- *Corresponding Author:
- N. Darbndi
Department of Biology, Faculty of Science, Arak University, Arak 38156-8-8349, Iran
E-mail: n-darbandi@araku.ac.ir
| Date of Submission | 12 April 2017 |
| Date of Revision | 07 November 2017 |
| Date of Acceptance | 05 June 2018 |
| Indian J Pharm Sci 2018;80(4):668-675 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The current study illustrated the possible positive effects of flavonoids present in the leaves of Mespilus germanica on cognitive performance, learning and memory function in an intra-cerebroventricular streptozotocin-induced Alzheimer’s disease model in male Wistar rats. Five groups (saline-saline control, streptozotocin-saline, and streptozotocin with different doses of flavonoids, 5, 10 and 20 mg/kg) of rats were examined. Rats received different doses of Mespilus germanica flavonoids or saline over three weeks starting one day before surgery. Next, they were assessed using a learning and memory test. After subjected to the behavioral test, the animals were perfused and their brains were fixed with paraformaldehyde 4 % and the tissue was further processed. Finally, the density of intact neurons in the hippocampal CA1 area in all groups was investigated. The results revealed that injection of streptozotocin significantly reduced cognitive function, memory retention as well as CA1 intact neurons compared to the control group. Flavonoids extracted by Mespilus germanica considerably eradicated the negative effects of streptozotocin. Accordingly, Mespilus germanica leaf flavonoids can improve cognitive deficits resulted from injection of streptozotocin.
Keywords
Mespilus germanica flavonoids, streptozotocin, cognitive performance, learning and memory, hippocampal CA1 neurons, Alzheimer’s disease
Alzheimer's disease (AD) is a progressive neurodegenerative disease that results in functional loss and cognitive decline. It was reported that in addition to aging as the main risk factor for the AD, lifestyle and nutritional habits play crucial roles in its onset [1]. Based on other existing evidence, it was depicted that oxidative stress has a considerably important role to play in this process [2-4]. The hippocampal network, which is the main part of the brain has such a prominent role in long-term memory and spatial navigation, and it is also one of the strikingly first regions of the brain that can meticulously show damages, memory problems and disorientation in AD patients [5].
Beneficial impacts of flavonoids in reducing oxidative stress and inflammatory cytokine levels beside their other useful features such as decreasing neural death, glucose levels, and blood lipids have recently become noticeable. Flavonoids eliminate the risk of developing various diseases such as different types of cancers, cardiovascular diseases, strokes and finally AD [6-8]. Flavonoids could scavenge free radicals through several mechanisms; some would inhibit free radicals directly, while others would absorb free radicals indirectly and end up preventing their oxidation [9]. These beneficial properties of flavonoids are involved in many different ways to reduce certain mechanisms of neural loss, diminish inflammatory cytokines and hence flavonoids could be effective in restricting the rapid development of AD [10,11].
Mespilus germanica L. (medlar) is a folk plant, large, white and flowery shrub of Rosaceae family, Maloideae subfamily and Mespilus genus [12]. It is a spiny wildtype shrub growing to a height of 2 to 3 m. Its fruit is extremely popular especially among people who live in the northern region of Iran, southeastern Europe and Turkey [13]. This precious plant is rich in antioxidants, flavonoids, vitamins and antiviral properties [14]. The present study examined the possible beneficial effects of M. germanica flavonoids on memory retention, cognitive performance and hippocampal CA1 neurons in an intra-cerebroventricular streptozotocin (STZ)- induced experimental AD model in the male Wistar rats.
Materials and Methods
Extraction and identification of flavonoids from the leaves of M. germanica
M. germanica leaves were collected from the forests of northern regions of Iran (Rasht). It was identified and authenticated using valuable and available references [15]. Leaves were dried in the shade for a week, powdered, and 250 g of the leaf powder was extracted with 70 % ethanol and the extract was vacuum concentrated to dryness in a rotary evaporator. The flavonoids in the extract were isolated and detected using two-dimensional paper and thin-layer chromatography according to reported methods [16]. The leaf flavonoid extract was kept in dark vials and stored in cool conditions until further use.
Two-dimensional paper chromatography (2-D PC)
Two hundred milligrams of the leaf powder was boiled for 2 min in 5 ml of 70 % EtOH, then cooled and left to extract for 24 h. The extract was then filtered, evaporated to dryness by rotary evaporation at 40° and redissolved in 2 ml of 80 % MeOH for performing 2-D PC. About 2 μl of the extract was applied and rutin (quercetin 3-O-rutinoside) was used as a standard, the paper was developed in mixture of n-butanol:acetic acid:water (BAW; first direction). HOAc 15 % was used for the second direction. The dried chromatogram was studied using UV at 366 nm and Rf values were calculated for both dimensions. After acid hydrolysis, co-chromatography was done by applying hydrolyzed flavonoid extract and standards on thin-layer cellulose chromatogram. TLC plate was run in a mixed solvent, viewed under UV at 245 nm and the Rf -values and colour was recorded for each spot in comparison with standards.
Experimental animals
Male Wistar rats (Pasteur Institute, Tehran, Iran) weighing 220 to 250 g at the time of surgery were used. The animals were kept in an animal house under a 12-h light/12-h dark cycle and controlled (22±2°) temperature. The rats had free access to food and water and were allowed to adapt to laboratory conditions for at least 1 w before surgery. They were handled for 5 min per day. Eight animals were used in each experimental group and all procedures for the treatment of the animals were approved by the Research and Ethics Committee of the Biology School of Arak University and performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (publication no. 80-23, revised 1996).
Surgical and infusion procedures
Rats were anesthetized under 50 mg/kg ketamine- 5 mg/kg xylazine [17]. Stainless steel, 22-gauge guide cannula were bilaterally implanted 1 mm above the intended site of injection according to coordinates given by Paxinos and Watson atlas [18]. Stereotaxic coordinates for lateral ventricle were incisor bar (–3.3 mm), 0.8 mm posterior to the bregma, ±1.4 mm lateral to the sagittal suture and 3.4 mm from the top of the rats’ skull. In order to inject drugs, the animals were gently restrained by hand and injected using 27-gauge injection needle (1 mm below the tip of the guide cannula). Each injection unit was injected by polyethylene tubing attached to a 25 μl Hamilton syringe. The left and right ventricles were infused with a 10 μl solution on each side (20 μl per rat) over 2 to 3 min.
Behavioural test
Shuttle box consisted of two chambers, light (white compartment, 20×20×30 cm), and the dark one (black compartment, 20×20×30 cm). A guillotine door was made on the floor in the centre of the partition between the two compartments. Stainless steel grades (2.5 mm in diameter) were placed at 1-cm intervals (distance between the centers of the grades) on the floor of the dark compartment to produce foot shock. Intermittent electric shocks (1 Hz, 3 s, 1/5 mA) were delivered to the grid floor of the dark compartment from an insulated stimulator.
Training
The method for training was based on our previous studies [19]. In the habituation stage, each rat was placed in the bright room for approximately 30 min prior to experiments. The acquisition trail was carried out 30 min after the habituation trial. The rat was placed in the bright compartment, and 5 s later the guillotine door was opened. Immediately after entering animal into the dark compartment, the door was closed and a foot shock (1 Hz, 1/5 mA, and 3 s) was delivered to their feet.
Retention test
Twenty-four hours after the training, a retention test due to the investigation of long-term memory was carried out. Every animal was placed into the bright compartment and the door was opened; time latency to the entry of the dark chamber and the time spent, stepthrough latency (STL) and total dark chamber (TDC) were evaluated.
Tissue preparation and hematoxylin-eosin staining
Rats were deeply anesthetized with 3.5 % chloral hydrate (35 mg/100 g, i.p.), and perfused by intracardiac infusion with phosphate buffer solution (0.1 M, pH 7.4) followed by 200 ml of 4 % paraformaldehyde fixative in phosphate buffer solution (0.1 M, pH 7.4) for 15 min. Next, their brains were removed, isolated, postfixed in 4 % paraformaldehyde (24 h), and embedded in paraffin. Coronal sections (10 μm in thickness) were taken from the dorsal hippocampus and stained with haematoxylin and eosin. Finally, the numbers of intact neurons, in which one can vividly consider a distinctive nucleus, in the hippocampal C A1 pyramidal layer were counted [20] using a light microscope (BX40, Olympus, New York, USA) connected to a camera (Olympus, DP12), and quantitatively analysed by Image J software.
Experimental procedure
The drugs used in the current study were STZ (Sigma- Aldrich, USA) and flavonoids extracted from the M. germanica leaf (5, 10 and 20 mg/kg/day). STZ powder was dissolved in sterile 0.9 % saline just prior to the experiment [21-24]. STZ was administered intracerebroventricularly (ICV) into the lateral ventricles, and flavonoids from the M. germanica leaf were injected intraperitoneally.
The therapeutic period was 21 d (3 w) for every group. In STZ group and STZ+flavonoids group, rats were injected on the first and third days with STZ (3 mg/kg; ICV; bilateral; 10 μl volume on each side) while in control group, they were injected with saline (ICV; bilateral with 10 μl volume on each side) on the first and third days. All STZ or saline recipients were infused over 3 w with different doses of flavonoids (5, 10, 20 mg/kg i.p.) or saline (1 ml/kg i.p.). Immediately after 3 w, rats were trained in the stepthrough apparatus. Twenty-four hours later, a retention test was performed to determine their memories. Immediately after the behavioural test all rats were anesthetized with 3.5 % chloral hydrate (35 mg/ 100 g intraperitoneal) and perfused via the left ventricle heart. Eventually, in order to examine the effects of M. germanica on hippocampal CA1 pyramidal neurons, their brains were removed and stained with hematoxylin-eosin.
Statistical analysis
All results were expressed as the mean±SEM. For the behavioural test STL and TDC were analysed by one-way analysis of variance (ANOVA) and Tukey’s posthoc test. Statistical results were assessed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Differences between groups in the average number of intact neurons in the hippocampal CA1 pyramidal layer were analysed using Graph Pad Prism Software (GraphPad Software Inc., San Diego, CA, USA; version 5.00). A value of p<0.05 was considered statistically significant.
Results and Discussion
The concentration of flavonoids in M. germanica leaf was 24 g/kg on a dry mass basis involving flavonoid sulfates and C and C-O glycosides of flavones. Moreover, quercetin, myricetin, luteolin, kaempferol, and chrysin were identified as the main constituents of the leaf (Table 1).
| Constituent Present/Sample applied | MEL |
|---|---|
| Total flavonoids | 7 |
| Flavonoid C- and C/O- glycosides | 5 |
| Flavonoid sulphates | 2 |
| Aglycones | 0 |
| Apigenin | |
| Chrysin | + |
| Genistein | |
| Isorhamnetin | |
| Kaempferol | + |
| Luteolin | + |
| Morin | |
| Myricetin | + |
| Naringenin | |
| Quercetin | + |
| Rhamnetin | |
| Rutin | |
| Tricin | |
| Vitexin |
Table 1: Two-dimensional paper and thin layer chromatographical data of M. germanica leaf extract
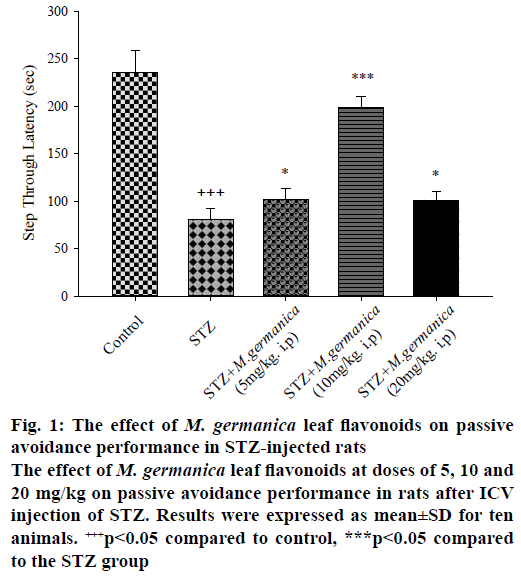
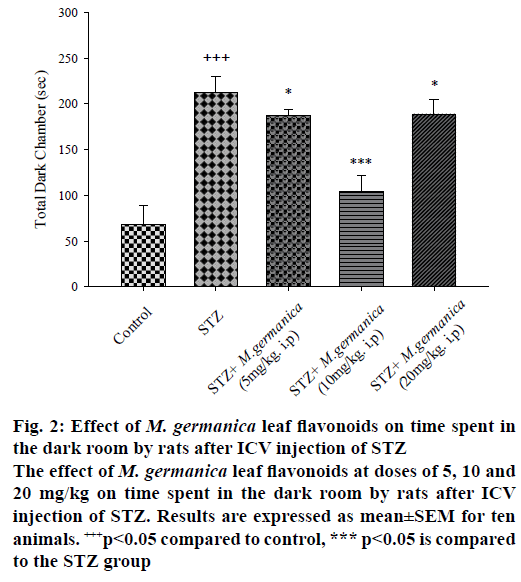
Results of passive avoidance test in the STZ group showed that in contrast the significant reduction of STL, TDC was remarkably escalated indicating memory impairment (Figures. 1 and 2) while oneway ANOVA revealed that the administration of various doses of M. germanica flavonoids (5, 10 and 20 mg/kg i.p.), over 21 d, significantly improved cognitive performance with different ratios. Additionally, the Tukey test depicted that treatment with different doses of M. germanica flavonoids noticeably increased STL (Figure 1) and decreased TDC (Figure 2) as well. The highest positive impact of M. germanica flavonoids was observed with 10 mg/kg dose (F(4,50) = 23/17, p<0.05).
Figure 1: The effect of M. germanica leaf flavonoids on passive avoidance performance in STZ-injected rats
The effect of M. germanica leaf flavonoids at doses of 5, 10 and 20 mg/kg on passive avoidance performance in rats after ICV injection of STZ. Results were expressed as mean±SD for ten animals. +++p<0.05 compared to control, ***p<0.05 compared to the STZ group
Figure 2: Effect of M. germanica leaf flavonoids on time spent in the dark room by rats after ICV injection of STZ
The effect of M. germanica leaf flavonoids at doses of 5, 10 and 20 mg/kg on time spent in the dark room by rats after ICV injection of STZ. Results are expressed as mean±SEM for ten animals. +++p<0.05 compared to control, *** p<0.05 is compared to the STZ group
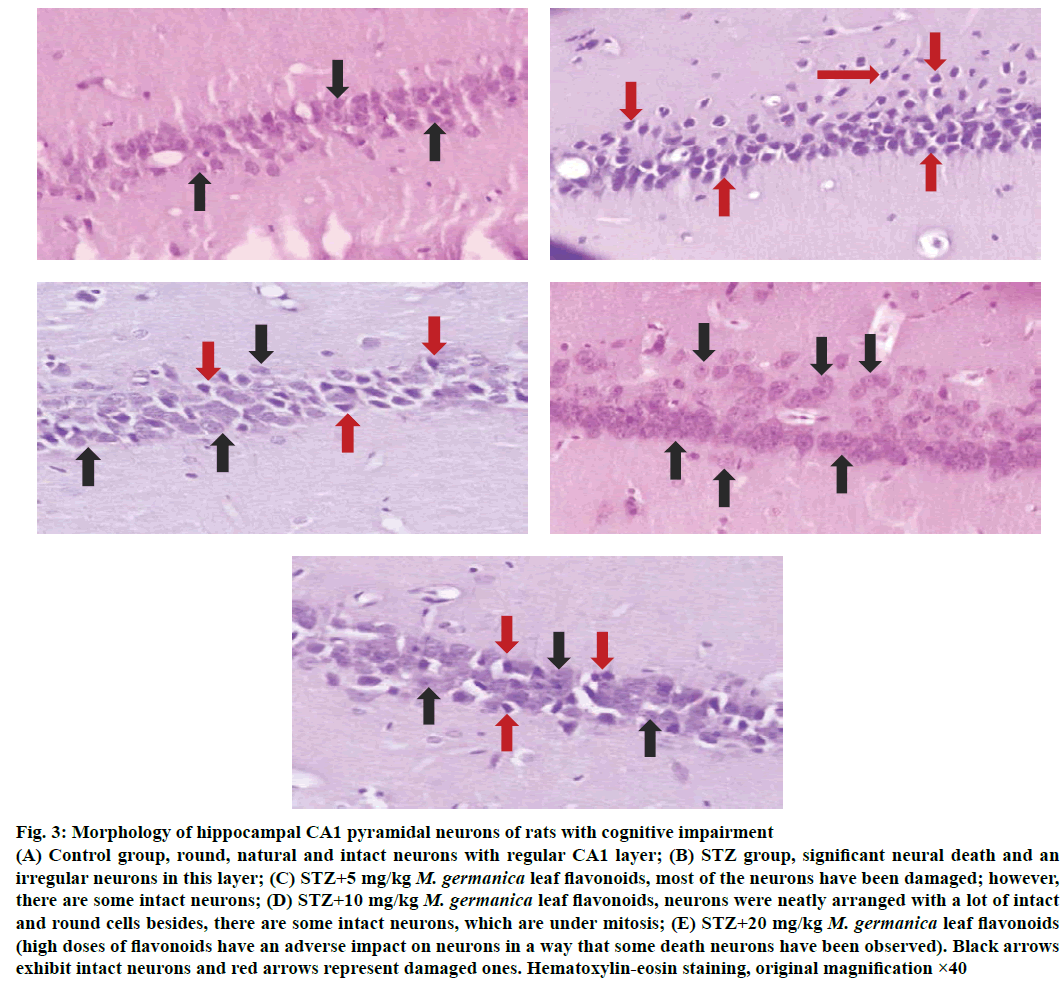
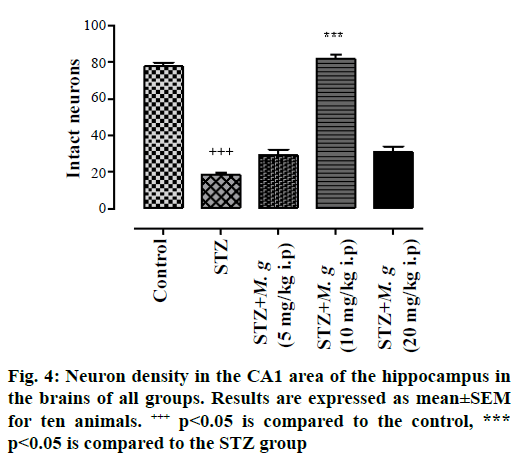
As histological surveys showed (Figure 3A-E) the number of intact neurons in the hippocampal CA1 pyramidal layer was markedly fell in the STZ group compared to the control ones (p<0.05). Despite the fact that STZ greatly injured neurons (Figure 3), M. germanica flavonoids could protect neurons from STZ-induced damage, so treatment with M. germanica flavonoids (5, 10 and 20 mg/kg i.p.) substantially enhanced the number of intact neurons containing large, round, regular and intact nucleus (Figure 3) though this cure was most conspicuous in the dose of 10 mg/kg/day flavonoids (Figure 3), which could raise neuronal recovery and simultaneously improve neurogenesis in the hippocampal CA1 pyramidal layer.
Figure 3: Morphology of hippocampal CA1 pyramidal neurons of rats with cognitive impairment
(A) Control group, round, natural and intact neurons with regular CA1 layer; (B) STZ group, significant neural death and an irregular neurons in this layer; (C) STZ+5 mg/kg M. germanica leaf flavonoids, most of the neurons have been damaged; however, there are some intact neurons; (D) STZ+10 mg/kg M. germanica leaf flavonoids, neurons were neatly arranged with a lot of intact and round cells besides, there are some intact neurons, which are under mitosis; (E) STZ+20 mg/kg M. germanica leaf flavonoids (high doses of flavonoids have an adverse impact on neurons in a way that some death neurons have been observed). Black arrows exhibit intact neurons and red arrows represent damaged ones. Hematoxylin-eosin staining, original magnification ×40
In fact, the intact neurons were defined as roundshaped, cytoplasmic membrane-intact cells, without any nuclear condensation or distorted aspect. Regarding the numbers of neurons those which were counted in the hippocampal CA1 area, M. germanica flavonoids significantly increased the numbers of intact cells in diverse groups of flavonoids compared with STZ group (F(4,60) = 112, p<0.0; Figure 4).
Results indicated that the ICV injection of STZ (3 mg/kg; 10 μl in each ventricle) led to the significant detriment of memory over 3 w. Conversely, treatment of these animals with the flavonoids of M. germanica leaf (5, 10, 20 mg/kg, i.p.) significantly elevated learning and memory as well as cognitive functions in AD animals. On the other hand, high doses of flavonoids (STZ+20 mg/kg flavonoids groups) have an adverse impact on neurons in a way that some death neurons have been observed, and it can confirm previous studies, which have shown flavonoids in high doses have potentially toxic effects resulting in the generation of free radicals [25,26]. Besides, the results of other studies illustrated that flavonoid diets in some circumstances presumably become involved in the cancer process [27].
It has also been confirmed that the central ICV administration of STZ caused a significant reduction in cognitive function and a remarkable amplification in the abnormal aggregation of Aβ and total tau proteins. It is noteworthy that these alternations were accompanied by a decline in glycogen synthase kinase (GSK-3) alpha/beta ratio (phosphorylated/total) in animal brains [28,29]. Furthermore, injection of ICV-STZ induced high levels of neuroinflammation, oxidative stress, and biochemical changes considered to be a valid experimental model of the early pathophysiological shift in neurodegenerative disease [30]. Thus, STZ administration could show many aspects of AD [31].
In other studies, it has been shown that injection of ICVSTZ strengthened blood glucose and triglyceride levels consistently [32,33]. In another study by Chu and Qian, it has been displayed that ICV-STZ injection decreased cerebral glucose uptake and produced some negative impacts, which were able to recreate the molecular, pathological, and behavioural characteristics of AD. Consequently, ICV-STZ damaged memory strikingly and a reduction in insulin expression in the hippocampus and cerebral cortex were also observed in AD patients [34]. It has also been reported that when the expression of genes such as insulin-like growth factor-1 (IGF-1) is reduced, IGF-1 receptors were present in the frontal cortex, hippocampus, and hypothalamus of AD rat brains [25]. Lee et al. reported that the expression of insulin/IGF signalling-related genes has basically been destroyed in the hippocampus of STZinjected monkey brains, similar to the early stages of AD in human [35]. Leaves, fruit, bark and wood of M. germanica L (medlar) has been used as a herbal medicine. The medicinal uses included treatment of diarrhoea, oral abscess, stomach bloating, intestinal infections, as a hematopoietic, treatment of internal haemorrhage, regurgitation disposal cholera, strengthen fine skin, development and improvement of nerves, treatment of intestinal inflammation and infections, menstrual disorders, cutaneous leishmaniasis and as a diuretic [36-38].
Accumulating evidence clarified that flavonoids, because of their high amount of antioxidant, have a noticeable role to play in ameliorating the cognition [37-41]. The most influential feature of flavonoids, which was the ability to reduce free radical formation and scavenge free radicals is related to their antioxidant activity [42,43]; hence, they were reported to be potentially effective against some diseases such as Parkinson's disease, epilepsy, depression, schizophrenia, and finally AD [44-46].
Flavonoids modulated transcription factors and affected the expression of genes involved in cell survival in some particular areas in the Dentate Gyrus and CA3 regions of the hippocampus via the huge impact of lipid kinase, the most famous pathways of PI3/Akt and MAP kinase. They also generated LTP through improving neuronal connections [47]. Flavonoids prompted some significant changes in cerebral blood flow resulting particularly in angiogenesis, neurogenesis, and morphogenesis of neurons in the hippocampus and into the brain. It is unanimously believed that flavonoids played a significant role in the up-regulation of brainderived neurotrophic factor (BDNF), a vital protein that facilitated the survival of neurons and performed a major important function in the growth, differentiation, and evolution of cells [48].
The present study depicted that treatment with M. germanica flavonoids extracted from the leaves significantly treated the cognitive impairment, learning and memory defects induced by ICV injection of STZ. Besides, flavonoids extracted increased dosedependently the numbers of intact cells and also marvellously decreased the number of dead cells in CA1 hippocampus area.
The results of 2-DPC and TLC showed M. germanica leaf contains flavonoid sulfates, flavones C and C-/O glycosides. Aglycones were not found. Chrysin, kaempferol, myricetin, quercetin, and rutin were found in the leaf. These compounds are important as antioxidants. As Kolodziejczyk et al. reported that antioxidative activity of some Trifolium species may also result from the abundance of flavonoids in these plants [49].
The ICV injection of STZ (3 mg/kg)-induced cognitive deficits and decreased the number of pyramidal neurons in the hippocampal CA1 area in the STZ group. In this study, the injection of ICV-STZ and treating by M. germanica leaf flavonoids significantly improved memory retention and cognitive functions as well. Furthermore, the number of intact cells in hippocampal CA1 area gradually increased and the number of dead cells decreased.
Acknowledgements
This work was supported by Grants from Arak University (Arak, Iran).
Conflicts of interest
The authors have no conflict of interest.
Financial support and sponsorship
Nil.
References
- Rios JA, Cisternasa P, Arresea M, Barjac S, Inestrosaa NC. Is Alzheimer's disease related to metabolic syndrome? A Wnt signaling conundrum. Prog Neurobiol 2014;121:125-46.
- Darbandi N, Hashemi A, Noori M, Momeni HR. Effect of Cornus mas fruit flavonoids on memory retention, level of plasma glucose and lipids in an intracerebroventricular streptozotocin-induced experimental Alzheimer’s disease model in Wistar rats. Environ Exp Biol 2016;14:113-20.
- Razay G, Vreugdenhil A, Wilcock G. The metabolic syndrome and Alzheimer disease. Arch Neurol 2007;64(1):93-6.
- Newman M, Musgrave IF, Lardelli M. Alzheimer disease: Amyloidogenesis, the presenilins and animal models. Biochim Biophys Acta 2007;1772:285-97.
- Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J. Hippocampal Neuroanatomy: The Hippocampus Book. Oxford: Oxford University Press; 2006. p. 37-115.
- Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther 2011;90(2-3):157-77.
- Chen L, He T, Han Y, Sheng JZ, Jin S, Jin MW. Pentamethylquercetin Improves Adiponectin Expression in Differentiated 3T3-L1 Cells via a Mechanism that Implicates PPARγ together with TNF-α and IL-6. Molecules 2011;16(7):5754-768.
- Yao Y, Lin G, Xie Y, Ma P, Li G, Meng Q, et al. Preformulation studies of myricetin: a natural antioxidant flavonoid. Pharmazie 2014;69:19-26.
- Choi YJ, Kang JS, Park JH, Lee YJ, Choi JS, Kang YH. Polyphenolic flavonoids differ in their antiapoptotic efficacy in hydrogen peroxide-treated human vascular endothelial cells. J Nutr 2003;133(4):985-91.
- Shukitt-Hale B, Lau FC, Carey AN, Galli RL, Spangler EL, Ingram DK, et al. Blueberry polyphenols attenuate kainic acid-induced decrements incognition and alter inflammatory gene expression in rat hippocampus. Nutr Neurosci 2008;11:172-82.
- Rendeiro C, Guerreiro JD, Williams CM, Spencer JP. Flavonoids as modulators of memory and learning: molecular interactions resulting in behavioural effects. Proc Nutr Soc 2012;71(2):246-62.
- Pollmann B, Jacomet S. First evidence of Mespilus germanica L. (medlar) in Roman Switzerland. Vege Hist Archaeobot 2012;21:61-8.
- Nabavi SF, Nabavi SM, Ebrahimzadeh MA, Asgarirad H. The antioxidant activity of wild medlar (Mespilus germanica L.) fruit, stem bark and leaf. Afr J Biotechnol 2011;10:283-89.
- Gülçin I, Topal F, Sarıkaya SBO, Bursal E, Bilsel G, Gören AC. Polyphenol Contents and Antioxidant Properties of Medlar (Mespilus germanica L.). Rec Nat Prod 2011;5-3:158-75.
- Mozaffarian V. Trees and shrubs of Iran. Tehran: Farhang Moaser Press; 2005.
- Markham KR. Techniques of flavonoid identification. Cambridge, Massachusetts: Academic press; 1982. p. 16-55.
- Rezayof A, Darbandi N, Zarrindast MR. Nicotinic acetylcholine receptors of the ventral tegmental area are involved in mediating morphine-state-dependent learning. Neurobiol Learn Mem 2008;90:255-60.
- Paxinos G, Watson C. The Rat Brain in Stereotaxic. 4th ed. San Diego: Academic Press; 1998. p. 21 .
- Darbandi N, Rezayof A, Zarrindast MR. Modulation of morphine state-dependent learning by muscarinic cholinergic receptors of the ventral tegmental area. Physiol Behav 2008;94:604-10.
- Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol 2008;172:454-69.
- Ashrafpour1 M, Parsaei S, Sepehri H. Quercetin improved spatial memory dysfunctions in rat model of intracerebroventricular streptozotocin-induced sporadic Alzheimer’sdisease. Natl J Physiol Pharm Pharmacol 2015;5(5):411-15.
- Chen Y, Liang Z, Blanchard J, Dai CL, Sun S, Lee MH, et al. A non-transgenic mouse model (icv-STZ mouse) of Alzheimer’s disease: Similarities to and differences from the transgenic model (3xTg-AD mouse). Mol Neurobiol 2013;47(2):711-25.
- Chen Y, Liang Z, Tian Z, Blanchard J, Dai CL, Chalbot S, et al. Intracerebroventricular streptozotocin exacerbates Alzheimerlike changes of 3xTg-AD mice. Mol Neurobiol 2014;49(1):547-62.
- Ramezani M, Darbandi N, Khodagholi F, Hashemi A. Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer’s disease. Neural Regen Res 2016;11(12):1976-80.
- Skibola CF, Smith MT. Potential health impacts of excessive flavonoid intake. Free Radic Biol Med 2000;29(3-4):375-83.
- Dickancaité E, Nemeikaitè A, Kalvelytè A, Cènas N. Prooxidant character of flavonoid cytotoxicity: structure-activity relationships. Biochem Mol Biol Int 1998;45(5):923-30.
- Knekt P, Järvinen R, Seppänen R, Hellövaara M, Teppo L, Pukkala E, et al. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am J Epidemiol 1997;1;146(3):223-30.
- Paweł G. Intracerebroventricular Streptozotocin Injections as a Model of Alzheimer ’s disease: in Search of a Relevant Mechanism. Mol Neurobiol 2016;53:1741-52.
- Bedse G, Di Domenico FD, Serviddio G, Cassano T. Aberrant insulin signaling in Alzheimer's disease: current knowledge. Front Neurosci 2015;9:204.
- Kamat PK. Streptozotocin-induced Alzheimer’s disease like changes and the underlying neural degeneration and regeneration mechanism. Neural Regen Res 2015;10(7):1050-52.
- Chen Y, Tian Z, Liang Z, Sun S, Dai CL, Lee MH, et al. Brain gene expression of a sporadic (icv-STZ Mouse) and a familial mouse model (3xTg-AD mouse) of Alzheimer's disease). PLoS One 2012;7(12):e51432.
- Komolafe O, Adeyemi D, Adewole S, Obuotor E. Streptozotocin-Induced Diabetes Alters The Serum Lipid Profiles Of Adult Wistar Rats. IJCVR 2008;7(1):7.
- Jiao L, Xiujuan S, Juan W, Song J, Lei X, Guotong X, et al. Comprehensive Experiment–Clinical Biochemistry: Determination of Blood Glucose and Triglycerides in Normal and Diabetic Rats. Biochem Mol Biol Educ 2015;43(1):47-51.
- Chu WZ, Qian CY. Expressions of Abeta1-40, Abeta1-42, tau202, tau396 and tau404 after intracerebroventricular injection of streptozotocin in rats. Di Yi Jun Yi Da Xue Xue Bao 2005;25(2):168-70.
- Lee CH, Ahn JH, Park JH, Yan BC, Kim IH, Lee DH, et al. Decreased insulin-like growth factor-I and its receptor expression in the hippocampus and somatosensory cortex of the aged mouse. Neurochem Res 2014;39:770-6.
- Bibalani GH, Mosazadeh-Sayadmahaleh F. Medicinal benefits and usage of medlar (Mespilus germanica) in Gilan Province (Roudsar District), Iran. J Med Plant Res 2012;6(7):1155-9.
- Kim JK, Choi SJ, Cho HY, Hwang HJ, Kim YJ, Lim ST, et al. Protective Effects of Kaempferol (3,4′,5,7- tetrahydroxyflavone) against Amyloid Beta Peptide (Aβ)-Induced Neurotoxicity in ICR Mice. Biosci Biotechnol Biochem 2010;74(2):397-401.
- Choi GN, Kim JH, Kwak JH, Jeong CH, Jeong HR, Lee U, et al. Effect of quercetin on learning and memory performance in ICR mice under neurotoxic trimethyltin exposure. Food Chem 2012;132(2):1019-24.
- Jung WY, Park SJ, Park DH, Kim JM, Kim DH, Ryu JH. Quercetin impairs learning and memory in normal mice via suppression of hippocampal phosphorylated cyclic AMP response element-binding protein expression. Toxicol Lett 2010;197(2):97-105.
- Rendeiro C, Rhodes JS, Spencer JP. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem Int 2015;89:1-14.
- Wang H, Wang H, Cheng H, Che Z. Ameliorating effect of luteolin on memory impairment in an Alzheimer's disease model. Mol Med Rep 2016;13(5):4215-422.
- Pietta PG. Flavonoids as antioxidants. J Nat Prod 2000;63(7):1035-42.
- Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011;82(4):513-23.
- Grosso C, Valentão P, Ferreres F, Andrade PB. The use of flavonoids in central nervous system disorders. Curr Med Chem 2013;20(37):4694-719.
- Diniz TC, Silva JC, de Lima-Saraiva SR, Ribeiro FP, Pacheco AG, de Freitas RM, et al. The Role of Flavonoids on Oxidative Stress in Epilepsy. Oxid Med Cell Longev 2015;2015:171756.
- Magalingam KB, Radhakrishnan AK, Haleagrahara N. Protective Mechanisms of Flavonoids in Parkinson’s Disease. Oxid Med Cell Longev 2015;2015;314560.
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 2002;12(5):578-84.
- Jeremy P. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr 2007;2(3):257-73.
- Kolodziejczyk AA, Kim JK, Svensson V, Marioni JC, Teichmann SA. The Technology and Biology of Single-Cell RNA Sequencing. Mol Cell 2011:58(21):610-20.