- *Corresponding Author:
- Chunyan Xue
Department of Obstetrics and Gynecology, The Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu 213001, China
E-mail: 15895081921@139.com
| This article was originally published in a special issue,“Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “67-74” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Chondrocytes play a crucial role in modern tissue engineering; however, their in vitro cultivation often encounters challenges associated with dedifferentiation, posing a significant obstacle in the extracorporeal cultivation of these cells. The extracellular environment in vitro profoundly influences cellular states, encompassing cell morphology, proliferation, and gene expression. This study aimed to investigate the impact of different basal culture media and supplement factors on the proliferation and gene expression of fetal chondrocytes in vitro. The goal was to identify an optimal complete culture medium that retarded the dedifferentiation process during the extracorporeal cultivation of fetal chondrocytes. Chondrocytes were isolated and extracted from fetal cartilage tissue and cultured in vitro, and the morphological changes of chondrocytes were observed. The effects of various basal culture media and supplement factors on chondrocyte proliferation and gene expression were assessed using cell counting kit-8 and quantitative polymerase chain reaction, respectively. Chondrocytes with high viability could be successfully isolated from fetal cartilage tissue and exhibited adherence to in vitro surfaces. The addition of transferrin and ethanolamine to low-serum Dulbecco’s modified eagle medium/F12 culture medium promoted the expression of chondrocyte marker genes and cell proliferation. The supplementation of transferrin and ethanolamine in low-serum Dulbecco’s modified eagle medium/F12 culture medium was advantageous for enhancing the proliferation of fetal chondrocytes and suppressing dedifferentiation during cultivation.
Keywords
Fetal chondrocytes, basal culture media, supplement factors, dedifferentiation
The microenvironment of cell culture significantly influences the degree and direction of differentiation of chondrocytes in extracorporeal cultivation. As the duration of in vitro cultivation increases, the degree of chondrocyte differentiation also rises, leading to notable differences in cell proliferation activity and cellular functionality between highly differentiated cells and those in earlier stages[1,2]. Studies have demonstrated that suitable culture media, supplement factors, and other additives can maintain cells in an early stage of differentiation, preserving cell proliferation activity and biological characteristics effectively[3-7]. Different cells thrive in different culture environments, and research on the in vitro cultivation environment of chondrocytes is currently limited.
Articular cartilage is primarily composed of chondrocytes and cartilage matrix, including collagen fibers composed of type II collagen, proteoglycans and water. Type II collagen and proteoglycans have long been considered characteristic markers of articular chondrocytes. In the in vitro cultivation of human fetal chondrocytes, early-passaged chondrocytes closely resemble the in vivo conditions in terms of morphology, function and biological features, making them suitable seed cells for cartilage tissue engineering. However, with increasing passage numbers during in vitro expansion, chondrocyte morphology gradually transitions from a granular shape to a spindle shape, transforming into fibroblast-like cells[8]. Concurrently, significant metabolic changes occur, characterized by a reduction in the characteristic protein type II collagen and an increase in types I and III collagen, along with a decrease in aggregated proteoglycans, indicating chondrocyte dedifferentiation[9]. Moreover, the longer the in vitro cultivation period, the more pronounced the phenomenon of chondrocyte dedifferentiation becomes.
Insulin-Transferrin-Selenium-Ethanolamine (ITSE) is a commonly used additive in cell culture, effectively promoting cell proliferation and maintaining cell status[10]. ITSE consists of four components; I which stimulates cell proliferation, T involved in the isolation and transport of iron ions across the cell membrane[11], S as an essential trace nutrient in living organisms[12], and E, a precursor in phospholipid synthesis. Research indicates that the components of ITSE can to some extent inhibit chondrocyte dedifferentiation. However, the composition of ITSE remains complex. Whether its components can be dissected and recombined, using a simplified additive with fewer components to achieve optimal or comparable effects in inhibiting chondrocyte dedifferentiation, thus simplifying the in vitro cultivation system, is a topic worth exploring. The primary objective of this study was to mitigate or minimize the occurrence of dedifferentiation in the in vitro cultivation of chondrocytes and streamline the cultivation system. The aim was to facilitate the extracorporeal proliferation of fetal chondrocytes while identifying a straightforward combination of basal culture media and ITSE components that optimally preserved the expression of chondrocyte genes.
Materials and Methods
Experimental materials:
With informed consent and approval from the ethics committee (2023k33), fetal samples from 12 w to 16 w of gestation were obtained from Shanghai Pudong New Area People’s Hospital through medical abortions. Basal culture media (Dulbecco’s Modified Eagle Medium (DMEM) with high glucose, DMEM with low glucose, Alpha-Minimum Essential Medium (α-MEM), DMEM/F12, and serum-reduced DMEM/F12 (1:1)) were purchased from Shanghai BasalMedia Bio-Technology Co., Ltd. Collagenase II was obtained from Shanghai Yisheng Biotechnology Co., Ltd. Supplement factors I, sodium S, and T were purchased from Lianke Technologies. E was obtained from Sigma. A commercially available ITSE mixture was purchased from Biogems. Antibodies for cell surface markers Clusters of Differentiation (CD) 73, CD90, CD105, CD19, CD11b, CD45, Human Leukocyte Antigen (HLA)-DE, and CD34 were acquired from R&D systems. Quantitative Polymerase Chain Reaction (qPCR) primers for chondrocyte genes Aggrecan (ACAN) and Collagen Type II A1 (COL2A1) were synthesized by Shanghai Sangon Biotech. All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by a Full named institutional and/or licensing committee. Informed consent was obtained from our hospital.
Isolation and culture of fetal chondrocytes:
Twelve fetal samples aged 12 w to 16 w obtained from medical abortions were processed after obtaining informed consent and ethics committee approval. In a biosafety cabinet, skin, muscle and superficial fascia were removed from the samples to expose limb bones and joints. After removing fascia and perichondrium from the cartilage surface, semitransparent cartilage tissue was obtained. The tissue was then cut into small pieces, enzymatically digested with collagenase II and primary chondrocytes were obtained. The obtained chondrocytes were cultured at 37° in a 5 % Carbon dioxide (CO2) cell culture incubator.
Observation of cell morphology:
Cell growth was observed before each passage operation. When the cell confluence reached 80 % or more, chondrocyte morphology was observed using an inverted microscope, and images were captured. Two random fields at 40X and 100X magnifications under the same view were selected for photography and saved.
Cell proliferation:
Cell growth was observed, and when the cell confluence in the culture flask reached 80 % or more, cells were passaged. A cell counter recorded cell harvest, and cell multiplication and doubling time were calculated by comparing cell counts at the time of seeding.
Cell Counting Kit-8 (CCK-8) assay:
Cell growth was observed, and when the cell confluence in the culture flask reached 80 % or more, cells were collected and counted. Equal numbers of cells were seeded in 96-well plates, with five replicates. After 48 h, Optical Density (OD) values were measured using a microplate reader for data analysis.
Flow cytometry for cell surface markers:
Flow cytometry was used to detect chondrocyte surface markers CD73, CD90, CD105, CD19, CD11b, CD45, HLA-DE and CD34. The brief process included cell collection and counting, antibody incubation, washing, flow cytometry analysis and data interpretation.
qPCR for gene expression:
qPCR was used to measure the relative expression levels of chondrocyte marker genes ACAN and COL2A1. The concise process included cell collection and counting, total Ribonucleic Acid (RNA) extraction, reverse transcription of total RNA into complimentary Deoxyribonucleic Acid (cDNA), qPCR instrument analysis and data interpretation.
Results and Discussion
To assess the impact of different culture media on the dedifferentiation of fetal chondrocytes in vitro, twelve fetal samples aged 12 w to 16 w obtained from medical abortions were utilized for the isolation and culture of primary cells. All the information of samples from donors were recorded. Initially, within a biosafety cabinet, the skin, muscle and superficial fascia were removed from the samples to expose limb bones and joints. Subsequently, the perichondrium and fascia on the surface of the cartilage were carefully removed, resulting in semi-transparent cartilage tissue (fig. 1). After enzymatic digestion with collagenase II, primary chondrocytes were obtained. The cell yield and viability were 2.23±1.32×104 cells/mg and 90.81 %±3.88 %, respectively (Table 1), indicating that a substantial number of highly viable chondrocytes could be isolated from fetal articular cartilage tissue.
| Cartilaginous site | Amount of live cells harvested (mg) | Primary cell viability (%) | Cell diameter (µm) |
|---|---|---|---|
| Extremities and joints | 2.23±1.32×104 | 90.81±3.88 | 13.5±0.99 |
Table 1: Viability and yield of primary chondrocytes
The isolated cells were seeded at a density of 2×104 cells/cm2 and cultured in the incubator. The culture medium was changed every 3 d, and passaging was performed when the confluence reached 80 %.
Isolated fetal articular chondrocytes exhibited adherent growth. After passaging in vitro, earlypassage cells displayed a typical polygonal morphology, gradually transitioning into a fibroblastlike spindle shape with increasing passage numbers, a characteristic transformation observed in fibroblastlike cells (fig. 2). As fetal chondrocytes in in vitro culture closely resemble the in vivo conditions in terms of morphology and function during early passages, and literature reports indicate that human fetal chondrocytes express surface markers associated with mesenchymal stem cells, including positive markers CD73, CD90, CD105, and negative markers CD19, CD11b, CD45, HLA-DE, and CD34.
Flow cytometry was employed to evaluate the expression of surface markers at passages P0 and P2 of fetal chondrocytes. The findings, in line with existing literature, are detailed in Table 2. For P0, the expression of positive markers CD73, CD90 and CD105 was 99.95 %±0.03 %, 99.42 %±0.38 %, and 98.10 %±0.10 %, respectively, while the expression of negative markers CD19, CD11b, CD45, HLA-DR, and CD34 was 0.02 %±0.01 %, 0.01 %±0.01 %, 0.01 %±0.01 %, 0.01 %±0.01 % and 0.20 %±0.15 %, respectively. At P2, the expression of positive markers CD73, CD90, and CD105 was 99.96 %±0.08 %, 99.61 %±0.44 %, and 98.75 %±1.70 %, respectively, while the expression of negative markers CD19, CD11b, CD45, HLA-DR and CD34 was 0.01 %±0.01 %, 0.01 %±0.01 %, 0.01 %±0.01 %, 0.01 %±0.01 %, 0.24 %±0.10 %, respectively. In summary, this study successfully isolated chondrocytes from fetal articular cartilage, demonstrating in vitro proliferation and manifesting specific characteristics of mesenchymal stem cells.
|
Markers of mesenchymal stem cells | |||||||
|---|---|---|---|---|---|---|---|---|
| CD73 | CD90 | CD105 | CD19 | CD11b | CD45 | HLA-DR | CD34 | |
P0 |
99.95 %±0.03 % | 99.42 %±0.38 % | 98.10 %±0.75 % | 0.02 %±0.01 % | 0.01 %±0.01 % | 0.01 %±0.01 % | 0.01 %±0.01 % | 0.20 %±0.15 % |
P2 |
99.96 %±0.08 % | 99.61 %±0.44 % | 98.75 %±1.70 % | 0.01 %±0.01 % | 0.01 %±0.01 % | 0.01 %±0.01 % | 0.01 %±0.01 % | 0.24 %±0.10 % |
Table 2: Flow cytometric analysis of surface markers in fetal chondrocytes
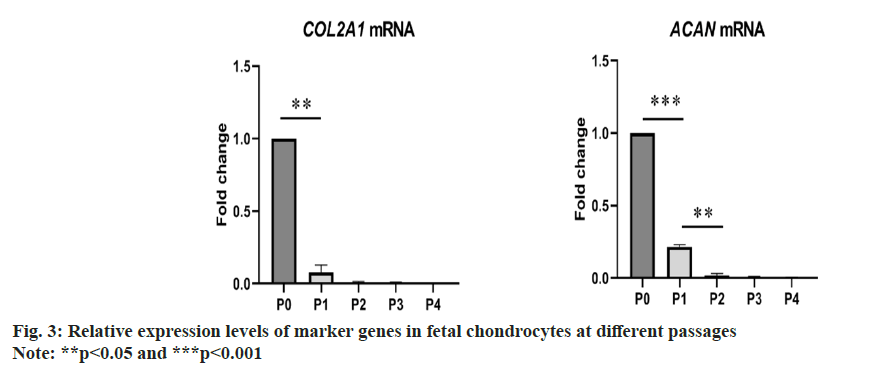
During the in vitro cultivation of fetal chondrocytes using commercial culture media, the expression levels of chondrocyte marker genes were found to be unstable. As depicted in fig. 3, the expression levels of chondrocyte markers rapidly decreased with passages, indicating dedifferentiation and loss of chondrogenic characteristics. To maintain the expression of chondrocyte markers and promote cell proliferation, subsequent efforts aimed to optimize the culture medium composition, including both basal culture media and supplement factors.
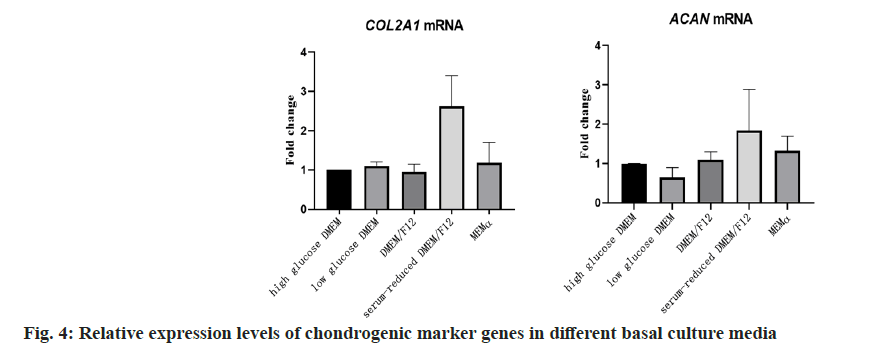
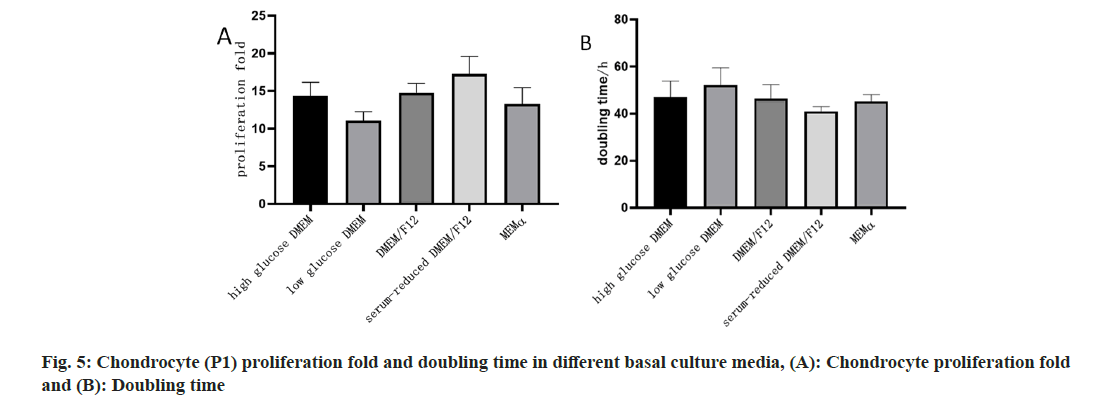
To investigate the impact of different basal culture media on the proliferation capacity and gene expression of chondrocytes, this study selected highglucose DMEM, low-glucose DMEM, DMEM/F12, serum-reduced DMEM/F12, and α-MEM as basal culture media. qPCR was employed to measure the relative expression levels of chondrocyte marker genes COL2A1 and ACAN in P1 passage chondrocytes. The results in fig. 4 demonstrated that chondrocytes cultured in serum-reduced DMEM/F12 exhibited higher relative expression levels of COL2A1 and ACAN. Simultaneously, in terms of cell proliferation, chondrocytes cultured in serum-reduced DMEM/F12 showed the highest amplification factor and the shortest doubling time, indicating stronger proliferation capacity (fig. 5).
The comprehensive analysis of these results suggested that different basal culture media could influence the gene expression and proliferation capacity of fetal chondrocytes. Among them, serumreduced DMEM/F12 was relatively favorable for maintaining the expression of chondrocyte marker genes and promoting cell proliferation. Therefore, serum-reduced DMEM/F12 was chosen as the basal culture medium for subsequent studies.
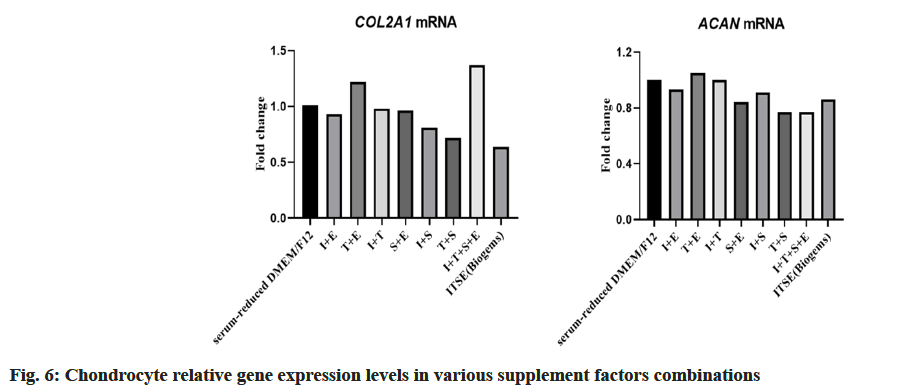
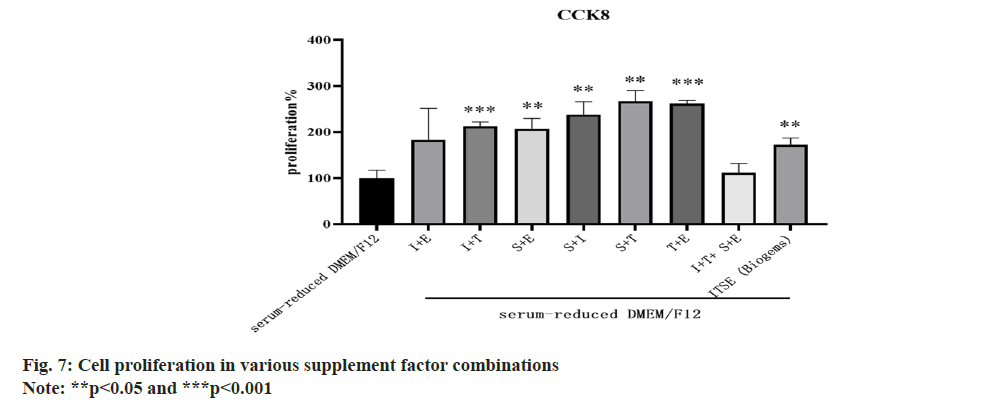
Based on the aforementioned research results, serum-reduced DMEM/F12 was chosen as the basal culture medium for chondrocyte cultivation. To further optimize the chondrocyte culture medium and investigate the effects of different supplement factors on chondrocyte proliferation and gene expression, four supplement factors ITSE were selected for this study. Various combinations of these factors (Table 3 and fig. 6) were tested using serum-reduced DMEM/ F12 as the control, and the relative expression levels of chondrocyte marker genes COL2A1 and ACAN were measured. The control group with serum-reduced DMEM/F12 was set as 1. Results >1 indicated a relative increase in chondrocyte gene expression levels, suggesting lower dedifferentiation. Results <1 indicated a relative decrease in chondrocyte gene expression levels, suggesting higher dedifferentiation. The results revealed that among the combinations, T+E exhibited a relative expression level of 1.22 for COL2A1 and 1.05 for ACAN, both >1, while other combinations showed relative expression levels below 1. This finding indicated that the T+E combination was relatively favorable for gene expression. Using serum-reduced DMEM/F12 as the control group, statistical analysis of the proliferation capacity of chondrocytes cultured under different combinations was conducted. In the bar chart, a higher column indicates a relatively faster growth rate and stronger proliferation capacity in the respective culture combination. The study found that the combinations of sodium S+T and T+E demonstrated relatively strong cell proliferation capacity (fig. 7).
| Control | s-r DMEM/F12 | Serum-reduced DMEM/F12 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| I+E | T+E | I+T | S+E | I+S | T+S | I+T+S+E | ITSE (Biogems) | ||
| COL2A1 | 1.01 | 0.93 | 1.22 | 0.98 | 0.96 | 0.81 | 0.72 | 1.37 | 0.64 |
| ACAN | 1 | 0.93 | 1.05 | 1 | 0.84 | 0.91 | 0.77 | 0.77 | 0.86 |
Note: s-r DMEM/F12 means serum-reduced DMEM/F12
Table 3: Relative gene expression levels in various factor combinations
In summary, considering both gene expression and cell proliferation, the addition of T+E to serumreduced DMEM/F12 was found to be advantageous for gene expression and promoting cell proliferation.
In the present study, we investigated the impact of different basal culture media and various combinations of components in ITSE on the morphology, proliferation and expression of cartilage marker genes in fetal chondrocytes during in vitro culture. The objective was to identify the optimal culture medium for chondrocyte cultivation. The results indicated that reducing serum in DMEM/F12 culture medium, supplemented with transferrin and ethanolamine, effectively minimized dedifferentiation during the in vitro culture of chondrocytes and enhanced their proliferative capacity.
Previous studies have demonstrated that chondrocytes undergo dedifferentiation during in vitro culture[13,14]. With increasing passage numbers and culture duration, chondrocytes cultured in monolayers lose their original phenotype, transitioning from a polygonal or round shape to a fibroblast-like spindle shape. Simultaneously, there is a change in the gene expression profile of the cells, marked by a decrease in the expression of type II collagen, proteoglycans, and the Y-chromosome Sex-determining region Y-Box 9 (SOX9) gene, along with an increase in the expression of type I collagen.
Additionally, research has identified significant differences in gene and protein expression[15], as well as phenotype, between normal and dedifferentiated chondrocytes. Dedifferentiated chondrocytes exhibit elevated levels of tenascin-C, Hypoxia-Inducible Factor (HIF)-1α, and fibroregulin, while normal chondrocytes express higher levels of type XI collagen alpha 1 and Superficial Zone Protein (SZP). Another study suggested that α-Smooth Muscle Actin (SMA) was expressed during the dedifferentiation process of chondrocytes and increased with the degree of dedifferentiation[16]. The ratio of CD90+ cells to CD105+ cells significantly decreases during chondrocyte dedifferentiation, serving as an indicator of dedifferentiation[17]. Furthermore, Fibroblast Growth Factor (FGF)-18 is highly expressed during chondrocyte dedifferentiation and serves as a dedifferentiation marker[18]. The phenomenon of chondrocyte dedifferentiation poses a bottleneck in cartilage cell transplantation therapy for joint cartilage diseases in cartilage tissue engineering research. Understanding dedifferentiation not only promotes further development in cartilage tissue engineering research but also provides a research model for other degenerative diseases such as intervertebral disc degeneration and osteoarthritis[19]. Under appropriate culture conditions, dedifferentiated chondrocytes can redifferentiate. Redifferentiated passaged chondrocytes exhibit consistency with primary chondrocytes and can be utilized to form cartilage tissue. The study of chondrocyte dedifferentiation and its molecular mechanisms has become a current focus in the field of cartilage tissue engineering.
Research suggests that human fetal chondrocytes are a potential source of cartilage regeneration cells. They can proliferate for over 15 generations in vitro without apparent growth inhibition. In comparison to young human chondrocytes and bone marrow mesenchymal stem cells, human fetal chondrocytes possess stronger multipotent differentiation capabilities. They can differentiate not only into chondrocytes but also into adipocytes and osteoblasts. Human fetal chondrocytes excel in cartilage tissue formation compared to bone marrow mesenchymal stem cells. In cartilage regeneration and tissue engineering, human fetal chondrocytes can serve as a suitable cell source[20]. This study demonstrated that the selection of culture media and added factors influenced the growth characteristics of chondrocytes, providing new guidance for chondrocyte culture and contributing thoughtful insights for utilizing chondrocytes in treating degenerative diseases through cartilage regeneration and tissue engineering. Different combinations of culture media and added factors in this study resulted in different expressions of cartilage genes in cultured chondrocytes, consistent with previous research findings[13]. The differential expression of typical surface markers of chondrocytes depends on the applied culture environment, offering a new research strategy for further investigating the impact of different matrix components on chondrocyte characteristics. The combination of basal culture media and added factors selected in this study effectively reduced chondrocyte dedifferentiation. This finding might offer a reference solution for minimizing dedifferentiation in chondrocyte cultures in the field of cartilage tissue engineering.
However, certain limitations exist in the current research on the cultivation of fetal chondrocytes. Our study was confined to reducing the degree of chondrocyte dedifferentiation under two-dimensional culture conditions. While an increasing number of studies seek optimal conditions for chondrocyte culture, the in vivo environment for cells to function is three-dimensional. Hence, compared to threedimensional culture systems, the effectiveness of chondrocyte therapy for diseases under twodimensional culture is far below expectations. More and more studies are leaning towards threedimensional culture systems. The required matrix for three-dimensional culture is still under exploration to obtain a matrix material that is more costeffective and yields better results. Therefore, in the future, combining the optimal culture medium composition with three-dimensional culture systems may maximally improve the dedifferentiation phenomenon in the in vitro culture of chondrocytes.
Funding:
Pudong New Area Health and Family Planning Commission Discipline Construction Project (No: PWZxk2022-28) and Changzhou Health Green Seedling Talent Program (No: CZQM2020036).
Author’s contributions:
The authors confirm contribution to the paper as follows; study conception and design by Chenyan Xue, Wenfeng Ye, Yuhua Wang; data collection by Wenfeng Ye, Linlin Chen, Xiangnan Chen; analysis and interpretation of results by Wanmin Chen, Wenfeng Ye, Jiaping Fang; draft manuscript preparation by Wenfeng Ye, Lihua Zhu. All authors reviewed the results and approved the final version of the manuscript. Wenfeng Ye and Wanmin Chen are co first authors.
Conflict of interests:
The authors declared no conflict of interests.
References
- Bianchi VJ, Lee A, Anderson J, Parreno J, Theodoropoulos J, Backstein D, et al. Redifferentiated chondrocytes in fibrin gel for the repair of articular cartilage lesions. Am J Sports Med 2019;47(10):2348-59.
[Crossref] [Google Scholar] [PubMed]
- von der Mark K, Gauss V, von der Mark H, Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 1977;267(5611):531-2.
[Crossref] [Google Scholar] [PubMed]
- Duan L, Ma B, Liang Y, Chen J, Zhu W, Li M, et al. Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy. Am J Transl Res 2015;7(2):194.
[Google Scholar] [PubMed]
- Jiang J, Altammar J, Cong X, Ramsauer L, Steinbacher V, Dornseifer U, et al. Hypoxia Preconditioned Serum (HPS) promotes proliferation and chondrogenic phenotype of chondrocytes in vitro. Int J Mol Sci 2023;24(13):10441.
[Crossref] [Google Scholar] [PubMed]
- Jeyakumar V, Niculescu-Morzsa E, Bauer C, Lacza Z, Nehrer S. Platelet-rich plasma supports proliferation and redifferentiation of chondrocytes during in vitro expansion. Front Bioeng Biotechnol 2017;5:75.
[Crossref] [Google Scholar] [PubMed]
- Pei M, Seidel J, Vunjak-Novakovic G, Freed LE. Growth factors for sequential cellular de- and re-differentiation in tissue engineering. Biochem Biophys Res Commun 2002;294(1):149-54.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Zhang W, Li X, Zhong D, Li Y, Li J, et al. Strategies to modulate the redifferentiation of chondrocytes. Front Bioeng Biotechnol 2021;9:764193.
[Crossref] [Google Scholar] [PubMed]
- Davies RL, Kuiper NJ. Regenerative medicine: A review of the evolution of Autologous Chondrocyte Implantation (ACI) therapy. Bioengineering 2019;6(1):22.
[Crossref] [Google Scholar] [PubMed]
- Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vecsei V, et al. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthr Cartil 2002;10(1):62-70.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Zhang T, Wang R, Shi P, Pan B, Pang X. Insulin-transferrin-selenium as a novel serum-free media supplement for the culture of human amnion mesenchymal stem cells. Ann Clin Lab Sci 2019;49(1):63-71.
[Google Scholar] [PubMed]
- Wen X, Wang Y, Gu Y. Transferrin promotes chondrogenic differentiation in condylar growth through inducing autophagy via ULK1-ATG16L1 axis. Clin Sci 2023;137(18):1431-49.
[Crossref] [Google Scholar] [PubMed]
- Dai X, Li Y, Zhang R, Kou Y, Mo X, Cao J, et al. Effects of sodium selenite on c-Jun N-terminal kinase signalling pathway induced by oxidative stress in human chondrocytes and c-Jun N-terminal kinase expression in patients with Kashin-Beck disease, an endemic osteoarthritis. Br J Nutr 2016;115(9):1547-55.
[Crossref] [Google Scholar] [PubMed]
- Ecke A, Lutter AH, Scholka J, Hansch A, Becker R, Anderer U. Tissue specific differentiation of human chondrocytes depends on cell microenvironment and serum selection. Cells 2019;8(8):934.
[Crossref] [Google Scholar] [PubMed]
- Duval E, Leclercq S, Elissalde JM, Demoor M, Galéra P, Boumédiene K. Hypoxia-inducible factor 1α inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: Hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1α-dependent redifferentiation of chondrocytes. Arthritis Rheum 2009;60(10):3038-48.
[Crossref] [Google Scholar] [PubMed]
- Karlsen TA, Shahdadfar A, Brinchmann JE. Human primary articular chondrocytes, chondroblasts-like cells, and dedifferentiated chondrocytes: Differences in gene, microRNA, and protein expression and phenotype. Tissue Eng Part C Methods 2011;17(2):219-27.
[Crossref] [Google Scholar] [PubMed]
- Parreno J, Raju S, Wu PH, Kandel RA. MRTF-A signaling regulates the acquisition of the contractile phenotype in dedifferentiated chondrocytes. Matrix Biol 2017;62:3-14.
[Crossref] [Google Scholar] [PubMed]
- Pei M, He F. Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol 2012;227(5):2163-74.
[Crossref] [Google Scholar] [PubMed]
- Yamaoka H, Nishizawa S, Asawa Y, Fujihara Y, Ogasawara T, Yamaoka K, et al. Involvement of fibroblast growth factor 18 in dedifferentiation of cultured human chondrocytes. Cell Prolif 2010;43(1):67-76.
- Choi WH, Kim HR, Lee SJ, Jeong N, Park SR, Choi BH, et al. Fetal cartilage-derived cells have stem cell properties and are a highly potent cell source for cartilage regeneration. Cell Transplant 2016;25(3):449-61.
[Crossref] [Google Scholar] [PubMed]
- Kim HR, Kim J, Park SR, Min BH, Choi BH. Characterization of human fetal cartilage progenitor cells during long-term expansion in a xeno-free medium. Tissue Eng Regen Med 2018;15:649-59.
[Crossref] [Google Scholar] [PubMed]