- *Corresponding Author:
- Mala Majumdar
Department of Biotechnology, Center for Post Graduate Studies
Jain University, Bengaluru-560 011, India
E-mail: mala.majumdar@jainuniversity.ac.in
| Date of Submission | 01 June 2017 |
| Date of Revision | 04 April 2018 |
| Date of Acceptance | 11 October 2018 |
| Indian J Pharm Sci 2018;80(6):1155-1159 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Leaves of Exacum bicolor are traditionally used as a remedy for the treatment of inflammatory disorders in the form of a tonic by the tribal community of Western Ghats of Karnataka. The present study was designed to evaluate the antiinflammatory activity of the methanol extract of Exacum bicolor leaf using the carrageenan-induced mice paw oedema model. The methanol extract which was shown earlier to possess in vitro antiinflammatory activity was further screened for in vivo antiinflammatory activity at doses of 250 and 500 mg/kg. Diclofenac sodium 10 mg/kg was used as the reference standard. The plant extract showed significant dose-dependent reduction in paw edema when compared to the control at all the time intervals, which was also comparable to that produced by diclofenac. Histopathological analysis revealed clear and distinguished cellular damage for all the groups. The results of the current study showed that the methanol extract of the leaf of Exacum bicolor possessed significant antiinflammatory potential, which provided supporting evidence to the folklore use of the plant leaves as an antiinflammatory drug.
Keywords
Antiinflammatory activity, Exacum bicolor Roxb., carrageenan, oedema, histopathology
Exacum bicolor Roxb. (family: Gentianaceae), a perennial herbaceous medicinal plant with attractive flowers is endemic to peninsular India [1,2]. This species has been used traditionally for curing diabetes [1,3], skin disorders [4], inflammation, blood purification to treat malaria [5], as a stomachic [6] and asthma [7]. The plants also exhibited anthelmintic, antioxidant, antiinflammatory and thrombolytic activities [8-10]. Preliminary phytochemical screening of the extracts showed the presence of alkaloids, coumarins, flavonoids, saponin, steroids, terpenoids, glycosides and phenols [8].
Oxidative stress plays a major role in today’s modern life style causing diseases such as inflammation, rheumatoid arthritis, cardiovascular, Alzheimer’s, diabetes, respiratory, liver, autoimmune, kidney and skin problems [11]. Inflammation is a key factor in all aspects of coronary disease including the initiation and progression of atherosclerotic plaque, plaque rupture and thrombosis where the oxidative stress plays a significant role [12]. Oxidative stress and inflammation are intimately linked with the evolution of cardiovascular disease and acute coronary syndromes [13]. Due to short comings of most synthetic drugs, current research is directed towards the development of herbal medicine, which is considered to be safe and less toxic. Herbal source have been the source of a wide variety of biologically active compounds used as potent therapeutic agents for treating not only inflammation but also related disease conditions where inflammation plays a vital role [14]. Carrageenan is a natural polysaccharide obtained from edible red seaweed is used in experimental medicine, pharmaceutical formulations, cosmetics and industrial applications. A special use of carrageenan is in experimental pharmacology for the testing of antiinflammatory drugs [15], which is followed in the present study. In vitro antiinflammatory activity of the methanol extract of E. bicolor leaves was reported earlier [10]. According to literature survey, there has been no report on the in vivo antiinflammatory activity of E. bicolor. The present study was aimed to evaluate the antiinflammatory activity of methanol extract of the leaf in carrageenan-induced mouse paw oedema model.
E. bicolor leaves were collected in November 2012 from Kumara Parvatha, at Kukke Subramanya in Sullia taluk, Western Ghats, Karnataka, India. The leaves were identified and authenticated at the National Ayurveda Dietetics Research, Jayanagar, Bengaluru, India (Accession No. SMPU/NADRI/BNG/2010- 11/557) and a voucher specimen was deposited in the Herbarium of Biotechnology Department of the Jain University, Bengaluru. The leaves were shade dried and were extracted by continuous hot percolation in a Soxhlet apparatus (50±2°) using methanol (yield: 13 %). The resulting extract was dried using a rotary evaporator and was stored at 4° for further studies.
In the present study Swiss albino mice (20-30 g) were used, which were obtained from the Veterinary College, Hebbal, Bengaluru. Six animals were housed in each polyvinyl cage and were maintained under standard laboratory conditions. The animal house was maintained at 25±2° with 12/12 h dark/light cycle. The mice were provided feed (Vet care; Bangalore) and water (Bisleri, Bangalore) ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Nargund College of Pharmacy, Bengaluru (ethical clearance number IAEC/ NCP/94/2015). Extracts and the standard drugs were administered in the form of suspension in water with 1 % dimethyl sulfoxide as suspending agent before 30 min of carrageenan treatment. Acute oral toxicity study was performed as per Organization for Economic Co-operation and Development-423 (OECD-423) guidelines. The methanol extract was found to be safe up to a dose of 2000 mg/kg. Hence, 250 and 500 mg/kg doses were used for the evaluation of in vivo antiinflammatory activity. Mouse carrageenan-induced hind paw oedema model was used to study the in vivo antiinflammatory activity of the methanol extract [16]. The animals were observed for mortality, signs for gross toxicity and behavioural changes at least once daily for 14 d. Body weights were recorded prior to administration of the plant extract and again on 7th and 14th d. The animals were divided into five groups each composed of six animals. Group I- control group given saline (0.9 %) orally. Group II- carrageenan (1 %) in saline was administered into subplantar region of right hind paw. Group III- standard group was given diclofenac sodium (10 mg/kg) orally. Group IVtest group to which E. bicolor methanol leaf extract (250 mg/kg) was administered orally. Group V- test group to which E. bicolor methanol leaf extract (500 mg/kg) was administered orally.
Paw oedema was induced by injecting 50 μl [17] of 1 % carrageenan (Sigma-Aldrich) in physiological saline into subplantar tissues of hind paw to produce acute inflammation [18-21]. The volume of oedema was measured at intervals of 0, 2, 4, 8 and 24 h by the mercury displacement method using a Plethysmograph (Letica, Comella, Spain). Percent inhibition (% IE) of oedema was calculated using the Eqn., % IE = (Vc− Vt)/Vc×100, where Vc is the inflammatory increase in paw volume in control group of animals and Vt is the inflammatory increase in paw volume in drug-treated animals. Inhibition of paw volume in drug-treated group was compared with carrageenan control group (group 1).
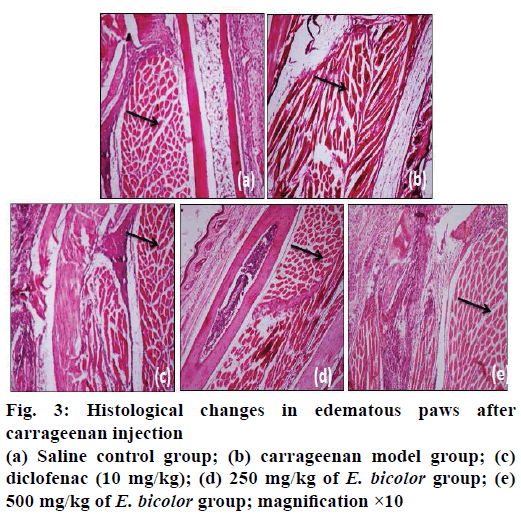
Histopathological examination of mice paws were carried out to assess the effects of inflammation. After 24 h of carrageenan injection, mice were sacrificed and paws were fixed in 10 % formaldehyde. The tissues were cut into 4 μm thick slices with microtome and placed on adhesive glass. Standard haematoxylin and eosin (H and E) staining was performed for morphological observation.
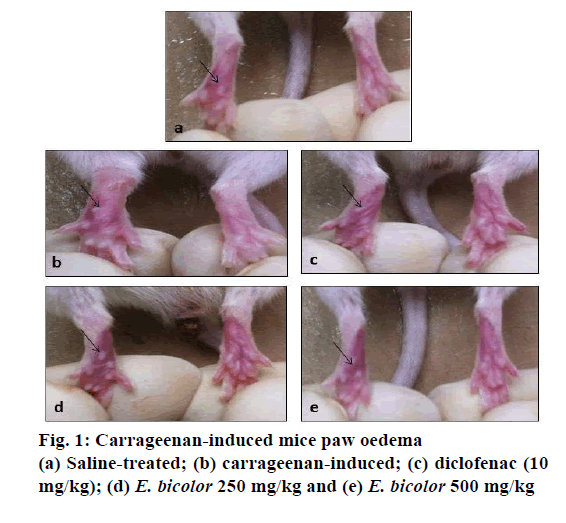
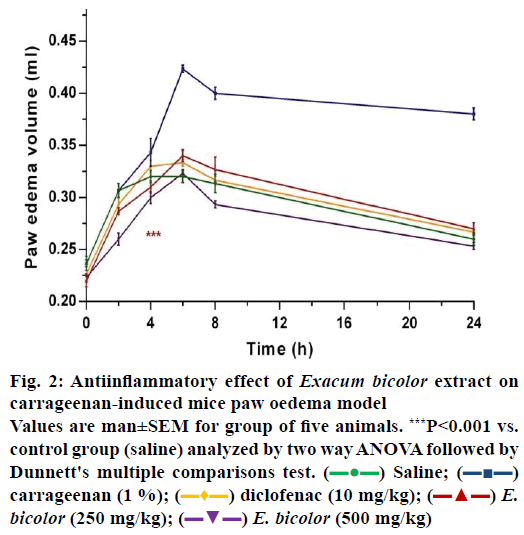
In the present study it was observed that there were no signs of mortality and none of the mice showed clinical toxic signs such as anorexia, depression, lethargy, dermatitis throughout the examination with a dose level up to 2000 mg/kg of E. bicolor leaf methanol extract when administered to mice. A carrageenan-induced mouse paw oedema model was used to study in vivo antiinflammatory activity and the volume of paw oedema was measured using a Plethysmometer. Carrageenan showed increase in paw oedema volume (0.42 ml) at 6th h (Figures. 1 and 2) whereas saline group showed an increase in volume of 0.32 ml (Figure. 2). The development of oedema in the rat hind paw following the injection of carrageenan has been described as a biphasic, age-weightdependent event in which various mediators operate in sequence to produce the inflammatory response [15]. The present study with E. bicolor the leaf extracts at the doses of 250 and 500 mg/kg showed 0.3 and 0.33 ml of increase in volume of paw oedema, respectively. E. bicolor extracts at different doses were significant and comparable to control group (saline). Diclofenac and E. bicolor extracts (250 and 500 mg/kg) showed 96, 90 and 91 % inhibition, respectively at 6 h. According to previous reports, the methanol extract of Enicostemma axillare was assessed for antiinflammatory activity by in vitro methods using albumin denaturation assay, proteinase inhibitory activity, membrane stabilization and antilipoxygenase activities, which showed a significant inhibitory activity [22]. E. bicolor leaf methanol extract exhibited significant in vitro membrane stabilization on human red blood cell with IC50 value of 37.4 μg/ml and there was also a significant correlation (R2>0.98) between total phenolic content and antiinflammatory activity [10]. In the present study, E. bicolor leaf methanol extract exhibited significant and in vivo antiinflammatory activity at 250 and 500 mg/kg with percent inhibition of 90 and 91 %, respectively. Diclofenac (10 mg/kg) inhibited inflammation by 96 %. In the histopathological sections of mice paw (Figure. 3) there was no cellular infiltration (Figure. 3a), which represented that saline did not cause any damage to the cells. In contrast, there was swelling followed by cellular damage due to the carrageenan injection (Figure. 3b). After treatment with diclofenac, the cellular damage was not much prominent as the cells were intact. E. bicolor at the doses of 250 and 500 mg/kg significantly reduced the oedema and also the cellular damages when compared to carrageenan (Figure. 3d and e). In contrast to the present study, E. littorale extract at 100 mg/100 g exhibited 54 % antiinflammatory activity in carrageenan-induced inflammation [20]. Alcohol and petroleum ether extracts of Gentiana lutea rhizomes at 500 and 1000 mg/kg showed dose-dependent antiinflammatory activities in carrageenan-induced rat paw oedema. Both extracts showed significant dose-dependent antiinflammatory activities [21]. Antiinflammatory activity was assessed using carrageenan-induced rat paw oedema model in ethanol extract of Swertia chirata root. The extract was found to reduce the formation of oedema significantly (p<0.001) at the 400 mg/kg dose level and showed 57.81 % (p<0.001) inhibition of oedema volume at the end of 3 h [22].
Figure 2: Antiinflammatory effect of Exacum bicolor extract on
carrageenan-induced mice paw oedema model
Values are man±SEM for group of five animals. ***P<0.001 vs.
control group (saline) analyzed by two way ANOVA followed by
Dunnett's multiple comparisons test. ( ) Saline; (
) Saline; ( )
carrageenan (1 %); (
)
carrageenan (1 %); ( ) diclofenac (10 mg/kg); (
) diclofenac (10 mg/kg); ( ) E.
bicolor (250 mg/kg); (
) E.
bicolor (250 mg/kg); ( ) E. bicolor (500 mg/kg)
) E. bicolor (500 mg/kg)
In a previously reported study of E. bicolor leaf methanol extract, Fourier-transform infrared spectroscopy spectral analysis revealed the identity of the functional groups such as alcohols, phenols, alkanes, amines, aromatic compound, aldehyde and ethers. Gas chromatography mass spectrometry analysis revealed the presence of phytoconstituents such as erythrocentaurin, neophytadiene, hexadecanoic acid, 6-octadeccenoic acid, (+–)-inophylum D, 4,6,8(14)-cholestatriene and methyl 3,4-diphenylpyrrolo [2,1,5-cd]indolizine- 1-carboxylate. These phytocompounds might be responsible for the cause of antiinflammatory response in E. bicolor leaf methanol extracts [10].
The present investigation has provided the scientific basis for the traditional uses of E. bicolor Roxb. leaves in inflammation having significant antiinflammatory activity. This report on in vivo antiinflammatory study on carrageenan-induced mice paw oedema was for the first time. Further pharmacological investigations are underway to find out the exact active constituents responsible for antiinflammatory activity and to elucidate its mechanism of action.
Acknowledgements
The authors are grateful to the Center for Post Graduate Studies, Jain University, Bangalore, India, for providing financial assistance to the first author to carry out the research work, lab facilities and also infrastructural facilities. We also thank Nargund College of Pharmacy, Bangalore for ethical clearance and providing animal house facility to carry out in vivo studies.
Conflict of interest
Nil.
References
- Sreelatha U, Baburaj TS, Kutty NC, Nazeem P, Bhaskar J. Cultivation prospects of Exacum bicolor Royle: an endangered, ornamental & anti-diabetic herb. Nat Prod Rad2007;6:402-4.
- Brilliant R, Vincy MV, Joby P, Pradeepkumar AP. Vegetation analysis of Montane forest of Western Ghats with special emphasis on RET species. Int J Biodivers Conserv 2012;4:652-64.
- Marles RJ, Farnsworth NR. Antidiabetic plants and their active constituents. Phytomedicine 1995;2:137-9.
- Reddi STV, Naidu BVAR, Prasanthi S. Herbal remedies for diabetes. In: Alikhan I, Khanum A, editors. Herbal remedies for diseases. Hyderabad: Ukaz Publications; 2005. p. 67-134.
- Rao MRR. Ethnobotanical studies in Nagaland medicinal plants used by the Angami Nagas. J Econ Bot 1983;4:167-72.
- Shiddamallayya N, Yasmeen A, Gopakumar K. Medico-botanical survey of Kumara parvatha Kukke Subramanya. Indian J Tradit Know 2010;9:96-9.
- Lingaraju DP, Sudarshana MS, Rajashekar N. Ethnopharmacological survey of traditional medicinal plants in tribal areas of Kodagu district, Karnataka. India. J Pharm Res 2013;6(2):284-97.
- Ashwini AM, Majumdar M. Qualitative phytochemical screening and in vitro anthelmintic activity of Exacum bicolor Roxb., an endemic medicinal plant from Western Ghats in India. Acta Biol Indica 2014;3:510-4.
- Ashwini AM, Mala M. Quantification of phytochemical contents and in vitro antioxidant activity of Exacum bicolor (Roxb.), an endemic medicinal plant. Int J Pharm Pharm Sci 2015;7(6):225-30.
- Ashwini AM, Puttarudrappa L, Ravi R, Mala M. GC-MS analysis, evaluation of phytochemicals, antioxidant, thrombolytic and anti-inflammatory activities of Exacum bicolor (Roxb.) leaves. Bangladesh J Pharmacol 2015;10:745-52.
- Galli F, Piroddi M, Annetti C, Aisa C, Floridi E, Floridi A. Oxidative stress and reactive oxygen species. Contrib Nephrol 2005;149:240-60.
- Freedman JE. Oxidative stress and platelets. Arterioscler Thromb Vasc Biol 2008;28:11-6.
- Pashkow FJ. Oxidative stress and inflammation in Heart Disease: Do antioxidants have a role in treatment and/or prevention? Int J Inflam 2011;2011:514623.
- Dasilva EJ. Medicinal plants: a re-emerging health aid. Electron J Biotechnol 1999;2:57-70.
- Necas J, Bartosikova L. Carrageenan: a review. Vet Med 2013;58(4):187-205.
- Winter CA, Risley EA, Nuss GW. Carrageenan-induced oedema in hind paws of the rats as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 1962;111:544-57.
- Muhammad N, Saeed M, Khan H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement Altern Med 2012;12:59.
- Turner RA. Screening methods in pharmacology. New York, London: Academic Press; 1965.
- Leelaprakash G, Dass SM. In vitro anti-inflammatory activity of methanol extract of Enicostemma axillare. Int J Drug Dev Res 2011;3:189-96.
- Sadique J, Chandra T, Thenmozhi V, Elango V. The antiinflammatory activity Enicostemma littorale and Mullogo cerviana. Biochem Med Metab Biol 1987;37:167-76.
- Mathew A, Taranalli AD, Torgal SS. Evaluation of anti-inflammatory and wound healing activity of Gentiana lutea rhizome extracts in animals. Pharm Biol 2004;42(1):8-12.
- Das SC, Bhadra S, Roy S, Saha SK, Islam MS, Bachar SC. Analgesic and anti-inflammatory activities of ethanolic root extract of Swertia chirata (Gentianaceae). Jordan J Biol Sci 2012;5:31-6.