- *Corresponding Author:
- K. Huang
Department of Anesthesiology, Jinhua Maternal and Child Health Hospital, Jinhua, Zhejiang 321000, China
E-mail: kk_219@126.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “136-142” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to investigate the impact of adding dexmedetomidine to ropivacaine in patients undergoing laparoscopic myomectomy on pain control, comfort levels, stress markers, and sleep quality. 96 patients undergoing laparoscopic myomectomy were randomly assigned to receive either ropivacaine alone or ropivacaine combined with dexmedetomidine for transversus abdominis plane block. General anesthesia was administered using standardized protocols. Blood samples were collected before nerve blockade (T0) and postoperatively at 1 h (T1) and 24 h (T24) for quantification of stress markers. Pain scores were assessed using the visual analogue scale. Comfort levels were evaluated using the general comfort questionnaire and perianesthesia comfort scale. Sleep quality was assessed using the numeric rating scale and Athens insomnia scale on 1st and 3rd d post operation. Visual analogue scale scores showed significant differences between the groups at T1 and T24, with lower scores observed in the ropivacaine+dexmedetomidine group. General comfort questionnaire scores were significantly higher in the ropivacaine+dexmedetomidine group at both T1 and T24 compared to the ropivacaine group. Levels of cortisol, norepinephrine, interleukin-6, interleukin-1 beta, and tumor necrosis factor-alpha, were lower in the ropivacaine+dexmedetomidine group compared to the ropivacaine group. Patients receiving ropivacaine combined with dexmedetomidine demonstrated improved sleep quality on 1st and 3rd d of post operation. compared to those receiving ropivacaine alone. Adding dexmedetomidine to ropivacaine for transversus abdominis plane block in laparoscopic myomectomy patients resulted in improved pain control, comfort levels, stress reduction and sleep quality, suggesting potential benefits for perioperative management.
Keywords
Laparoscopy, myomectomy, ropivacaine, dexmedetomidine, fentanyl, anesthesia, analgesia
In a society favoring delayed parenthood, the widespread occurrence of uterine fibroids (70 %-80 % lifetime prevalence) necessitates nuanced approach, considering symptomatology, fibroid characteristics, quality of life impact and pregnancy goals[1]. With the rising preference of minimally invasive surgical interventions, laparoscopic myomectomy has gained prominence. This procedure involves enucleation, myoma bed preparation and extraction entirely performed through laparoscopy, showcasing advancements in intracorporeal suturing techniques that enhance surgical proficiency[2,3]. In the realm of laparoscopic surgeries, the intraperitoneal instillation of local anesthetics, often supplemented with adjuvants, has emerged as a potent multimodal analgesia strategy. This approach effectively blocks the visceral nociceptive receptors on the exposed peritoneum, reducing nociception through systemic absorption[4].
Ropivacaine, which is a long-acting amide local anesthetic with the chemical formula, C17H26N2O has become a key foundation in post-operative pain management due to its extended duration of action, low cardiac toxicity and high safety profile[5] (fig. 1A). Its advantages include the separation of sensory and motor blockade, producing dual effect of both anesthesia and analgesia[6,7]. Widely administered through epidural infusion, ropivacaine finds applications in various procedures, including abdominal surgery[8,9]. Recent trends have seen its intraoperative injection or use in Patient-Controlled Epidural Analgesia (PCEA) during laparoscopic myomectomy[10,11].
Dexmedetomidine (DEX) whose chemical formula is C13H16N2, selectively binds and activates presynaptic Alpha (α)-2 adrenoceptors in the brain and it represents a unique sedative with analgesic, sympatholytic and respiratory-preserving properties[12,13] (fig. 1B). Several studies underscored the potential benefits of incorporating DEX into Transversus Abdominis Plane (TAP) blocks. These investigations have demonstrated enhanced analgesia, prolonged duration of pain relief and improved patient comfort when DEX is used alongside local anesthetics in TAP blocks[14-16]. The rationale behind combining ropivacaine and DEX lies in the potential for synergistic effects, aiming not only for effective pain relief but also for preserving neural integrity.
This study encompasses 96 individuals undergoing laparoscopic myomectomy, randomly assigned to either the ropivacaine+DEX (DEX with ropivacaine for TAP block) or ropivacaine group (ropivacaine for TAP block), for assessing postoperative pain control. By unraveling the complexities of combining ropivacaine and DEX, it aspires to contribute to the evolving landscape of anesthesia practices and pain relief strategies.
Materials and Methods
General information:
A total of 96 patients undergoing laparoscopic myomectomy who were enrolled between January 2021 and October 2022 were selected, each consenting individual, aged (18-60) y. All the individuals were randomly assigned into two groups, ropivacaine+DEX group and ropivacaine group alone, with 48 patients in each group using computer-generated randomization tables. The clinical experiments strictly adhere as per the informed consent procedures of ethics committee of our hospital and detailed informed consent was obtained from all patients.
Inclusion criteria: All the individuals underwent preoperative exclusion of gynecological diseases other than uterine fibroids and were scheduled for laparoscopic myomectomy under general anesthesia according to the classification of American Society of Anesthesiologists (ASA) physical status I and II. Postoperative pathological examination confirmed the absence of other specific types of uterine lesions or malignancies.
Exclusion criteria: Patients who had the history of allergy to local anesthetics or study drugs, pregnancy, and the presence of systemic illnesses such as cardiovascular and pulmonary diseases limiting physical activity and patients experiencing unexpected complications during surgery leading to conversion to open procedures were excluded from the study.
Treatment method:
Patients in the ropivacaine group received TAP block with 1 ml of saline solution and 30 ml of 0.2 % ropivacaine while ropivacaine+DEX group had a TAP block with 1 ml saline solution and 30 ml of 0.2 % ropivacaine+0.5 mcg/kg DEX[4,17]; all the patients were operated under general anesthesia. Induction of anesthesia included 0.05 mg/kg of midazolam, 0.2 μg/kg of fentanyl, 2 mg/kg of propofol and vecuronium bromide intravenously. Maintenance comprised continuous infusion of 0.1-0.2 μg/kg/min of fentanyl, 4-8 mg/kg/h of propofol and 0.05 mg/ kg of intermittent vecuronium bromide injections for muscle relaxation. Bispectral Index (BIS) was controlled between 40-60 min. 30 min before the surgery, 0.1 μg/kg of sufentanil and 1 mg/kg of flurbiprofen were injected intravenously. Further, 4 mg of ondansetron injection was given to the individuals which prevented postoperative nausea and vomiting.
Outcome measurements:
Inflammatory biomarkers: 3 ml of peripheral venous blood was collected from all the patients before nerve blockade (T0), postoperatively after 1 h (T1) and after 24 h (T24). After centrifugation at 4000 rpm at 4° for 10 min, plasma was carefully extracted and stored at -80° for the quantification of cortisol, norepinephrine, Interleukin-6 (IL-6), IL-Beta (β) and Tumor Necrosis Factor (TNF)-α levels. All the biomarkers were performed and detected using Enzyme-Linked Immunosorbent Assay (ELISA) (Boster Biotechnology, Co., Ltd.).
Pain assessment: Visual Analogue Scale (VAS) pain score, involving a straight line with endpoints representing extreme opposites of the characteristic were measured for pain intensity, where score 0 denoted no pain while score 10 denoted worst imaginable pain[18]; the pain intensities were recorded at T1 and T24.
Assessment of quality of life: Perianesthesia Comfort Scale (PCS) assessed the patients’ comfort level and requirements during the perioperative period using a three-level, four-dimensional questionnaire consisting of 24 items. Responses were scored on a Likert scale ranging from 1 to 6, with expressions ranging from strongly disagrees to strongly agree. The total score was divided by the number of items to obtain a mean value between 1 and 6, where lower scores indicated poor comfort and higher scores indicated better comfort[19]. General Comfort Questionnaire (GCQ) scale comprises 48 items rated on a four-point Likert scale, including a combination of positive and negative items. The total score was divided by the number of items to yield a value between 1 and 4, where a score of 1 indicates low comfort level and 4 indicates high comfort level[20].
Sleep conditions: It was assessed among the patients of both the groups using Numeric Rating Scale (NRS) and Athens Insomnia Scale (AIS) on Postoperative Day 1 (POD1) and POD3. NRS measures the sleep quality on a scale of 0 (excellent or good sleep) to 10 (inability to fall asleep all night), while the AIS evaluates the various aspects of sleep difficulties with a score ranging from 0 to 24 points [21].
Statistical analysis:
GraphPad prism was utilized for the statistical analysis. The normality of data distribution was assessed using the Shapiro-Wilk test. Normally distributed metric data was presented as mean±Standard Deviation (SD) and multiple group comparisons were conducted using t-tests or Analysis of Variance (ANOVA), followed by Tukey’s multiple comparisons test. In instances where the data significantly deviated from normality (mean±Interquartile Range (IQR)) and non-parametric tests such as the Mann-Whitney Test (U-test) were applied to assess the changes between the ropivacaine group and the ropivacaine+DEX group at different time points. Wilcoxon matchedpairs signed-rank test were employed to evaluate intergroup changes in each group over time. A significance level of p<0.05 was considered statistically significant.
Results and Discussion
Demographic and perioperative characteristics between the ropivacaine and ropivacaine+DEX groups were studied. Table 1, presents the demographic and perioperative characteristics of patients in both the groups. There were no significant differences in their age ((41.02±11.25) y vs. (41.44±12.16) y and p=0.862), Body Mass Index (BMI) (18.25, IQR: 17.53-18.78 vs. 18, IQR: 17.43-18.98 and p=0.525), surgical duration (102.5, IQR: 78.5-125.8 vs. 111, IQR: 89.25-142, p=0.195) and blood loss (49, IQR: 38-59 vs. 43, IQR: 38.25-54.5, p=0.1299) between the two groups. The time interval from the end of surgery to the first press of the analgesic pump was significantly longer in the ropivacaine+DEX group compared to the ropivacaine group (5, IQR: 4-6 vs. 3, IQR: 2-3 and p<0.001). Additionally, the number of analgesic pump presses at T24 was significantly lower in the ropivacaine+DEX group compared to the ropivacaine group (7.896±2.354 vs. 11.83±3.867 and p<0.001).
| Demographic characteristics | Ropivacaine | Ropivacaine+DEX | p |
|---|---|---|---|
| Age (y) | 41.02±11.25 | 41.44±12.16 | 0.862 |
| BMI (kg/cm2) | 18.25 (17.53-18.78) | 18 (17.43-18.98) | 0.525 |
| Surgical duration (min) | 102.5 (78.5-125.8) | 111 (89.25-142) | 0.195 |
| Blood loss (ml) | 49 (38-59) | 43 (38.25-54.5) | 0.13 |
| Initial analgesic pump (h)* | 3 (2-3) | 5 (4-6) | <0.001 |
| Number of analgesic pump presses# | 11.83±3.867 | 7.896±2.354 | <0.001 |
Note: (*): Denotes time interval from the end of surgery to the initial press and (#): Indicates total number of analgesic pump presses from post-operation to 24 h
Table 1: The demographic and perioperative characteristics between the two groups
Comparison of postoperative pain control between ropivacaine alone and ropivacaine combined with DEX. Postoperatively, the VAS scores showed significant differences between the two groups at T1, with a median of 3 (IQR: 2-3) in the ropivacaine group and a median of 2 (IQR: 1.25-3) in the ropivacaine+DEX group (p=0.020). At T24, the median VAS score was 2 (IQR: 2-3) in the ropivacaine group and 1 (IQR: 0.25-3) in the ropivacaine+DEX group, indicating significant differences between the groups (p=0.014) (Table 2). Further analysis using the Wilcoxon matched-pairs signed-rank test revealed significant differences in VAS scores between T1 and T24 in both the ropivacaine group (p=0.008) and the ropivacaine+DEX group (p=0.015).
| Postoperative pain scores | Ropivacaine | Ropivacaine+DEX | p |
|---|---|---|---|
| VAS | |||
| T1 | 3 (2-3) | 2 (2-3) | 0.02 |
| T24 | 2 (1.25-3) | 1 (0.25-3) | 0.014 |
| p | 0.008 | 0.015 | |
| PCS | 5 (4-5) | 5 (4.25-5) | 0.5333 |
| GCQ | |||
| T1 | 2 (1-3) | 3 (3-3) | <0.001 |
| T24 | 3 (3-3) | 4 (3-4) | <0.001 |
| p | <0.001 | <0.001 |
Table 2: Comparison of postoperative pain control and comfort levels between the two groups
Comparison of comfort levels between the two groups was studied. According to the statistical analysis (Table 2), GCQ scores at both T1 and T24 were markedly higher in the ropivacaine+DEX group compared to the ropivacaine group. Specifically, at T1, the median GCQ score for the ropivacaine group was 2 (range: 1-3), while for the ropivacaine+DEX group, it was 3 (range: 3-3), showing a significant difference (p<0.001). At T24, the median GCQ score for the ropivacaine group was 3 (range: 3-3), whereas for the ropivacaine+DEX group, it increased to 4 (range: 3-4), also demonstrating a significant difference (p<0.001). Conversely, there was no significant difference in PCS scores between the two groups, with median scores of 5 (range: 4-5) for the ropivacaine group and 5 (range: 4.25-5) for the ropivacaine+DEX group (p=0.5333).
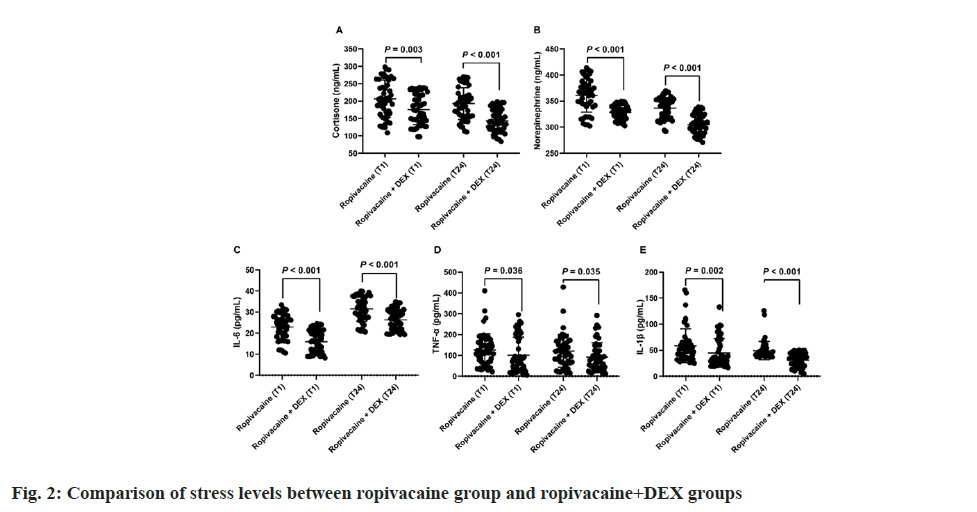
Comparison of stress levels between ropivacaine alone and ropivacaine combined with DEX was also observed. Further, plasma levels of cortisone, norepinephrine, IL-6, IL-1β, and TNF-α between the two groups were observed which showed no statistically significant differences at T0 (data not shown). Fig. 2 illustrated stress levels, as indicated by various biomarkers, among patients administered either ropivacaine alone or ropivacaine combined with DEX at distinct postoperative time points (T1 and T24). Comparing both groups, it’s evident that at T1, median cortisone levels were 203.9 ng/ml (range: 162.4-262.2) for ropivacaine alone and 173.6 ng/ml (range: 140.5-219.1) for ropivacaine+DEX, showing a statistically significant difference (p=0.003). Similarly, at T24, median cortisone levels decreased to 190.8 ng/ml (range: 156.1- 221.0) for ropivacaine and 139.5 ng/ml (range: 118.5-171.6) for ropivacaine+DEX (p<0.001), indicating a further decrease in stress levels in the ropivacaine+DEX group. Similar trends were observed for norepinephrine, IL-6, TNF-α and IL- 1β at both T1 and T24, with significantly lower levels in the ropivacaine+DEX group compared with the ropivacaine group (p<0.05).
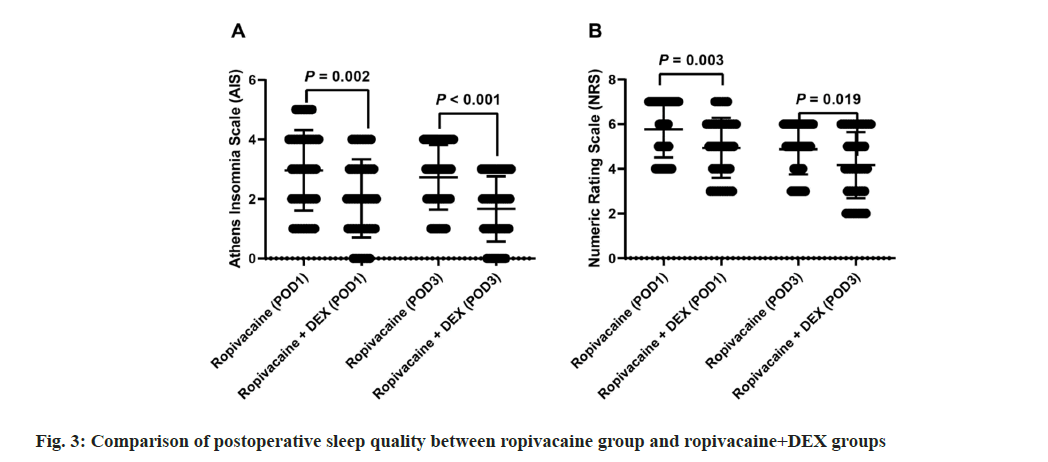
Comparison of postoperative sleep quality between ropivacaine alone and ropivacaine combined with DEX. Fig. 3 compares postoperative sleep quality between patients receiving ropivacaine alone and ropivacaine combined with DEX on POD1 and POD3. For the AIS, median scores were 3 (range: 2-4) for ropivacaine and 2 (range: 1-3) for ropivacaine+DEX on both POD1 and POD3, with significant differences observed (p=0.002 and p<0.001, respectively). Similarly, for the NRS, median scores were 6 (range: 4.25-7) for ropivacaine and 5 (range: 4-6) for ropivacaine+DEX on POD1, and 5 (range: 4-6) for ropivacaine and 4 (range: 3-6) for ropivacaine+DEX on POD3, with significant differences noted (p=0.003 and p=0.019, respectively). These findings indicate that the addition of DEX to ropivacaine leads to improved sleep quality postoperatively.
The results of the study comparing the effects of ropivacaine alone vs. ropivacaine combined with DEX in laparoscopic myomectomy patients undergoing general anesthesia provide valuable insights into the potential mechanisms underlying the observed differences in perioperative outcomes. One significant finding is the difference in postoperative pain control between the two groups. The study reports lower VAS pain scores at both 1 h and 24 h postoperatively in the ropivacaine+DEX group compared to the ropivacaine group. This suggests that the addition of DEX to ropivacaine may enhance analgesic efficacy, leading to better pain relief in the early and late postoperative periods. These clinical improvements align with studies in various surgical contexts. For instance, ropivacaine+DEX in epidural injections enhances analgesia and sedation in preeclampsia patients[22]. Meta-analysis support the superior postoperative pain control and longer analgesia duration with DEX and ropivacaine combinations in different surgical settings[23]. Additionally, continuous DEX infusion during femoral and sciatic nerve block with ropivacaine protects the liver in type 2 diabetes mellitus patients[24].
The inflammatory response is also modulated, as seen in the inhibitory effect on IL-6 levels with DEX in femoral nerve block after knee arthroplasty[25]. Furthermore, the study demonstrates lower plasma levels of stress hormones, such as cortisol and norepinephrine, in the ropivacaine+DEX group compared to the ropivacaine group at both T1 and T24. This suggests that the addition of DEX may attenuate the neuroendocrine stress response to surgery, leading to reduced perioperative physiological stress and potentially contributing to improved postoperative outcomes, including better pain control and faster recovery. Several mechanisms could explain this enhanced analgesic effect. Firstly, DEX’s selective activation of α2-adrenergic receptors in the central nervous system may modulate pain processing pathways, resulting in central analgesia[26]. Secondly, DEX’s peripheral analgesic properties, such as inhibiting the release of norepinephrine from peripheral nerve endings, may complement the local anesthetic action of ropivacaine, leading to synergistic pain relief[27]. Additionally, DEX’s anti-inflammatory effects[28], as indicated by the significant reductions in pro-inflammatory cytokine levels observed in the study, may contribute to decreased pain perception by reducing tissue inflammation at the sur gical site.
Moreover, the study reports higher scores on the GCQ in the ropivacaine+DEX group, indicating better overall comfort levels compared to the ropivacaine group. This improvement in comfort could be attributed to DEX’s sedative and anxiolytic properties, which may alleviate perioperative anxiety and promote relaxation, enhancing patient comfort during the surgical procedure and recovery period. The study evaluated postoperative sleep quality on postoperative days. The results showed that patients receiving ropivacaine combined with DEX had significantly better sleep quality compared to those receiving ropivacaine alone, as indicated by lower NRS and AIS scores on both POD1 and POD3. This improvement in sleep quality could be attributed to several factors. Firstly, DEX’s sedative properties may promote relaxation and facilitate sleep initiation, leading to improved sleep onset latency and overall sleep efficiency[29]. Secondly, DEX’s anxiolytic effects may reduce postoperative anxiety and apprehension, which are known contributors to sleep disturbances[12]. By alleviating anxiety, DEX may help patients achieve deeper and more restful sleep, enhancing subjective perceptions of sleep quality[30]. Additionally, the analgesic efficacy of the ropivacaine+DEX combination may indirectly influence sleep quality.
However, several limitations should be considered when interpreting these findings. Firstly, the study’s sample size may not be sufficient to detect subtle differences in outcomes or account for potential confounding variables. Additionally, long-term follow-up data beyond the immediate postoperative period are lacking, making it challenging to assess the sustainability of the observed benefits.
The addition of DEX to ropivacaine in laparoscopic myomectomy patients undergoing general anesthesia not only improves perioperative pain control, comfort, and stress response but also enhances postoperative sleep quality. By exerting sedative, anxiolytic, analgesic, and stress-modulating effects, DEX plays a crucial role in optimizing sleep outcomes following surgery, contributing to overall patient well-being and recovery.
Conflict of interests:
The authors declared no conflict of interests.
References
- Dumitrascu MC, Nenciu CG, Nenciu AE, Călinoiu A, Neacșu A, Cirstoiu M, et al. Laparoscopic myomectomy: The importance of surgical techniques. Front Med 2023;10:1-11.
[Crossref] [Google Scholar] [PubMed]
- Andou M, Yanai S, Shirane A, Kanno K. Laparoscopic myomectomy. Surg J 2020;6:35-43.
[Crossref] [Google Scholar] [PubMed]
- Ochi Y, Semba S, Sawada M, Kanno K, Sakate S, Yanai S, et al. Clinical use of mixed reality for laparoscopic myomectomy. Int J Gynaecol Obstet 2023;162(1):364-5.
[Crossref] [Google Scholar] [PubMed]
- Panda A, Das M, Dhatri D, Satapathy GC. Efficacy of intraperitoneal 0.2 % ropivacaine with dexmedetomidine vs. 0.2 % ropivacaine with ketamine in laparoscopic surgeries: A randomized controlled trial. Cureus 2023;15(4):1-10.
[Crossref] [Google Scholar] [PubMed]
- Santos M, Arroja S, Antunes AL, Mariz J, Teixeira J. Ropivacaine: An unusual cause of neuroleptic malignant-like syndrome. Eur J Case Rep Intern Med 2021;8(11):1-11.
[Crossref] [Google Scholar] [PubMed]
- Nedeljkovic SS, Kett A, Vallejo MC, Horn JL, Carvalho B, Bao X, et al. Transversus abdominis plane block with liposomal bupivacaine for pain after cesarean delivery in a multicenter, randomized, double-blind, controlled trial. Anesth Analg 2020;131(6):1830-9.
[Crossref] [Google Scholar] [PubMed]
- Kamel AAF, Amin OAI, Ibrahem MAM. Bilateral ultrasound-guided erector spinae plane block vs. transversus abdominis plane block on postoperative analgesia after total abdominal hysterectomy. Pain Physician 2020;23(4):375-82.
[Google Scholar] [PubMed]
- Patil P, Dhulkhed PV, Dhulkhed VK. Isobaric forms of ropivacaine vs. bupivacaine in lower abdominal surgeries: A hospital-based, prospective, comparative study. Med Gas Res 2023;13(3):123-7.
[Crossref] [Google Scholar] [PubMed]
- Kumar A, Kumar R, Kumar R, Koshire A. The comparative study of epidural anesthesia between isobaric ropivacaine 0.5 % and isobaric bupivacaine 0.5 % for lower abdominal surgery. Anesth Essays Res 2019;13(4):688-91.
[Crossref] [Google Scholar] [PubMed]
- Kwack JY, Ahn KH, Kwon YS. Postoperative pain control with ropivacaine following laparoscopic myomectomy: A randomized double-blind, pilot study. J Obstet Gynaecol Res 2019;45(4):871-76.
[Crossref] [Google Scholar] [PubMed]
- Shimohata K, Shimohata T, Ikeda N, Sato Y, Ono T, Motegi R, et al. Effectiveness of low dose PCEA for postoperative pain after laparoscopic gynecological surgeries-A comparison of laparoscopic ovarian cystectomy and myomectomy. Masui 2011;60(6):666-70.
[Google Scholar] [PubMed]
- Lee S. Dexmedetomidine: Present and future directions. Korean J Anesthesiol 2019;72(4):323-30.
[Crossref] [Google Scholar] [PubMed]
- Yuki K. The immunomodulatory mechanism of dexmedetomidine. Int Immunopharmacol 2021;97:1-11.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Hao D. Effect of transversus abdominis plane block combined with low-dose dexmedetomidine on elderly patients undergoing laparoscopic colectomy. Wideochir Inne Tech Maloinwazyjne 2023;18(1):524-32.
[Crossref] [Google Scholar] [PubMed]
- Elfadl GMA, Ali WN, Ahmed FN, Abd el-Rady NM, Ali AM, Rady MMA. Add dexmedetomidine to levobupivacaine for transversus abdominis plane block in elderly patients undergoing inguinal hernia repair: Could it make a difference? A randomised trial. J Perioper Pract 2023;33(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Sun Q, Liu S, Wu H, Ma H, Liu W, Fang M, et al. Dexmedetomidine as an adjuvant to local anesthetics in transversus abdominis plane block: A systematic review and meta-analysis. Clin J Pain 2019;35(4):375-84.
[Crossref] [Google Scholar] [PubMed]
- Vaghela SS, Chaurasiya MK, Prakash R, Khan MP. Ultrasound-guided quadratus lumborum block vs. transversus abdominis plane block for laparoscopic inguinal hernia repair and appendicectomy using ropivacaine with dexmedetomidine. Cureus 2023;15(1):1-12.
[Crossref] [Google Scholar] [PubMed]
- Heller GZ, Manuguerra M, Chow R. How to analyze the visual analogue scale: Myths, truths and clinical relevance. Scand J Pain 2016;13(1):67-75.
[Crossref] [Google Scholar] [PubMed]
- Yilmaz M, Karabulut N. How can we improve the comfort level and sleep quality after surgery? J Perianesth Nurs 2022;37(1):100-4.
[Crossref] [Google Scholar] [PubMed]
- Gois JA, Freitas KS, Kolcaba K, Mussi FC. Cross-cultural adaptation of the general comfort questionnaire to Brazilian patients with myocardial infarction. Rev Bras Enferm 2018;71(6):2998-3005.
[Crossref ] [Google Scholar] [PubMed]
- Qiu D, Wang XM, Yang JJ, Chen S, Yue CB, Hashimoto K, et al. Effect of intraoperative esketamine infusion on postoperative sleep disturbance after gynecological laparoscopy: A randomized clinical trial. JAMA Netw Open 2022;5(12):1-11.
[Crossref] [Google Scholar] [PubMed]
- Wu S, Zhong C, Huang A, Li J, Chen C, Yuan H. Feasibility of epidural injection of ropivacaine and dexamethasone for labor analgesia in women with preeclampsia. Am J Transl Res 2021;13(7):7921-7.
[Google Scholar] [PubMed]
- Zhu M, Sun W. Analgesic effects of ropivacaine combined with dexmedetomidine in transversus abdominis plane block in patients undergoing laparoscopic cholecystectomy: A systematic review and meta-analysis. J Perianesth Nurs 2023;38(3):493-503.
[Crossref] [Google Scholar] [PubMed]
- Zeng L, Liu J, Zhang T, Liu Y, Liao L, Chen X, et al. Study on the protective mechanism of dexmedetomidine on the liver of perioperative diabetic patients: A randomized controlled trial. Medicine 2022;101(41):1-10.
[Crossref] [Google Scholar] [PubMed]
- Li J, Wang H, Dong B, Ma J, Wu X. Adding dexmedetomidine to ropivacaine for femoral nerve block inhibits local inflammatory response. Minerva Anestesiol 2017;83(6):590-7.
[Crossref] [Google Scholar] [PubMed]
- Yu X, Franks NP, Wisden W. Sleep and sedative states induced by targeting the histamine and noradrenergic systems. Front Neural Circuits 2018;12:1-9.
[Crossref] [Google Scholar] [PubMed]
- Zhou M, Luo Q, Xu Y. As an inhibitor of norepinephrine release, dexmedetomidine provides no improvement on stroke-associated pneumonia in mice. Front Pharmacol 2023;14:1-11.
[Crossref] [Google Scholar] [PubMed]
- Yamazaki S, Yamaguchi K, Someya A, Nagaoka I, Hayashida M. Anti-inflammatory action of dexmedetomidine on human microglial cells. Int J Mol Sci 2022;23(17):1-13.
[Crossref] [Google Scholar] [PubMed]
- Weerink MA, Struys MM, Hannivoort LN, Barends CR, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet 2017;56(8):893-913.
[Crossref] [Google Scholar] [PubMed]
- Li J, Zhang H, Deng B, Wang X, Liang P, Xu S, et al. Dexmedetomidine improves anxiety-like behaviors in sleep-deprived mice by inhibiting the p38/MSK1/NFκB pathway and reducing inflammation and oxidative stress. Brain Sci 2023;13(7):1-10.
[Crossref] [Google Scholar] [PubMed]