawistowski H, Latortue MC, Kelly ET, et al. Multivitamin therapy for recurrent aphthous stomatitis: A randomized, double-masked, placebo-controlled trial. J Am Dent Assoc 2012;143(4):370-6.
[Crossref] [Google scholar] [Pubmed]
- Bijina Rajan JA, Shenoy N, Denny C, Ongole R, Binnal A. Assessment of quality of life in patients with chronic oral mucosal diseases: A questionnaire-based study. Perm J 2014;18(1):e123-7.
[Crossref] [Google scholar] [Pubmed]
- Rivera C. Immune system and zinc are associated with recurrent aphthous stomatitis. An assessment using a network-based approach. J Oral Res 2017;6(9):245-51.
- Wu J, Chen ZP, Shang AQ, Wang WW, Chen ZN, Tao YJ, et al. Systemic bioinformatics analysis of recurrent aphthous stomatitis gene expression profiles. Oncotarget 2017;8(67):111064-72.
[Crossref] [Google scholar] [Pubmed]
- Neville BW, Damm DD, Chi AC, Allen CM. Oral and maxillofacial pathology. 4th ed. Elsevier Health Sciences; 2015.
- Landesberg R, Fallon M, Insel R. Alterations of T helper/inducer and T suppressor/inducer cells in patients with recurrent aphthous ulcers. Oral Surg Oral Med Oral Pathol 1990;69(2):205-8.
[Crossref] [Google scholar] [Pubmed]
- Greenspan JS, Gadol N, Olson JA, Hoover CI, Jacobsen PL, Shillitoe HJ, et al. Lymphocyte function in recurrent aphthous ulceration. J Oral Pathol Med 1985;14(8):592-602.
[Crossref] [Google scholar] [Pubmed]
- Buno IJ, Huff JC, Weston WL, Cook DT, Brice SL. Elevated levels of interferon gamma, tumor necrosis factor α, interleukins 2, 4 and 5, but not interleukin 10, are present in recurrent aphthous stomatitis. Arch Dermatol 1998;134(7):827-31.
[Crossref] [Google scholar] [Pubmed]
- Albanidou-Farmaki E, Markopoulos AK, Kalogerakou F, Antoniades DZ. Detection, enumeration and characterization of T helper cells secreting type 1 and type 2 cytokines in patients with recurrent aphthous stomatitis. Tohoku J Exp Med 2007;212(2):101-5.
[Crossref] [Google scholar] [Pubmed]
- Komisarenko YI, Bobryk MI. Vitamin D deficiency and immune disorders in combined endocrine pathology. Front Endocrinol 2018;9:600.
[Crossref] [Google scholar] [Pubmed]
- Glowka E, Stasiak J, Lulek J. Drug delivery systems for vitamin D supplementation and therapy. Pharmaceutics 2019;11(7):347.
[Crossref] [Google scholar] [Pubmed]
- Balachandar R, Pullakhandam R, Kulkarni B, Sachdev HS. Relative efficacy of vitamin VD2 and vitamin D3 in improving vitamin D status: Systematic review and meta-analysis. Nutrients 2021;13(10):3328.
[Crossref] [Google scholar] [Pubmed]
- Tripkovic L, Wilson LR, Hart K, Johnsen S, De Lusignan S, Smith CP, et al. Daily supplementation with 15 μg vitamin VD2 compared with vitamin D3 to increase wintertime 25-hydroxyvitamin D status in healthy South Asian and white European women: A 12 w randomized, placebo-controlled food-fortification trial. Am J Clin Nutr 2017;106(2):481-90.
[Crossref] [Google scholar] [Pubmed]
- Holmberg I, Berlin T, Ewerth S, Björkhem I. 25-Hydroxylase activity in subcellular fractions from human liver. Evidence for different rates of mitochondrial hydroxylation of vitamin VD2 and D3. Scand J Clin Lab Investig 1986;46(8):785-90.
[Crossref] [Google scholar] [Pubmed]
- Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem 1984;21(1):81-6.
[Crossref] [Google scholar] [Pubmed]
- Houghton LA, Vieth R. The case against ergocalciferol (vitamin VD2) as a vitamin supplement. Am J Clin Nutr 2006;84(4):694-7.
[Crossref] [Google scholar] [Pubmed]
- Horst RL, Reinhardt TA, Ramberg CF, Koszewski NJ, Napoli JL. 24-Hydroxylation of 1,25-dihydroxyergocalciferol. An unambiguous deactivation process. J Biol Chem 1986;261(20):9250-6.
[Crossref] [Google scholar] [Pubmed]
- Ramasamy I. Vitamin D metabolism and guidelines for vitamin D supplementation. Clin Biochem Rev 2020;41(3):103.
[Crossref] [Google scholar] [Pubmed]
- Pludowski P, Holick MF, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol 2018;175:125-35.
[Crossref] [Google scholar] [Pubmed]
- De Niet S, Coffiner M, Da Silva S, Jandrain B, Souberbielle JC, Cavalier E. A randomized study to compare a monthly to a daily administration of vitamin D3 supplementation. Nutrients 2018;10(6):659.
[Crossref] [Google scholar] [Pubmed]
- Płudowski P, Karczmarewicz E, Bayer M, Carter G, Chlebna-Sokół D, Czech-Kowalska J, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe-recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol 2013;64(4):319-27.
- Mazahery H, Von Hurst PR. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients 2015;7(7):5111-42.
[Crossref] [Google scholar] [Pubmed]
- Žmitek K, Hribar M, Hristov H, Pravst I. Efficiency of vitamin D supplementation in healthy adults is associated with body mass index and baseline serum 25-hydroxyvitamin D level. Nutrients 2020;12(5):1268.
[Crossref] [Google scholar] [Pubmed]
- Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int 2009;20(8):1407-15.
[Crossref] [Google scholar] [Pubmed]
- Klinedinst BS, Meier NF, Larsen B, Wang Y, Yu S, Mochel JP, et al. Walking in the light: How history of physical activity, sunlight and vitamin d account for body fat-A UK Biobank study. Obesity 2020;28(8):1428-37.
[Crossref] [Google scholar] [Pubmed]
- Bratel J, Hakeberg M, Jontell M. The effect of LongoVital on recurrent aphthous stomatitis in a controlled clinical trial. Oral Health Prev Dent 2005;3(1).
[Google scholar] [Pubmed]
- Pedersen A, Klausen B, Hougen HP, Ryder L, Winther K. Immunomodulation by LongoVital in patients with recurrent aphthous ulceration. J Oral Pathol Med 1990;19(8):376-80.
[Crossref] [Google scholar] [Pubmed]
- Adeeb F, Khan MU, Li X, Stack AG, Devlin J, Fraser AD. High vitamin D levels may downregulate inflammation in patients with Behçet’s disease. Int J Inflam 2017;2017.
[Crossref] [Google scholar] [Pubmed]
- Aslan N, Demirci K, Güler T, Dörtbaş F, Kale E. The effect of vitamin D on clinical manifestations and activity of Behçet’s disease. Postepy Dermatol Alergol 2017;34(1):15.
[Crossref] [Google scholar] [Pubmed]
- Stagi S, Bertini F, Rigante D, Falcini F. Vitamin D levels and effects of vitamin D replacement in children with periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis (PFAPA) syndrome. Int J Pediatr Otorhinolaryngol 2014;78(6):964-8.
[Crossref] [Google scholar] [Pubmed]
- Rodes AR, Bermúdez GS, Vidal AL, Minagorre PJ. Periodic fever, aphtous stomatitis, pharyngitis and adenopathy syndrome and vitamin D: A possible treatment option? Reumatol Clin 2016;12(6):363-4.
[Crossref] [Google scholar] [Pubmed]
 ) 0 mo; (
) 0 mo; ( ) 12 mo
) 12 mo