- *Corresponding Author:
- G. Murtaza
Department of Pharmacy, COMSATS University Islamabad, Lahore Campus, Lahore 54000, Pakistan
E-mail: gmdogar356@gmail.com
| This article was originally published in a special issue, “XXXXXX” |
| Indian J Pharm Sci 2020:82(1)spl issue1;XX-XX |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of the current study was to evaluate the antioxidant, lethality and anticancer potential of root extracts of Berberis lycium. Equal weight of powdered roots was used to prepare water, methanol, ethyl acetate and n-hexane extracts. Methanol extract of Berberis lycium showed promising 2,2-diphenyl-1-picrylhydrazyl free radical scavenging potential with an IC50 of 144.31 µg/ml. In the brine shrimp lethality assay, the ethyl acetate extract produced 53.33 % mortality at 1000 µg/ml and its LD50 was 907.7 µg/ml. In the sulforhodamine B colorimetric cytotoxicity screening assay, methanol extracts of Berberis lycium caused 45 % inhibition in growth of HCT116, without producing much effect against MCF7 cell line. In addition, the potential mediators that could be involved in mediating these activities include AKT1, TP53, JUN, and TLR4. In conclusion, methanol extract of Berberis lycium exhibited excellent antioxidant, brine shrimp lethality, and cytotoxic activities, indicating the potential of this plant against colorectal and breast cancer.
Keywords
Antibacterial, antifungal, antioxidant, anticancer, Berberis lycium, Methicillin-resistant Staphylococcus aureus strain 10
Cancer, a condition of uncontrolled cell proliferation due to genetic mutations, can be divided into several types, including colon, colorectal, prostate and blood cancer. The most commonly used anticancer agents are platinum analogues, alkylating agents, and antimetabolites[1]. Because of toxicities associated with cancer chemotherapies and radiation therapy, screening of plants for discovering natural anticancer agents is widely under way. Several natural anticancer molecules such as vincristine and vinblastine have been already discovered from plants[2]. Berberis lycium (Berberidaceae) is famous for its medicinal and edible values. It is widely distributed in North West Himalayan region of Pakistan and included in both British and Indian pharmacopeias. Therapeutic potential is present in its fruits, root and root bark. Different parts of B. lycium are used for a number of medicinal purposes[3]. B. lycium is widely used by local community to treat jaundice, wounds and broken bones, intestinal colic, and diarrhea. It is used as an expectorant, diuretic and in chronic ophthalmic and throat inflammation. The roots are aperient, carminative, febrifuge and ophthalmic. They are utilized as a part of the treatment of eye conditions, menorrhagia, and abdominal disorders[4]. The leaves have been utilized as a part of the treatment of jaundice. The rhizomes of Berberis species have marked antibacterial activity. It is not absorbed by the body and it is utilized orally to treat different types of bacterial gastroenteritis. It ought not to be utilized with Glycyrrhiza species (liquorice) in the light of the fact that this invalidates the impacts of the berberine. Berberine has additionally indicated antitumor movement[4]. Various chemical constituent present in B. lycium are ascorbic acid, berberine, berbamine, chinabine, gilgitine karakoramine, maleic acid, palmatine, balauchistanamine, jhelumine, punjabine and sindamine. B. lycium contains the alkaloid berberine, which is an isoquinoline alkaloid. It is isolated from roots and bark. Berberine and palmatin are reported to have strong growth inhibitory and proapoptotic effect[3].
The present in vitro study is aimed at identifying a candidate plant drug source that could suppress the proliferation of malignant colorectal and breast cancers the have high incidence worldwide. Thus the objective of current study was not only to evaluate the antioxidant and lethality potential of root extracts of B. lycium, but also to assess the anticancer potential against colorectal and breast cancers. A sample of plant material was collected in June 2016, from Hazara region, Khyber Pakhtunkhwa. Plant material was identified a taxonomist. After identification, the plant sample was deposited in the herbarium. The roots of B. lycium were used for further analysis.
The roots of B. lycium were dried under shade. The dried material was chopped and ground to a powder. Five hundred grams of the powdered plant was macerated in different solvents (water, methanol, ethyl acetate and n-hexane) separately. After 24 h of maceration, sonication was carried out for 15 min at 35° and maceration was continued for another 6 d. After 7 d of maceration, a muslin cloth was used for initial filtration and then final filtrate was collected after passing through a Whatman filter paper. All the solvent was evaporated in a rotary vacuum evaporator. The crude extract was collected and percent yield was calculated for each extract using following formula[5], % yield = (M1–M2/M1)×100, where M1 is the mount of plant powder used for extraction, M2 is the amount of extract. The extracts were stored in refrigerator for further use.
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was used to evaluate the antioxidant activity of the prepared extracts a procedure previously reported[6] with slight modification. Stock solution of the water, methanol, ethyl acetate and n-hexane extracts were prepared by taking 2 mg of each extract, 1 ml of dimethyl sulfoxide (DMSO) was added and vortexed for 10 min. While for the preparation of stock solution of DPPH, 3.32 mg of DPPH was dissolved in 100 ml of methanol.
Initially, 20 μl each of the water, methanol, ethyl acetate and n-hexane extract stock solutions were taken in 96 well plates. In all wells, 180 μl of the DPPH stock solution was added. Final volume in each well of was 200 μl. These plates were then incubated for 45 min. After incubation, the absorbance was measured using a microplate reader at 517 nm. Each sample was tested in triplicate. Percent free radical scavenging activity was calculated using the formula, % free radical scavenging = Apc–As/Ac×100, where, Apc was the absorbance of the positive control and As was the absorbance of sample solution.
Cytotoxicity of all the extracts was screened using the brine shrimp lethality assay as describe previously[7] with a slight modification. Stock solutions of the water, methanol, ethyl acetate and n-hexane extracts were prepared by dissolving 10 mg of each plant extract in 100 μl of DMSO and making the final volume up to 1 ml with artificial sea water to obtain a final concentration of 10 mg/ml.
Artificial sea water was made by dissolving 38 g of sea salt in 1 l of distilled water with continuous stirring. This solution was placed for 1 h in a beaker on a magnetic mixer for proper mixing at room temperature. For hatching of brine shrimp eggs, a rectangular box was used that was pre-filled with artificial sea water. The rectangular box was divided into 2 compartments. Several holes of almost 2mm in diameter were made in the partition wall between two compartments. At one side of the box containing artificial sea water, eggs to be hatched were gently spread on its surface. The eggs side was covered with aluminium foil to make the side dark. Lamp was used on the other side to make it bright, so that hatched nauplii moved toward this compartment. Hatching process was started after 23-24 h. The newly hatched nauplii moved towards the other compartment (lighted area). The hatched nauplii were collected in a small beaker containing artificial sea water with the help of pipette.
Seven test tubes were used for each sample. Initially 550 μl of stock solution was added in test tube one and two and 550 μl of sea salt was added from test tube two to test tube seven. After proper mixing 550 μl of solution from test tube two was collected and added it in test tube three and process was continued up to test tube no seven. Then 4.5 ml of sea salt water was added in all test tubes and mix it properly. At last add 505 μl of sea salt water in all test tubes to makes final volume almost 5.5 ml. In each test tube 10 shrimps were added with the help of micropipette. Then these test tubes were placed at room temperature under bulb light. All samples were tested in triplicate.
The activity of the extracts against cancer cell lines was analysed via SRB assay by using the documented protocols[8]. Stock solutions (5 mg/ml) of plant extracts (water extract, methanol extract, ethyl acetate extract and n-hexane extract) were prepared by adding 5 mg plant extract in to 1 ml DMSO. Plant extract was dissolved in DMSO to make 5 mg/ml stock solution. Then two-fold serial dilution was made from 1 mg/ml to 0.03125 mg/ml in 10 % DMSO and sample solution was properly mixed after each transfer with the help of pipette. For each transfer, new pipette tip was used and add 10 μl test sample in 10 % of DMSO in each sample well of 96-well tissue culture plate (in triplicate) and then 10 μl of 10 % (v/v) DMSO was added into each negative control well. Medium from the cell monolayers was removed before starting assay, cells was washed with sterilized PBS and remove PBS. Then trypsin 0.25 % was added in versene EDTA and equally covered the cell growth surface, till the cells started to detach. The sterilized plastic pipettes were used to separate them from the surface of the culture with 10 fold volume of culture medium including FBS.
Mixing was performed to achieve a homogeneous cell suspension and cells were transferred to a pasteurized polypropylene tube. Cell concentration was determined by counting in a hemocytometer chamber under a microscope with the help of mixture of cell suspension and 0.4 % (w/v) trypan blue solution (1:1). Cell concentration was the adjusted with growth medium to achieve desired cell seeding density. After that, 190 μl cell suspension was added to the assay preprepared culture plates and cell suspension was mixed during plating. A plate containing only cell suspension in 3 columns for a no-growth control was incubated at 37° in an incubator with 5 % carbon dioxide till complete cell attachment. Remaining assay plates were incubated at 37° in a humidified incubator for next 72 h with 5 % carbon dioxide. One hundred microlitres of trichloro acetic acid was gently added to each well without removing the cell culture supernatant. Microplates were incubated for 1 h in an incubator.
Each plate was washed 3× with slowly flowing tap water through a plastic tubing connected directly to a faucet. Excess mount of water was removed using paper towels. Plates were dried at room temperature. In each well, 100 μl SRB solution was added and theses plates were kept at room temperature for next 30 min and then rapidly washed for 4× with acetic acid to eliminate dye that is not bound and kept them at room temperature to dry. In each well 100 μl of 10 mM Tris base solution was added. Each plate was placed on a gyratory shaker for 5 min. After solubilisation of protein bounded dye OD was measured at 510 nm in a microplate reader. Percent cell growth inhibition was calculated using the formulae, cell growth (%) = absorbance sample/ absorbance negative control or untreated×100, and growth inhibition (%) = 100–% cell growth.
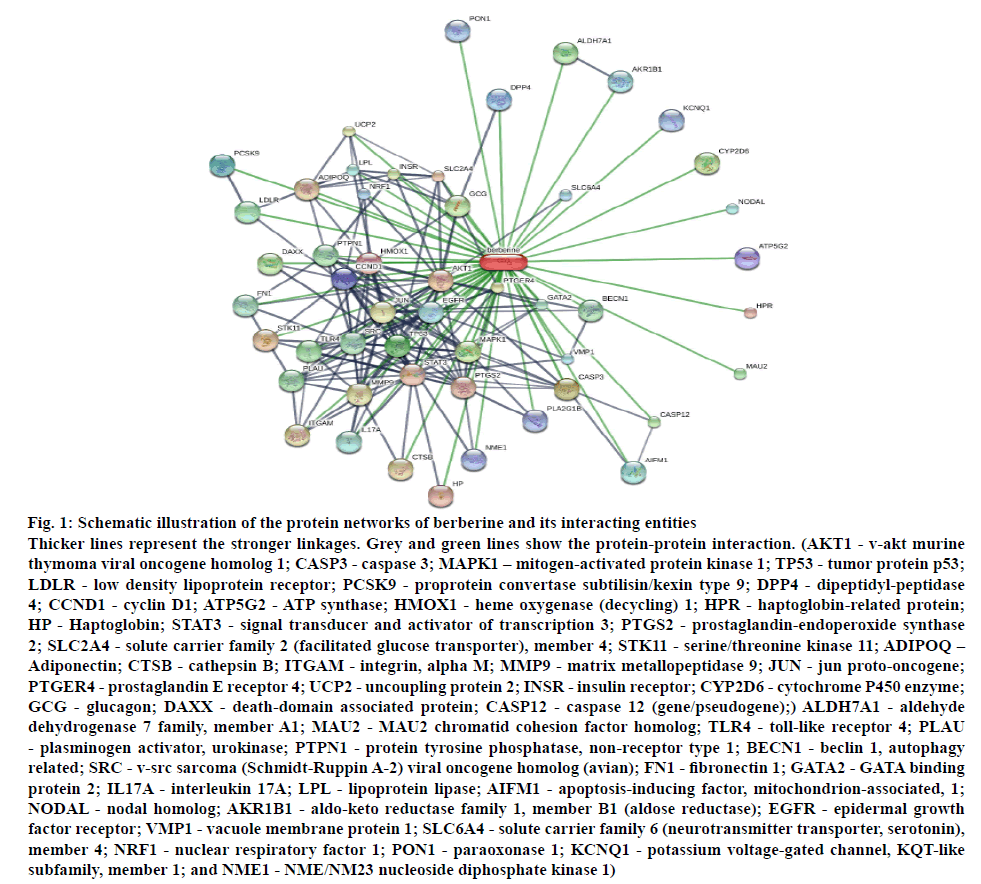
From literature, ascorbic acid, berberine, berbamine, chinabine, gilgitine, karakoramine, maleic acid, palmatine, balauchistanamine, jhelumine, punjabine and sindamine were retrieved as the active constituents of B. lycium. Among these, berberine, in particular and berbamine are the most prevalent bioactive compound that are effective in a wide array of conditions, including type II diabetes (T2D). T2D-associated molecular targets of these two alkaloids were ascertained and plotted as interaction network utilizing STITCH 5.0 database (http://stitch.embl.de/, accessed in May 2017)[3].
Paired t-test was applied using SPSS version 19.0, to compare the numerical differences in the findings with p<0.05. In cytotoxicity test, IC50 values were determined using GraphPad Prism 5 (GraphPad, San Diego, USA) from nonlinear regression based sigmoidal dose response curve.
Millions are annually affected by cancer. Thus, the current study is mainly focused on the investigation of a suitable plant sources to suggest a novel therapeutic candidate against colorectal and breast cancers. For several years, a wide variety of plants has been documented to possess therapeutic potential against various cancers[1].
According to the results, maximum yield (3.37 %) was found in water extract, while the least yield (0.089 %) was found in n-hexane extract. Methanol extract and ethyl acetate extract yield were 2.32 % and 0.54 %, respectively. Based on free radical scavenging assay results presented in Table 1, methanol extract showed maximum free radical scavenging activity of 67.32± 1.58 %, while n-hexane extract showed minimum activity of 14.33±2.10 %. Similar findings were reported for methanol extract of D. gangeticum by Veeru et al.[9], reporting 21.33 mg/ml as its antioxidant potential.
Table 1: Findings Acquired Through Different Experiments
| Different plant extracts | % DPPH Scavenging at 200 µg/ml | Brine Shrimp growth inhibition (LD50 µg/ml) | HCT-116 inhibition (IC50 mg/ml) | MCF-7 inhibition (IC50 mg/ml) |
|---|---|---|---|---|
| Water extract | 17.20±2.67 | 1278.60 | 4.12 | 7.67 |
| Methanol extract | 67.32±1.58 | 1905.03 | 1.12 | 5.55 |
| Ethyl acetate extract | 25.07±1.20 | 894.85 | 4.91 | 5.98 |
| n-Hexane extract | 14.33±2.10 | 0 | 2.67 | 11.73 |
According to the brine shrimp lethality bioassay (Table 1), methanol extract showed maximum brine shrimp growth inhibition with an LD50 of 1905.03 μg/ml, followed by water extract with 1278.60 μg/ml. No mortality was observed in animal group treated with n-hexane extract. In a previous study[10], the ethanol extract of Moringa oleifera Lam at a concentration of 80 μg/ml exhibited similar lethality potential. In another cytotoxicity study, 50 % aqueous ethanol extract of C. bonplandianum showed 100 % mortality at 1 mg/ml concentration[11].
According to HCT116 cell growth inhibition assay, the maximum IC50 value (1.12) was achieved with methanol extract followed by n-hexane extract (2.67, Table 1). Methanol extract (40 μg/ml) of Emblica officinalis has also been reported to inhibit HCT116 cell by 35.07 %[12]. Cytotoxic results of the present study were very important, since B. lycium methanol extract has little activity in brine shrimp lethality bioassay. Future work can be conducted on the methanol extract by making its different fractions, to increase the sample potency against HCT 116 cell lines.
It was observed from the results against MCF-7 cell line that B. lycium methanol extract showed the maximum cell growth inhibition with IC50 of 5.55 mg/ml (Table 1), while the minimum cell growth inhibition with IC50 of 11.73 mg/ml was seen for n-hexane extract. Previously, methanol extract of Cladonia convolute has also shown 90.1 μg/ml IC50 value against MCF-7 cell lines[13]. It is noteworthy that in the SRB assay, inhibition produced by DMSO at 1 mg/ml was 8 and 4 on HCT-116 and MCF7 cell lines, respectively.
From the above documented results, it can be concluded that the methanol extract exhibited best cytotoxic potential (with IC50 values 1.12 mg/ml and 5.55 mg/ml in HCT-116 and MCF-7 cell lines, respectively) supported by its promising antioxidant (with % DPPH scavenging at 200 μg/ml of 67.32±1.58) as well as brine shrimp lethal activity (with LD50 of 1905.03 μg/ml), verifying the encouraging anticancer potential of B. lycium to further explore and purify the targeted anticancer candidates.
By finding this new natural treatment of cancer, the present study appears to have demonstrated a great advance in the field of anticancer agents. B. lycium extracts should be further investigated at molecular level to recognize the compound having anticancer potential in colorectal and breast cancers. Plants can be used for treating human diseases, such as cancer[1]. The main purpose of this study was to explore the potential of B. lycium extracts against human colorectal and breast cancer cell lines. B. lycium root extracts in different solvents were used to assess its efficacy against different types of cancer and the methanol extract showed protective effect against malignant tumors. This plant contains various chemical ingredients including berberine, which is found to possess suppressive effect on the cultured cancer cells[14]. Although B. lycium has been recommended to be a candidate for anticancer drug, the major finding of this in vitro study shows that it can be an excellent source of a drug molecule against colorectal and breast cancers.
The in silico investigation revealed a wide array of molecules (figure 1) involved in the action of berberine, the important constituent of B. lycium. The most involved mediators in this activity are AKT1, TP53, JUN, and TLR4, which have already been reported to be involved in the anticancer activity[5,11].
Figure 1: Schematic illustration of the protein networks of berberine and its interacting entities
Thicker lines represent the stronger linkages. Grey and green lines show the protein-protein interaction. (AKT1 - v-akt murine thymoma viral oncogene homolog 1; CASP3 - caspase 3; MAPK1 – mitogen-activated protein kinase 1; TP53 - tumor protein p53; LDLR - low density lipoprotein receptor; PCSK9 - proprotein convertase subtilisin/kexin type 9; DPP4 - dipeptidyl-peptidase 4; CCND1 - cyclin D1; ATP5G2 - ATP synthase; HMOX1 - heme oxygenase (decycling) 1; HPR - haptoglobin-related protein; HP - Haptoglobin; STAT3 - signal transducer and activator of transcription 3; PTGS2 - prostaglandin-endoperoxide synthase 2; SLC2A4 - solute carrier family 2 (facilitated glucose transporter), member 4; STK11 - serine/threonine kinase 11; ADIPOQ – Adiponectin; CTSB - cathepsin B; ITGAM - integrin, alpha M; MMP9 - matrix metallopeptidase 9; JUN - jun proto-oncogene; PTGER4 - prostaglandin E receptor 4; UCP2 - uncoupling protein 2; INSR - insulin receptor; CYP2D6 - cytochrome P450 enzyme; GCG - glucagon; DAXX - death-domain associated protein; CASP12 - caspase 12 (gene/pseudogene);) ALDH7A1 - aldehyde dehydrogenase 7 family, member A1; MAU2 - MAU2 chromatid cohesion factor homolog; TLR4 - toll-like receptor 4; PLAU - plasminogen activator, urokinase; PTPN1 - protein tyrosine phosphatase, non-receptor type 1; BECN1 - beclin 1, autophagy related; SRC - v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (avian); FN1 - fibronectin 1; GATA2 - GATA binding protein 2; IL17A - interleukin 17A; LPL - lipoprotein lipase; AIFM1 - apoptosis-inducing factor, mitochondrion-associated, 1; NODAL - nodal homolog; AKR1B1 - aldo-keto reductase family 1, member B1 (aldose reductase); EGFR - epidermal growth factor receptor; VMP1 - vacuole membrane protein 1; SLC6A4 - solute carrier family 6 (neurotransmitter transporter, serotonin), member 4; NRF1 - nuclear respiratory factor 1; PON1 - paraoxonase 1; KCNQ1 - potassium voltage-gated channel, KQT-like subfamily, member 1; and NME1 - NME/NM23 nucleoside diphosphate kinase 1)
In the present study, methanol extract of B. lycium exhibited excellent antioxidant, brine shrimp lethality, and cytotoxic activities, revealing that this plant is a suitable candidate for colorectal and breast cancer drug. The nature of chemical and molecular mechanism of interaction for therapeutic activity against colorectal and breast cancers may be elucidated by further refinements. Moreover, the probable and potential mediators involved in its activity include AKT1, TP53, JUN, and TLR4. Though the clinical effectiveness and toxicity profiles of large number of anticancer agents are vague, understanding the basic role of herbal extracts play a crucial role in developing herbal products and using for cancer treatment.
Acknowledgment
The research was funded by the researchers supporting project (RSP-2019/98), King Saud University, Riyadh, Saudi Arabia.
References
- Hejmadi M. How cancer arises. In Introduction to cancer biology, Ed. Hejmadi M Ventus Publishing ApS, United Kingdom 2009;6-12.
- James RH. Natural Products; the Secondary Metabolites, the Royal Society of Chemistry, UK, 2003;10:1039-40.
- Sood P, Modgil R, Sood M. Berberis lycium a Medicinal Plant with Immense Value. Pharm Biol Res 2012;1:1-9.
- Gupta M, Singh A, Joshi HC. Berberis lycium multipotential medicinal application : An overview. IJCS 2015;3:10-3.
- Ahmad A, Alkarkhi AFM, Hena S, Khim LH. Extraction, Separation and Identification of Chemical Ingredients of Elephantopus Scaber L. Using Factorial Design of Experiment. Int J Chem 2009;1:36-49.
- Clarke G, Ting K, Wiart C, Fry J. High Correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging, Ferric Reducing Activity Potential and Total Phenolics Content Indicates Redundancy in Use of All Three Assays to Screen for Antioxidant Activity of Extracts of Plants from the Malaysian Rainforest. Antioxidants 2013;2:1-10.
- Olowa LF, Nuñeza OM. Brine Shrimp Lethality Assay of the Ethanolic Extracts of Three Selected Species of Medicinal Plants from Iligan City, Philippines. Mortality 2013;2:74-77.
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytoxicity screening Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 2006;1:1112-6.
- Veeru P, Kishor MP, Meenakshi M. Screening of medicinal plant extracts for antioxidant activity. J Med Plant Res 2009;3:608-12.
- Rafshanjani AS, Parvin S, Kader A. in vitro antibacterial activities and brine shrimp lethality bioassay of ethanolic extract from Moringa oleifera Lam leaves. Int Res J Pharm 2014;5:856-60.
- Ajoy G, Padma C. Brine shrimp cytotoxic activity of 50 % alcoholic extract of Croton bonplandianum baill. Asian J Pharm Clin Res 2013;6:40-1.
- Bandopadhyaya S, Ramakrishnan M, Thylur R. in vitro evaluation of plant extracts against colorectal cancer using HCT 116 cell line. Int J Plant Sci Ecol 2015;1:107-12.
- Bezivin C, Tomasi S, Lohézic-Le Dévéhat F, Boustie J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003;10:499-503.
- McCubrey JA, Lertpiriyapong K, Steelman LS, Abrams SL, Yang LV, Murata RM, et al. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging 2017;12:1477-1536.