- *Corresponding Author:
- S. Ning
Department of Pharmacy, Graduate School of Hebei North University, Qiaodong, Zhangjiakou 075000, China
E-mail: ning-shoubin@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “16-24” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of this research is to investigate the impact of butein on apoptosis, migration, invasion, and epithelial-mesenchymal transition in HT-29 colon cancer cells. HT-29 cancer cells were introduced into the suitable cell culture plates according to their density, and we exposed the cells to varying dose of butein for 24 h. Through the utilization of cell counting kit-8 assays, cell scratch, and cell invasion assays, we assessed the cells’ functionality, movement, and invasion capacity. The levels of apoptosis-related proteins B-cell lymphoma 2 and B-cell lymphoma 2 associated X, epithelial-mesenchymal transition-related proteins E-cadherin, N-cadherin, and snail, along with the growth arrest-specific 6 protein, were identified using Western blot. The proliferation, migration, and invasion of HT-29 cells were significantly suppressed by butein, along with the reduction in B-cell lymphoma 2 expression and B-cell lymphoma 2/B-cell lymphoma 2 associated X ratios (p<0.05). Furthermore, butein suppressed the expression of N-cadherin, enhanced the expression of E-cadherin, and inhibited the expression of snail, which is their upstream regulator (p<0.05). Additionally, we discovered that butein suppressed the expression of growth arrest-specific 6 (p<0.05). In conclusion, butein inhibited the proliferation, migration, invasion and promoted apoptosis, which may be related to the reversal of epithelial-mesenchymal transition process of HT-29 cells and this process, may be related to the inhibition the expression of growth arrest-specific 6 by butein.
Keywords
Butein, colon cancer, apoptosis, migration, invasion, epithelial mesenchymal transition
Colorectal Cancer (CRC) is the 2nd most common cause of cancer-related mortality in the United States. By the year 2023, it is estimated that around 153 020 people will receive a diagnosis of CRC, resulting in 52 550 fatalities caused by this illness. Among these cases, there will be 19 550 individuals below the age of 50 who will be affected, leading to 3750 deaths[1]. The prevalence of CRC continues to increase gradually as a result of economic progress and shifts in people's lifestyles and dietary patterns[2]. Because the initial stage of the lesion often lacks noticeable symptoms, a majority of patients receive a late diagnosis and consequently miss the optimal window for surgery, leading to an unfavorable prognosis and elevated mortality rate[3]. Chemotherapy is also an avenue for colon cancer, but drug toxicity limits its clinical application[4]. Consequently, it is indispensable to develop new, safer, and more effective drugs for preventing or treating CRC.
Phytocompounds are naturally present in plants, fruits, and vegetables, and they possess a wide range of biological significance, especially in terms of their medicinal attributes[5]. Butein, which is a naturally occurring polyphenolic compound is derived initially from the lacquer plant and is recognized for its medical benefits, particularly in Southeast Asia since antiquity. It has been utilized for the treatment of inflammation and cancer[6]. Growing evidence that butein inhibits proliferation, invasion, and chemoresistance in a wide range of cancer cells, including breast, pancreatic, bladder, and CRC[7-9]. Butein has shown a wide range of molecular targets, and some studies suggest that it can stop the movement and invasion of cancer cells by reversing the process of Epithelial-Mesenchymal Transition (EMT)[10,11].
EMT entails cells losing their tight organization characteristics and gaining mesenchymal traits, consequently boosting the cell mobility. Aggressive phenotype is developed as a result of the mechanical disruption of tight junctions, cell polarity, and reorganization of cytoskeletal structures in tumor cells. CRC is supported by substantial evidence which suggests that the importance of EMT relies within its progression and dissemination[12]. As a natural plant compound, the safety of butein has been widely recognized, and the side effects seem to be lower than that of chemotherapy drugs. The extraction technology of plant compounds is mature, saving cost for patients and additionally, as it has multi-molecular targets, it has high research value. Nevertheless, the research on the inhibitory impact of butein on CRC is scarce, and the underlying mechanisms are still not well understood. Hence the objective of this study was to investigate the impact of butein on the proliferation, infiltration, and EMT of HT-29 cells, along with any potential mechanisms involved.
Materials and Methods
Reagents and cell culture:
A solution of butein (TargetMol, China) was prepared by dissolving it in Dimethyl Sulfoxide (DMSO) at a concentration of 100 μM. HT-29 cells (Procell, China) were cultured in complete medium consisting of 10 % Fetal Bovine Serum (FBS) and 1 % penicillin-streptomycin mixture. The cells were grown at 37° in incubator with 5 % Carbon dioxide (CO2), and the medium was refreshed for every 48 h. Once the cells reach an approximate density of 80 %, the passage and experiment were conducted. The cells were grouped as a control group and a treatment group. The control group received treatment using a medium without serum (Aoqing, China), while the treatment group was subjected to various concentrations of butein for 24 h. The butein was mixed with serum-free Dulbecco's Modified Eagle Medium (DMEM) before dilution.
Further, rabbit B-cell lymphoma 2 (Bcl-2) (68103-1-lg), Bcl-2-Associated X (BAX) protein (60267-1-lg), Neural (N)-cadherin (22108-1-AP), Epithelial (E)-cadherin (20874-1-AP), snail (13099-1-AP), Growth Arrest-Specific 6 (GAS6) antibody (13795-1-AP) (ProteinTech, China) and mouse Beta (β)-actin antibody (Affbiotech, China) were obtained.
Cell Counting Kit-8 (CCK-8) assay for cellular activity:
HT-29 cells were seeded in 96-well plates at a density of 1.5×104 cells/well and were cultured with medium lacking serum in an incubator for 24 h. Once the cells adhered to the plate, the liquid in the wells was removed, and butein was introduced at concentrations of 10, 20, 30, 40, and 50 μM, and the control group was substituted with serum-free DMEM, with 5 wells/group for replication. The cells were then incubated in the incubator for another 24 h[13]. Cell viability was evaluated using the CCK-8 assay, followed by measuring the absorbance of the cells at 450 nm using a microplate reader (BioTek, Co., Ltd., China).
Terminal deoxynucleotidyl transferase Deoxyuridine Triphosphate (dUTP) Nick-End Labeling (TUNEL) assay:
HT-29 cells were seeded at a density of 5×104 cells/well in 24-well plates and cultured in serum-free medium for 24 h. Once the cells adhered to the surface, the liquid in the wells was removed and the HT-29 cells were treated with different concentrations (10, 20, 30, 40 and 50 μM) of butein for 24 h. We found that apoptosis staining increased significantly when butein concentration was 30 μM.
This was consistent with the results of the above tests of cell activity, and met our expectations, so we selected the concentration gradients of 20, 30 and 40 μM as the treatment concentration. Subsequently, different concentrations (0, 20, 30, and 40 μM) of butein were introduced for 24 h based on preliminary findings. The control group was replaced with serum-free DMEM. Apoptosis was detected following the instructions of the TUNEL kit (Roche, China), and subsequently, the fluorescence microscope (ZEISS microscopy, China) was used to capture the images.
Cell scratch assay:
HT-29 cells were inoculated into a 6-well plate. When the cell density was about 100 %, a straight line was drawn at the bottom of the hole with the tip of a 200 μl pipette. Various levels of butein (5, 10 and 20 μM) were introduced to the culture and incubated at 37° for 24 h, while the control group was substituted with DMEM lacking serum. Microscopic images of the damaged region were captured using a microscope (Carl Zeiss NTS Ltd., China)[14].
Cell invasion assay:
To test the invasion experiment, a Transwell cell (with an aperture of 8 μM) coated with Matrigel gel (Coring, China) was applied. HT-29 cells were exposed to various concentrations of butein like 0, 5, 10 and 20 μM for 24 h. Subsequently, the cells were placed in the upper chamber with a density of 5000/well, while the lower chamber was supplemented with DMEM medium containing 10 % FBS.
Following incubation of the cells at 37° for duration of 24 h, the cells in the upper chamber were gently cleaned using a cotton swab, then immobilized using 4 % paraformaldehyde, and finally stained with crystal violet. An optical microscope was used to observe and count the cells in the outer surface of the lower chamber[14].
Western blot assay:
HT-29 cells were exposed to various levels of butein for 24 h. Subsequently, the cells were lysed using cell lysate, and the protein concentration was assessed using the Bicinchoninic Acid (BCA) technique. Equal quantities of protein was extracted from every sample and subsequently moved onto a Polyvinylidene Difluoride (PVDF) membrane (Merck Millipore, China) following electrophoresis using a 10 % Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel (Solarbio Life Sciences, China). The membrane was left to incubate overnight at 4° with the primary antibody, subsequently followed by the corresponding secondary antibodies, and finally, the protein signal was visualized using Enhanced Chemiluminescence (ECL) reagent[11].
Statistical analysis:
GraphPad Prism (version 9.3.1) software was utilized for statistical analysis and the outcomes were presented as mean±Standard Deviation (SD). Statistical significance was determined using one-way Analysis of Variance (ANOVA), with a significance level of p<0.05.
Results and Discussion
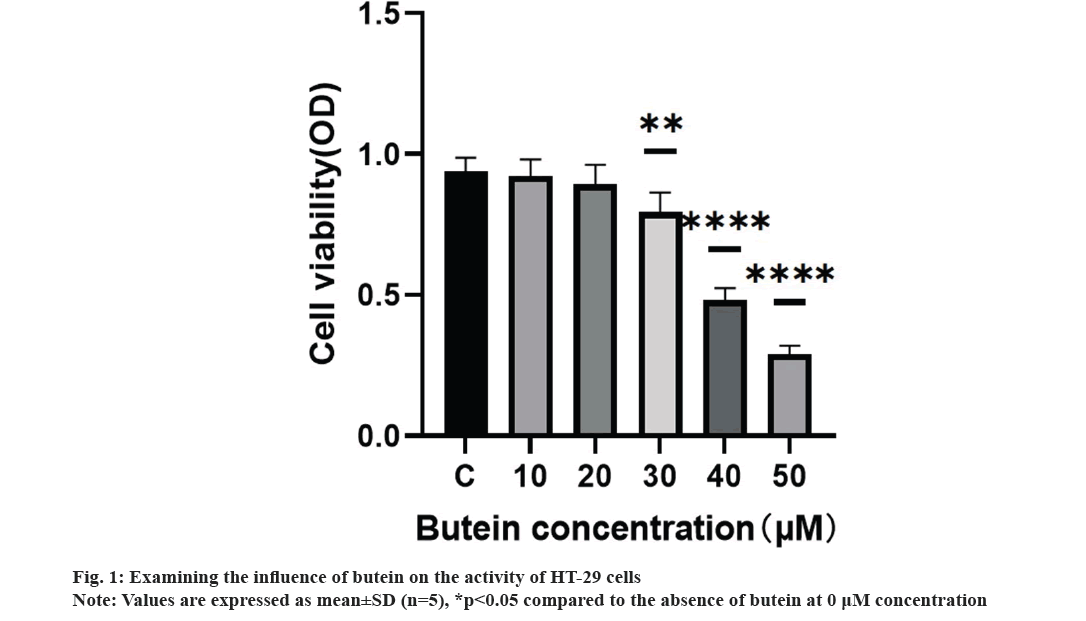
We systematically studied the impact of butein on the growth of HT-29 cells. HT-29 cells were treated with different concentrations of butein (0, 10, 20, 30, 40, and 50 μM) for 24 h. The CCK-8 assay results demonstrated the inhibitory effect of butein on the proliferation of HT-29 cells (fig. 1). Exposing the cells to 30, 40, and 50 μM concentrations of butein for 24 h resulted in a significant decrease in cell activity, when compared to the control group (p<0.05), except for 10 and 20 μM concentrations of butein.
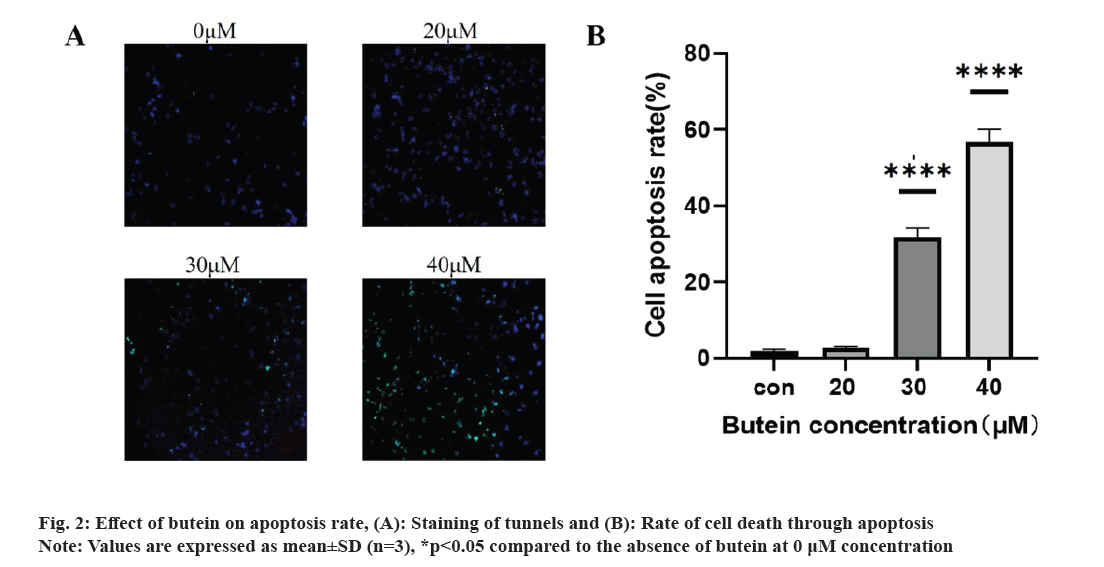
The impact of butein on apoptosis was studied. Following a 24 h treatment of HT-29 cells with different concentrations of butein (0, 20, 30, and 40 μM), apoptosis was assessed using TUNEL staining. The results revealed a significantly higher apoptosis rate in the groups treated with 30 and 40 μM of butein, compared to the control group (p<0.05) (fig. 2A and 2B), except for the 20 μM concentration of butein. The findings indicate that butein might play a role in inducing apoptosis in HT-29 cells.
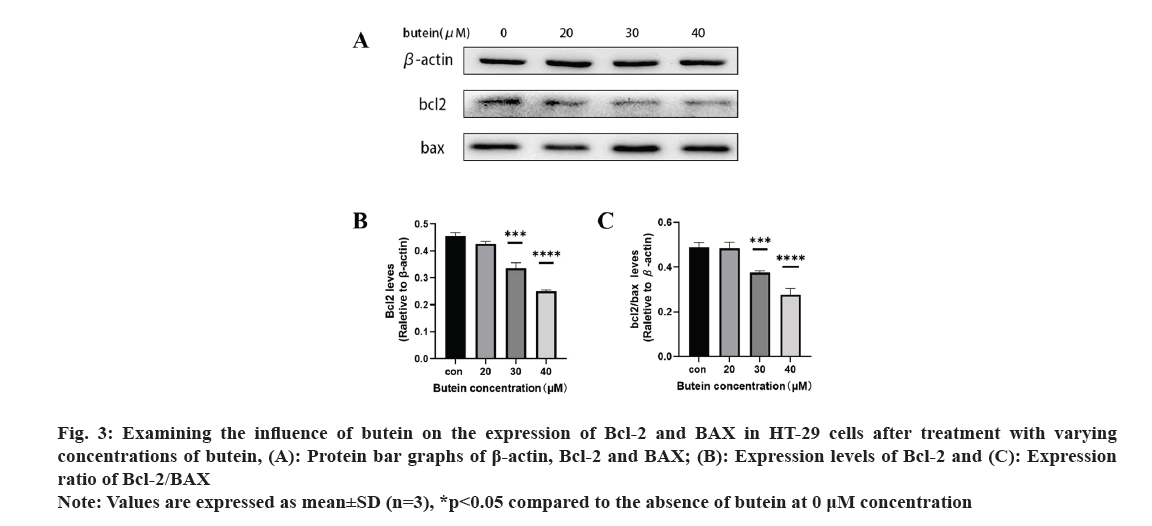
Further, the impact of butein on apoptotic protein expression in HT-29 cells was also observed. After exposing HT-29 cells to different concentrations of butein for 24 h, Western blotting analysis (fig. 3A) revealed the presence of apoptosis-related proteins Bcl-2 and BAX. The results showed that, except for the 20 μM butein concentration, the level of the anti-apoptotic protein Bcl-2 was greatly decreased after 24 h of treatment with 30 and 40 μM concentrations of butein, compared with the control group (p<0.05) (fig. 3B). Additionally, the ratio of Bcl-2 expression to that of the pro-apoptotic protein BAX was significantly reduced (p<0.05) (fig. 3C), indicating that butein might enhance apoptosis.
Fig. 3: Examining the influence of butein on the expression of Bcl-2 and BAX in HT-29 cells after treatment with varying concentrations of butein, (A): Protein bar graphs of β-actin, Bcl-2 and BAX; (B): Expression levels of Bcl-2 and (C): Expression ratio of Bcl-2/BAX.
Note: Values are expressed as mean±SD (n=3), *p<0.05 compared to the absence of butein at 0 μM concentration.
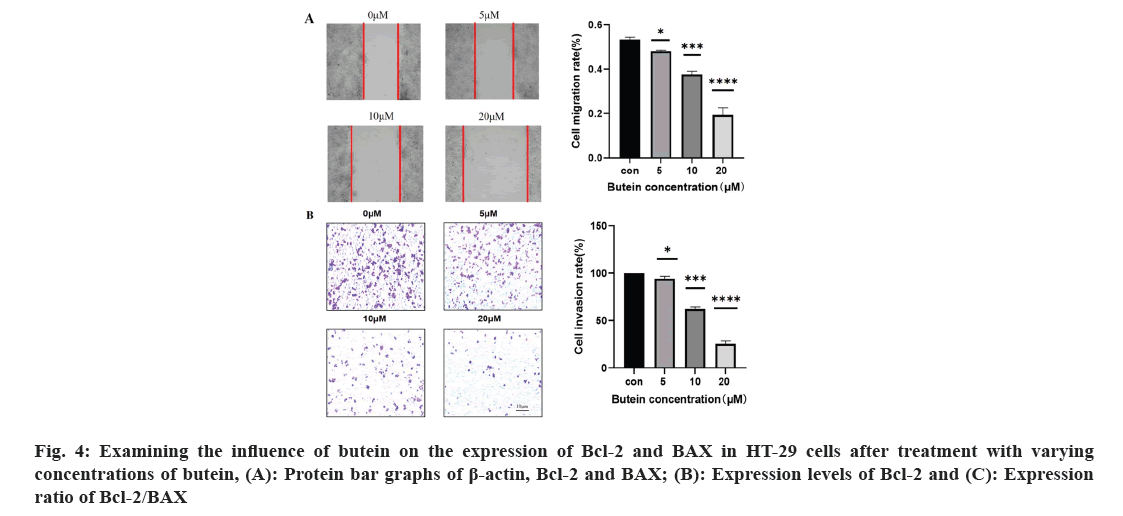
The effect of butein on the rates of migration and invasion of HT-29 cells was studied. To examine the movement and infiltration rates of HT-29 cells were exposed to 0, 5, 10, and 20 μM of butein for 24 h; the cell scratch and cell invasion assays were employed (fig. 4). Cells treated with 5, 10, and 20 μM concentrations of butein for 24 h exhibited a noteworthy decrease in migration and invasion rates when compared to the control group (p<0.05). According to the results, butein was found to have the ability to inhibit the movement and infiltration of HT-29 cells.
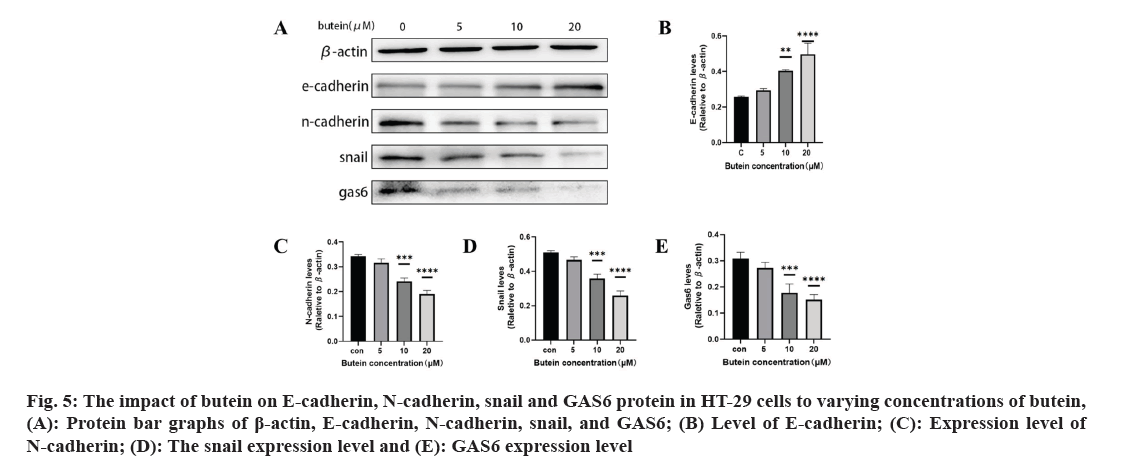
The impact of butein on EMT-associated proteins E-cadherin, N-cadherin, snail, and GAS6 protein in HT-29 cells was studied. After 24 h of treatment with butein, we investigated the impact of snail, E-cadherin, N-cadherin, and GAS6 proteins on HT-29 cells (fig. 5A). The results showed that, except for the 5 μM butein concentration, the level of E-cadherin increased significantly (p<0.05) (fig. 5B) with butein 10 and 20 μM concentrations of butein after 24 h. However, the expression of N-cadherin decreased significantly (p<0.05) (fig. 5C), and the expression of their upstream regulator, snail, was significantly reduced (p<0.05) (fig. 5D). The results indicate that butein potentially hindered the migration and invasion of HT-29 cells by suppressing the process of EMT. In the meantime, after being treated with butein at concentrations of 10 and 20 μM for 24 h, the GAS6 protein expression showed a significant decrease in comparison to the control group (p<0.05) (fig. 5E). The results indicated that butein may suppress the activation of the GAS6/Anexelekto (Axl) signaling pathway.
Fig. 5: The impact of butein on E-cadherin, N-cadherin, snail and GAS6 protein in HT-29 cells to varying concentrations of butein, (A): Protein bar graphs of β-actin, E-cadherin, N-cadherin, snail, and GAS6; (B) Level of E-cadherin; (C): Expression level of N-cadherin; (D): The snail expression level and (E): GAS6 expression level.
The incidence of CRC, a common neoplasm discovered in the gastrointestinal system, is steadily increasing. Neoplasms remain the main killer worldwide[15-19]. CRC has a high mortality rate, ranking 2nd among all malignant tumors globally[20]. Therefore, it is pivotal to develop new effective anti-CRC drugs. In multiple biological studies butein, a compound found naturally in various plants has been extensively researched for its numerous biological implications, including its potential anticancer properties against different types of human cancer cells[21,22]. Huang et al. found that butein could inhibit the proliferation of colon cancer cells by inducing the cessation of mitosis and abnormal chromosome segregation. This research examined the impacts of varying butein concentrations on human CRC HT-29 cells. The results displayed that butein effectively suppressed the activity of cancer cells, as determined by the CCK-8 assay after 24 h (fig. 1). Results indicated that butein inhibited the proliferation of HT-29 cells. Nonetheless, the development of human cancerous growths is not solely attributed to the escalating cell count resulting from unregulated cell division[23], but also to the hindrance of the programmed cell death[24], whenever there is a disruption in the equilibrium between the regulation of cell division and apoptosis, tumors emerge.
The exposure of HT-29 cells to different concentrations of butein for 24 h resulted in a notable rise in apoptosis levels (fig. 2). The results indicate that butein can induce apoptosis in HT-29 cells. The Bcl-2 family of apoptotic proteins includes both the pro-apoptotic protein BAX and the anti-apoptotic protein Bcl-2. Their interaction significantly influences apoptosis, which is ultimately induced by activating the caspase signaling pathway[25]. According to the results of the study, it was found that butein has the ability to reduce the levels of Bcl-2 protein expression and decrease the ratio of Bcl-2 to BAX protein expression (fig. 3). By altering the Bcl-2 and BAX protein expression, butein has the potential to induce apoptosis in HT-29 cells.
The process of cancer metastasis is intricate and dynamic, encompassing several stages such as the dislodgement of primary tumor cells, invasion into nearby tissues and intrusion into blood and lymph vessels. Moreover, it encompasses the formation of lymphatic channels and intravascular neoplastic emboli, as well as the generation of fresh metastases in distant sites during the circulation of lymph and blood[26]. Several recent studies have shown a connection between the spread of different types of cancer cells, specifically CRC, and the process of EMT[27,28]. Epithelial cells lose characteristics such as cell adhesion and polarity in the course of EMT and acquire mesenchymal traits like motility[29]. During the process of EMT, tumor cells experience the disruption of cellular tight junctions, loss of cell polarity, and rearrangement of cytoskeletal structures, leading to the development of an invasive phenotype[30]. It is a crucial molecular mechanism which triggered the incursion and metastasis of various tumor cells, like CRC. Transcription factors such as snail and twist play a crucial role in the regulation of EMT. Its downstream consequences include the decrease in E-cadherin and other proteins responsible for maintaining tight cell-to-cell communication, and the increase in N-cadherin, which plays a role in building the cytoskeleton[31]. In our study, we confirmed that butein has the ability to impede the migration and invasiveness of HT-29 cells, as evidenced by the results of the cell scratch assay and cell invasion assay (fig. 4). The impact of butein on the expression of E-cadherin, N-cadherin, and snail were analyzed using Western blotting. According to the results, butein was found to enhance the E-cadherin expression, decrease the N-cadherin expression, and reduce the expression of snail, the regulator that above them (fig. 5). The results suggest that butein may hinder the process of EMT in HT-29 cells, thereby acting as a strong inhibitor of tumor invasion and metastasis. Since concentration gradients were used in this study to demonstrate that cell migration and invasion it was found that EMT processes were positively correlated with butein. Direct relationship between them was not further proved, and the experimental results were limited to some extent.
GAS6 is a 75 kDa soluble glycoprotein, and its downstream receptors are known as Tyrosine-protein kinase Receptor (TYRO-3) Axl and Mertk (Mer) (TAM) receptors, belong to the tyrosine kinase family. By connecting to the TAM receptors, GAS6 triggers downstream signaling cascade, including mechanisms like Protein Kinase-B (Akt), Extracellular signal-Regulated Kinase (ERK), and Phosphoinositide 3-Kinase (PI3K). Activation of signaling pathways like Akt, ERK, and PI3K promotes the growth and dissemination of tumor cells[32]. Furthermore, multiple studies have shown significant levels of GAS6 in bladder cancer, prostate cancer and pancreatic ductal adenocarcinoma, as well as various other tumor types. These findings indicate its involvement in promoting tumor growth and metastasis[33]. It has been demonstrated that the intrinsic connection between the GAS6/Axl signaling pathway and the EMT process, which actively facilitates the survival, invasion, and metastasis of various cancer cells[34]. The GAS6/Axl signaling pathway activation and the EMT process, which have been scientifically linked, are recognized to promote the survival, invasion, and metastasis of diverse cancer cells[35,36]. It is confirmed that an increase in butein concentration led to a decrease in the expression of gas6 protein in HT-29 cells during the current study (fig. 5). This suggests that butein could potentially impede the GAS6/TAM signaling pathway's activation within these cells. The presence of this intervention could potentially have a substantial impact on impeding the development of cancer cells and hindering EMT in HT-29 cells. However, this study did not further prove the direct correlation between GAS6 and EMT process, and the correlation of cancer cell progression, so the experimental results have some limitations.
Butein is a natural plant compound that is simple to extract at low cost and safe for human use. Limited research has been conducted on the impact of butein on human colon cancer, while the suppression of the EMT process by butein has been extensively examined and its inhibitory effects on human colon cancer cells remain unexplored. The present study discovered that butein has a suppressive impact on GAS6 protein in HT-29 cells, thereby broadening the investigation into the potential molecular mechanism of butein in combating colon cancer.
This research, however, has some limitations. For the purpose of this investigation, cell cultures were employed for in vitro experimentation. If primary tumor cells were used and in vivo experiments were completed, the research value and clinical significance would be better reflected. The research discovered that butein has a suppressive impact on GAS6. However, it did not establish a definite connection between the GAS6/TAM pathway, the advancement of colon cancer and EMT process. This connection can be further validated by controlling the expression of GAS6 protein.
The current study suggests that butein could hinder the enhancement of human CRC HT-29 cells. Additionally, it indicates that butein could potentially impede the growth of colon cancer cells by inducing apoptosis through the modulation of Bcl-2 and BAX pathways. Additionally, butein has been linked to a reduction in the migration and invasion of HT-29 cells, potentially due to its ability to prevent the EMT process in HT-29 cells. Moreover, the blocking of GAS6 protein by butein disrupted the activation of the GAS6/TAM signaling pathway, potentially serving as a molecular mechanism for butein's ability to inhibit the invasion of HT-29 cells and the EMT process.
Although the anti-tumor effects of butein have been extensively studied, the studies on its mechanism are mainly in vitro experiments, and in vivo experiments are relatively lacking. At the same time, whether butein has potential side effects on the human body and the effective dose of application is not clear. The specific mechanism of antitumor effect of butein is not clear, and whether it has the same effect on different cells of the same tumor remains to be studied. Therefore, the research on butein still needs to continue to deepen, carry out in vivo research through animal experiments, and also need a large number of clinical studies to verify its therapeutic effect and effective dose.
Acknowledgement:
The authors are grateful to the support of 2021 Clinical Research Program at Air Force Medical University (2021LC2201).
Author contributions:
Quanlong Yang wrote the paper draft. Xuehui Shi corrected the draft. Shoubin Ning and Chongxi Fan conceived the project and supervised all procedures of this study. Quanlong Yang performed the experiments and prepared the figures. Xiaoying Wang and Donglin Zhao have provided various intellectual and material support. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Conflict of interests:
The authors declared no conflict of interests.
References
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73(3):233-54.
[Crossref] [Google Scholar] [PubMed]
- Sun P, Sun D, Wang X. Effects of Scutellaria barbata polysaccharide on the proliferation, apoptosis and EMT of human colon cancer HT-29 cells. Carbohydr Polym 2017;167:90-6.
[Crossref] [Google Scholar] [PubMed]
- Wan ML, Wang Y, Zeng Z, Deng B, Zhu BS, Cao T, et al. Colorectal Cancer (CRC) as a multifactorial disease and its causal correlations with multiple signaling pathways. Biosci Rep 2020;40(3):1-15.
[Crossref] [Google Scholar] [PubMed]
- Mishra B, Chaurasia S. Design of novel chemotherapeutic delivery systems for colon cancer therapy based on oral polymeric nanoparticles. Ther Deliv 2017;8(1):29-47.
[Crossref] [Google Scholar] [PubMed]
- Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res 2014;34(11):907-29.
[Crossref] [Google Scholar] [PubMed]
- Kim KH, Moon E, Choi SU, Pang C, Kim SY, Lee KR. Identification of cytotoxic and anti-inflammatory constituents from the bark of Toxicodendron vernicifluum (Stokes) F. A. Barkley. J Ethnopharmacol 2015;162:231-7.
[Crossref] [Google Scholar] [PubMed]
- Chen YH, Yeh CW, Lo HC, Su SL, Hseu YC, Hsu LS. Generation of reactive oxygen species mediates butein-induced apoptosis in neuroblastoma cells. Oncol Rep 2012;27(4):1233-7.
[Crossref] [Google Scholar] [PubMed]
- Cho SG, Woo SM, Ko SG. Butein suppresses breast cancer growth by reducing a production of intracellular reactive oxygen species. J Exp Clin Cancer Res 2014;33(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Huang YT, Lin CI, Chien PH, Tang TT, Lin J, Chao JI. The depletion of securin enhances butein-induced apoptosis and tumor inhibition in human colorectal cancer. Chem Biol Interact 2014;220:41-50.
[Crossref] [Google Scholar] [PubMed]
- Janani G, Pillai MM, Selvakumar R, Bhattacharyya A, Sabarinath C. An in vitro 3D model using collagen coated gelatin nanofibers for studying breast cancer metastasis. Biofabrication 2017;9(1):1-23.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Chen W, Li X. A novel anticancer effect of butein: Inhibition of invasion through the ERK1/2 and NF-kappa B signaling pathways in bladder cancer cells. FEBS Lett 2008;582(13):1821-8.
[Crossref] [Google Scholar] [PubMed]
- Spaderna S, Schmalhofer O, Hlubek F, Berx G, Eger A, Merkel S, et al. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology 2006;131(3):830-40.
[Crossref] [Google Scholar] [PubMed]
- Lee SH, Seo GS, Jin XY, Ko G, Sohn DH. Butein blocks tumor necrosis factor alpha-induced interleukin 8 and matrix metalloproteinase 7 production by inhibiting p38 kinase and osteopontin mediated signaling events in HT-29 cells. Life Sci 2007;81:1535-43.
[Crossref] [Google Scholar] [PubMed]
- Lai YW, Wang SW, Chang CH, Liu SC, Chen YJ, Chi CW, et al. Butein inhibits metastatic behavior in mouse melanoma cells through VEGF expression and translation-dependent signaling pathway regulation. BMC Complement Altern Med 2015;15:1-10.
[Crossref] [Google Scholar] [PubMed]
- Sun M, Liu X, Xia L, Chen Y, Kuang L, Gu X, et al. A nine-lncRNA signature predicts distant relapse-free survival of HER2-negative breast cancer patients receiving taxane and anthracycline-based neoadjuvant chemotherapy. Biochem Pharmacol 2021;189:1-39.
[Crossref] [Google Scholar] [PubMed]
- Yang Z, Jiang S, Lu C, Ji T, Yang W, Li T, et al. SOX11: Friend or foe in tumor prevention and carcinogenesis? Ther Adv Med Oncol 2019;11:1-25.
[Crossref] [Google Scholar] [PubMed]
- Li T, Qiao T. Unraveling tumor microenvironment of small-cell lung cancer: Implications for immunotherapy. Semin Cancer Biol 2022;86:117-25.
[Crossref] [Google Scholar] [PubMed]
- Ma Z, Fan C, Yang Y, Di S, Hu W, Li T, et al. Thapsigargin sensitizes human esophageal cancer to TRAIL-induced apoptosis via AMPK activation. Sci Rep 2016;6:1-16.
[Crossref] [Google Scholar] [PubMed]
- Tang Z, Dong H, Li T, Wang N, Wei X, Wu H, et al. The synergistic reducing drug resistance effect of cisplatin and ursolic acid on osteosarcoma through a multistep mechanism involving ferritinophagy. Oxid Med Cell Longev 2021:1-17.
[Crossref] [Google Scholar] [PubMed]
- Cheng W, Feng YQ, Ren J, Jing D, Wang C. Anti-tumor role of bacillus subtilis fmbJ-derived fengycin on human colon cancer HT-29 cell line. Neoplasma 2016;63(2):215-22.
[Crossref] [Google Scholar] [PubMed]
- Yit CC, Das NP. Cytotoxic effect of butein on human colon adenocarcinoma cell proliferation. Cancer Lett 1994;82(1):65-72.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Chan FL, Chen S, Leung LK. The plant polyphenol butein inhibits testosterone-induced proliferation in breast cancer cells expressing aromatase. Life Sci 2005;77(1):39-51.
[Crossref] [Google Scholar] [PubMed]
- Yu J, Wang R, Chen J, Wu J, Dang Z, Zhang Q, et al. miR-340 inhibits proliferation and induces apoptosis in gastric cancer cell line SGC-7901, possibly via the AKT pathway. Med Sci Monit 2017;23:71-7.
[Crossref] [Google Scholar] [PubMed]
- Zhu Y, Jiang Y, Shi L, Du L, Xu X, Wang E, et al. 7-O-Geranylquercetin induces apoptosis in gastric cancer cells via ROS-MAPK mediated mitochondrial signaling pathway activation. Biomed Pharmacother 2017;87:527-38.
[Crossref] [Google Scholar] [PubMed]
- Renault TT, Teijido O, Antonsson B, Dejean LM, Manon S. Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-xL: Keep your friends close but your enemies closer. Int J Biochem Cell Biol 2013;45(1):64-7.
[Crossref] [Google Scholar] [PubMed]
- Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat Rev Cancer 2014;14(6):430-9.
[Crossref] [Google Scholar] [PubMed]
- Punzi S, Balestrieri C, D’Alesio C, Bossi D, Dellino GI, Gatti E, et al. WDR5 inhibition halts metastasis dissemination by repressing the mesenchymal phenotype of breast cancer cells. Breast Cancer Res 2019;21(1):1-18.
[Crossref] [Google Scholar] [PubMed]
- Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg 2000;87(8):992-1005.
[Crossref] [Google Scholar] [PubMed]
- Liu CH, Tang WC, Sia P, Huang CC, Yang PM, Wu MH, et al. Berberine inhibits the metastatic ability of prostate cancer cells by suppressing Epithelial-to-Mesenchymal Transition (EMT)-associated genes with predictive and prognostic relevance. Int J Med Sci 2015;12(1):63-71.
[Crossref] [Google Scholar] [PubMed]
- Vu T, Datta PK. Regulation of EMT in colorectal cancer: A culprit in metastasis. Cancers 2017;9(12):1-22.
[Crossref] [Google Scholar] [PubMed]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15(3):178-96.
[Crossref] [Google Scholar] [PubMed]
- Wu G, Ma Z, Cheng Y, Hu W, Deng C, Jiang S, et al. Targeting Gas6/TAM in cancer cells and tumor microenvironment. Mol Cancer 2018;17(1):20.
[Crossref] [Google Scholar] [PubMed]
- Ireland L, Luckett T, Schmid MC, Mielgo A. Blockade of stromal Gas6 alters cancer cell plasticity, activates NK cells, and inhibits pancreatic cancer metastasis. Front Immunol 2020;11:1-16.
[Crossref] [Google Scholar] [PubMed]
- Antony J, Huang RY. Axl-driven EMT state as a targetable conduit in cancer. Cancer Res 2017;77(14):3725-32.
[Crossref] [Google Scholar] [PubMed]
- Kong L, Lu X, Chen X, Wu Y, Zhang Y, Shi H, et al. Qigesan inhibits esophageal cancer cell invasion and migration by inhibiting Gas6/Axl-induced epithelial-mesenchymal transition. Aging 2020;12(10):9714-25.
[Crossref] [Google Scholar] [PubMed]
- Gjerdrum C, Tiron C, Høiby T, Stefansson I, Haugen H, Sandal T, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA 2010;107(3):1124-9.
[Crossref] [Google Scholar] [PubMed]