- *Corresponding Author:
- Tuan-Nghia Phan
Key Laboratory of Enzyme and Protein Technology, VNU University of Science, 334 Nguyen Trai, ThanhXuan, Vietnam
E-mail: phantuannghia@vnu.edu.vn
| Date of Submission | 23 January 2017 |
| Date of Revision | 28 October 2017 |
| Date of Acceptance | 30 May 2018 |
| Indian J Pharm Sci 2018;80(4):755-761 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Human immunodeficiency virus type-1 is the causative pathogen of acquired immunodeficiency syndrome and its protease is one of the primary targets for human immunodeficiency virus/acquired immune deficiency syndrome therapy. In this study, two seco-lanostane triterpenoids, 3,4-seco-9βH-lanost-4(28),7,24-trien-3-oic acid and 24(E)-3,4-seco-9βH-lanost-4(28),7,24-trien-3,26-dioic acid isolated from the roots of Kadsura coccinea, were found to significantly inhibit human immunodeficiency virus-1 protease, with IC50 values of 1.0±0.03 µM and 0.05±0.009 µM, respectively. Neither compound was toxic to human embryonic kidney 293T cells at concentrations effective against human immunodeficiency virus-1 protease. Our findings indicate that these triterpenoids are potential candidates for development of antihuman immunodeficiency virus/acquired immune deficiency syndrome drugs.
Keywords
HIV-1 protease inhibitor, Kadsura coccinea, seco-coccinic acid F, 24(E)-3,4-seco-9βH-lanost- 4(28),7,24-trien-3,26-dioic acid, seco-lanostane triterpenoid
The protease (EC 3.4.23.16) encoded by the human immunodeficiency virus type 1 (HIV-1) genome plays a crucial role in the life cycle of the virus. Inhibition of the HIV-1 protease leads to the formation of immature non-infectious virions; therefore, the enzyme has become an important target in HIV drug development [1]. HIV-1 protease inhibitors developed to this point are substrate-based and compete with the natural substrate for the active site of the enzyme. Most are peptide mimetic compounds. However, it was reported that these compounds have low bioavailability when administered orally and are too expensive to serve as a feasible treatment option for patients living in developing countries [2]. Several side effects associated with these drugs have been described, such as fat accumulation, kidney stone formation, and cutaneous effects. More importantly, a high mutation rate of the virus has been reported, which would result in the generation of new resistant strains of the virus [3]. To stop the rapid spread of the HIV epidemic, innovative combinations of drugs with diverse antiHIV mechanisms must be effectively used. Potential HIV-1 protease inhibitors obtained from natural sources are fast becoming a promising strategy for the development of new treatments [2,4].
Kadsura coccinea (Lem.) AC Smith is an evergreen member of the family Schisandraceae. The genus Kadsura is closely related to Schisandra and many of its species are extensively used as substitutes for Schisandra in Chinese medicinal formulations in Taiwan, Japan, and Vietnam [5,6]. Extracts of this plant have been used as tonics, decongestants, and digestive agents in Vietnamese traditional medicine. The roots are also used to treat chronic enteritis, acute gastritis, and rheumatic pain in the bones [7]. A number of triterpenoids, particularly of the lanostane type, were isolated from the roots of K. coccinea and shown to possess various biological activities such as inhibition of human leukaemia cell growth [8] and protective effects against haemocyte necrosis [7].
Wei et al. [9] reported the presence seco-triterpenoids with ring A from Stauntonia obovatifoliola Hayata subspecies. Intermediates have potent antiHIV-1 protease activity. The results of the study indicated that the 2,3-seco-2,3-dioc acid moiety in ring A of these triterpenoids is an important pharmacophore in the design and synthesis of HIV-1 protease inhibitors because some triterpenoids derivatives with a 3-O-acidic acyl group also exhibit strong antiHIV activity. It is of interest to us to examine the antiHIV activity of other seco-triterpenoids. In a previous study, two seco-lanostane-type triterpenoids, 3,4-seco- 9βH-lanost-4(28),7,24-trien-3-oic acid (seco-coccinic acid F) and 24(E)-3,4-seco-9βH-lanost-4(28), 7,24-trien-3,26-dioic acid (Figure 1), were isolated from the ethanol (EtOH) extract of the roots of K. coccinea [6]. In this study, we evaluated the inhibitory effects of these compounds on HIV-1 protease. Maslinic acid (oleanane triterpenoid), ursolic acid (ursane triterpenoid), and fetal calf serum were purchased from Sigma-Aldrich (USA). Human embryonic kidney (HEK 293T) cells were purchased from Corning, USA. Recombinant HIV-1 protease was prepared in our laboratory according to a previously reported procedure [10]. The fluorogenic substrate for HIV-1 protease (QXL 520-GABA-SFNFPQITKHiLyte Flour 488-NH2, designed based on the specific hydrolysis sequence in the Gag-Pol protein substrate of HIV-1 protease) was synthesized and purified by highperformance liquid chromatography (HPLC: to 95 % purity by AnaSpec, USA). Other reagents and solvents used were of analytical grade.
The roots of K. coccinea were collected in the TrangDinh district, Lang Son province in September 2009, identified, sliced, dehumidified at 50° to dryness, and stored as a voucher specimen no. VDL.NR01.2009 in the herbarium (a dry room, temperature <25°, insects and microbial avoided) of the National Institute of Medicinal Materials, Vietnam.
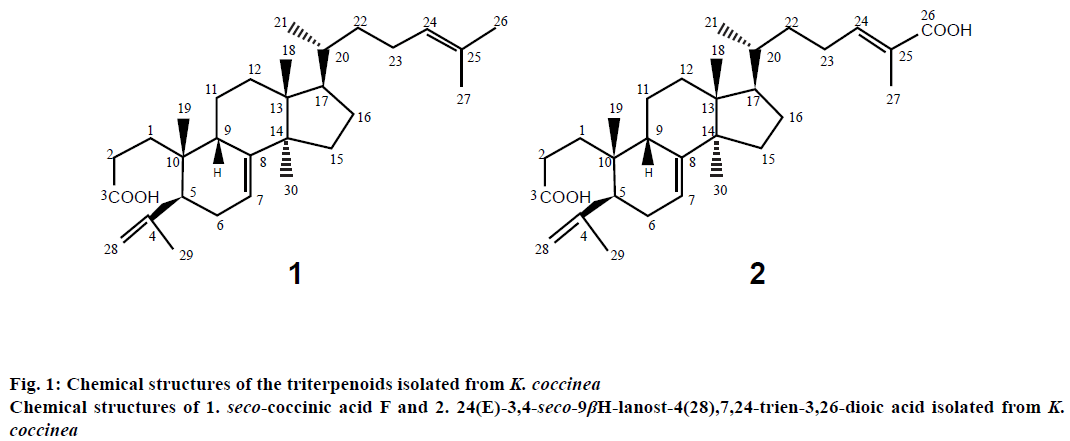
Two lanostane-type triterpenoids, 3,4-seco-9βHlanost- 4(28),7,24-trien-3-oic acid (seco-coccinic acid F, C30H48O2, molecular weight of 440, Figure 1) and 24(E)-3,4-seco-9βH-lanost-4(28),7,24-trien- 3,26-dioic acid (C30H46O2, molecular weight of 470, Figure 1), were isolated from the EtOH extract of the roots of K. coccinea according to the procedure described previously by Nguyen et al. [6]. Briefly, roots of K. coccinea (2 kg) were cut into small pieces and macerated three times with EtOH 80 % (v/v). Evaporation of the solvent under reduced pressure from the extract yielded the EtOH extract (150 g), which was then dissolved and suspended in H2O (500 ml), then partitioned in turn with n-hexane, ethyl acetate (EtOAc), and butanol (BuOH). The hexane extract (64 g) was subjected to silica gel column chromatography, eluted with hexane/EtOAc (100:1, 90:1, 80:1, 10:1, 1:1, 0:1, 500 ml each) to yield fractions I-V. Fraction III (12.6 g) was rechromatographed on a silica gel column and eluted with a gradient solvent system of hexane/EtOAc (8/1, 6/1, 4/1 and 1/2, 1000 ml each) to obtain 3 fractions (III.1-III.3): III.1 (hexane/ EtOAc 8/1, 6/1), III.2 (hexane/EtOAc 4/1), and III.3 (hexane/EtOAc 1/2). Fraction III.2 was repeatedly applied to a silica gel column and eluted with hexane/ EtOAc (4:1) to obtain compound 1 (seco-coccinic acid F, 125 mg). Fraction IV (10.8 g) was repeatedly applied to a silica gel column and eluted with a gradient solvent system of hexane/EtOAc (4:1, 2:1, 1:1, 0:1; 1000 ml each) to obtain 4 fractions (IV.1-IV.4): IV.1 (hexane/ EtOAc, 4:1), IV.2 (hexane/EtOAc, 2:1), IV.3 (hexane/ EtOAc, 1:1), and IV.4 (hexane/EtOAc, 0:1). Fraction IV.3 appeared as the major mark on the chromatogram and was treated with methanol (MeOH) to give compound 2 (24(E)-3,4-seco-9βH-lanost-4(28),7,24- trien-3,26-dioic acid, 50 mg). Compounds 1 and 2 had a purity of ≥ 93 %, as indicated by HPLC.
Seco-coccinic acid F (3,4-seco-9βH-lanost-4(28), 7,24-trien-3-oic acid, compound 1) was an amorphous powder, melting point 160-163°. UV (MeOH) λmax: 201 nm. IR νmax (cm-1): 2968, 1721, 1651, 1455, 1276, 1114. APCI-MS (m/z) 441 [M+H]+ (C30H48O2); 1H-NMR (500 MHz) and 13C-NMR (125 MHz) in dimethyl sulfoxide (DMSO; Table 1).
| Position | Compound 1 | Compound 2 | ||
|---|---|---|---|---|
| seco-coccinic acid F | 24(E)-3,4-seco-9ßH-lanost-4(28),7,24-trien-3,26-dioic acid | |||
| dH(m, J in Hz) | dC | dH(m, J in Hz) | dC | |
| 1 | * | 28.6 | * | 28.7 |
| 2 | * | 28.3 | * | 29.7 |
| 3 | - | 174.8 | - | 175.6 |
| 4 | - | 149.3 | - | 149.5 |
| 5 | * | 44.7 | * | 44.7 |
| 6 | * | 28.8 | * | 28.9 |
| 7 | 5.30 (1H, m) | 117.1 | 5.29 (1H, m) | 117.1 |
| 8 | - | 146.2 | - | 146.2 |
| 9 | * | 38.4 | * | 38.4 |
| 10 | - | 35.7 | - | 35.8 |
| 11 | * | 17.9 | * | 18 |
| 12 | * | 33.3 | * | 33.4 |
| 13 | - | 43.1 | - | 43.1 |
| 14 | - | 51 | - | 50.9 |
| 15 | * | 33.5 | * | 33.5 |
| 16 | * | 27.5 | * | 27.4 |
| 17 | * | 52.2 | * | 52.2 |
| 18 | 0.73 (3H, s) | 21.1 | 0.74 (3H, s) | 21.1 |
| 19 | 0.81 (3H, s) | 23.4 | 0.80 (3H, s) | 23.5 |
| 20 | * | 35.1 | * | 35.2 |
| 21 | 0.88 (3H, d, 6.0) | 17.9 | 0.90 (3H, d, 6.5) | 17.8 |
| 22 | * | 35.5 | * | 34.4 |
| 23 | * | 24.2 | * | 24.8 |
| 24 | 5.09 (1H, t, 7.0) | 124.7 | 6.54 (1H, t, 7.0) | 138.9 |
| 25 | - | 130 | - | 129.3 |
| 26 | 1.63 (3H, s) | 24.9 | - | - |
| 27 | 1.58 (3H, s) | 17.1 | 1.77 (3H, s) | 12.2 |
| 28 | 4.84 (1H, br s) | 111.3 | 4.83 (1H, br s) | 111.2 |
| 4.78 (1H, br s) | 4.77 (1H, br s) | |||
| 29 | 1.77 (3H, s) | 25.1 | 1.72 (3H, s) | 24.9 |
| 30 | 1.00 (3H, s) | 27 | 1.01 (3H, s) | 27 |
Spectra were recorded in DMSO; ‘*’ overlap signals; s- singlet; br s- broad singlet; d- doublet; t- triplet and m- multiplet
Table 1: Data for 1H- (500 MHz) and 13C-NMR (125 MHz) spectra of compounds 1 and 2
24(E)-3,4-seco-9βH-lanost-4(28),7,24-trien-3,26-dioic acid (compound 2) was amorphous powder, melting point 234-236°; UV (MeOH) λmax: 202 nm; IR νmax (cm-1): 3464; 2956; 1690; 1653; 1505; 1289; 1072; APCI-MS (m/z) 471 [M+H]+ (C30H46O2); 1H-NMR (500 MHz) and 13C-NMR (125 MHz) in DMSO (Table 1).
HIV-1 protease activity assay was performed using a fluorogenic substrate (QXL 520-GABA-SFNFPQITKHiLyte Flour 488-NH2). The assay mixture (30 μl) contained HIV-1 protease assay buffer (100 mM sodium acetate buffer, pH 4.7, containing 1 M NaCl, 1 mM ethylene diaminetetraacetic acid, 1 mM dithiothreitol, 5 % DMSO, 1 mg/ml bovine serum albumin, 150 ng HIV-1 protease, and 2 μM fluorogenic substrate). The assays were performed at 37° and the start of the reaction was recorded as the time at which the substrate was added into the mixture. The reaction mixture was excited at 490 nm and emitted at 525 nm using a Nanodrop 3300 fluorospectrometer (Thermo Fisher Scientific, USA).
For the HIV-1 protease inhibitory activity assay, the test compound (dissolved in 5 % DMSO) was preincubated with HIV-1 protease at different concentrations (0-10 μM seco-coccinic acid F and 0-1 μM 24(E)-3,4-seco-9βH-lanost-4(28),7,24-trien-3,26- dioic) for 5 min prior to the addition of the substrate. Based on residual activity ((Δexperimental×100%)/Δcontrol, where Δ was the change in emission at 525 nm per second of HIV-1 protease activity), the inhibitory activity of the compound was calculated as a percent of the residual activity of the control, in which inhibitor was absent (100 %).
A tetrazolium salt (MTS) assay was performed to determine the toxicity of two lanostane triterpenoids against HEK 293T cells. HEK 293T cells were maintained in culture flasks and grown at 37° in a CO2 incubator (5 %), as monolayers in Dulbecco’s modified Eagle’s medium (Corning) supplemented with 10 % (v/v) fetal calf serum and buffered with 3.7 g/l NaHCO3. Cells were inoculated into a flatbottomed clear 96-well microplate (Corning, USA) at a density of 1×104 cells/well in 180 μl of culture medium. The cultures were incubated for 24 h, then treated for 48 h with different concentrations of test compounds (0-125 μM seco-coccinic acid F and 0-37.5 μM 24(E)- 3,4-seco-9βH-lanost-4(28),7,24-trien-3,26-dioic). Each concentration was tested in triplicate at 37° under 5 % CO2. Thereafter, 20 μl of MTS solution (CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay, Promega, USA) was added to each well and the cells were incubated for an additional 2.5 h at 37° and 5 % CO2. The plate was gently shaken and absorbance at 490 nm was measured using a Model 680 Microplate reader (Bio-Rad, USA). All drug doses were tested using DMSO as the control in triplicate.
Data in all the figures are the mean±standard error of 3 determinations. Seco-coccinic acid F (3,4-seco- 9βH-lanost-4(28),7,24-trien-3-oic acid (compound 1) and 24(E)-3,4-seco-9βH-lanost-4(28),7,24-trien-3, 26-dioic acid (compound 2) were isolated from the EtOH extracts of the roots of K. coccinea. The compounds had purity of ≥93 %, as determined by HPLC and were confirmed by UV, IR, MS, and NMR analysis (Table 1) before testing the inhibitory activity of HIV-1 protease.
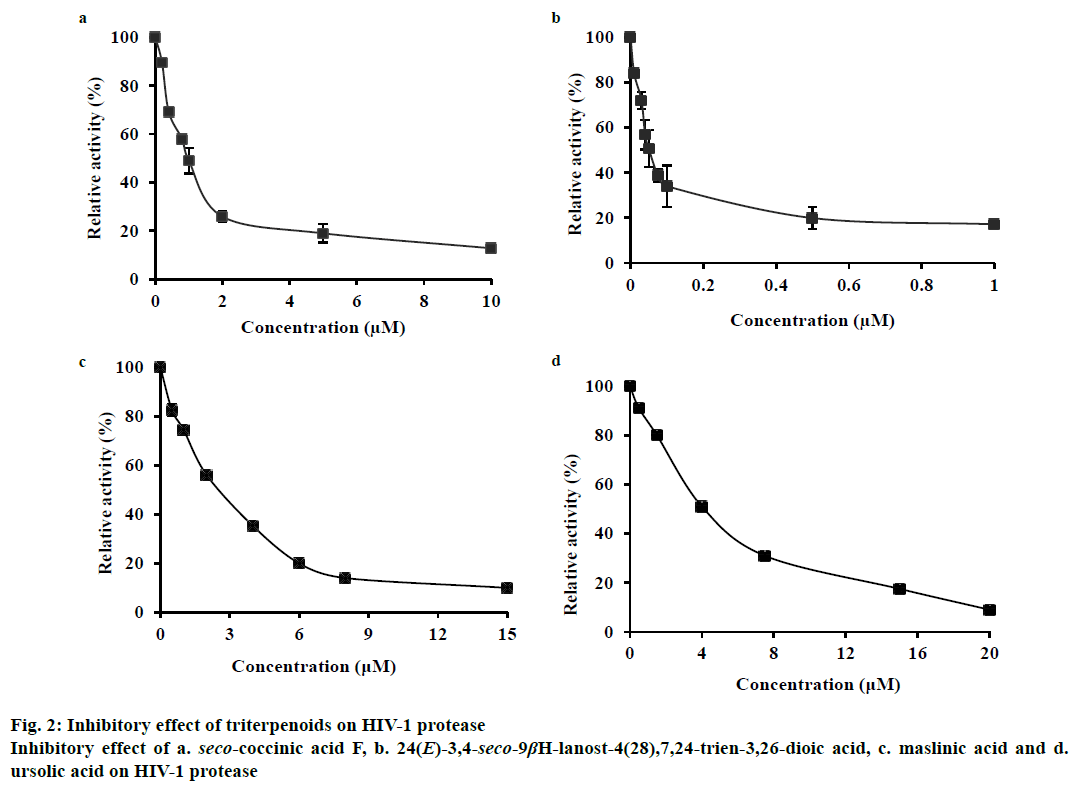
In this study, we found that these two seco-lanostane triterpenoids strongly inhibited HIV-1 protease, with IC50 values of 1.0±0.03 μM (Figure 2a) and 0.05± 0.009 μM (Figure 2b), respectively. As references, two other triterpenoids, maslinic acid (oleanane triterpenoid) and ursolic acid (ursane triterpenoid) that are known inhibitors of HIV-1 protease were tested. They yielded IC50 values of 2.6±0.5 μM (Figure 2c) and 4±0.5 μM (Figure 2d), respectively, under the same experimental conditions.
Maslinic acid and ursolic acid are pentacyclic triterpenoids found in a variety of natural sources [11,12] and are known non-peptide HIV-1 protease inhibitors, with IC50 values reported in the range of 2.5-8 μM [4,13]. However, their potency against HIV-1 protease was much lower than that of the two seco-lanostane triterpenoids evaluated in this study. The difference in the structures of the triterpenoids might have caused this discrepancy. The carboxylic moiety at the 3’ position and 4,4,14-trimethyl cholestan frame seems to have conferred higher inhibitory activity to the seco-lanostane triterpenoids (IC50, 0.05-1 μM) than that conferred by the hydroxy group to maslinic and ursolic acids (IC50, 2.6-4 μM). This result was consistent with the observation that the polar-function group of the C-3 side chain of triterpenoids played an important role in interacting with HIV-1 protease [4].
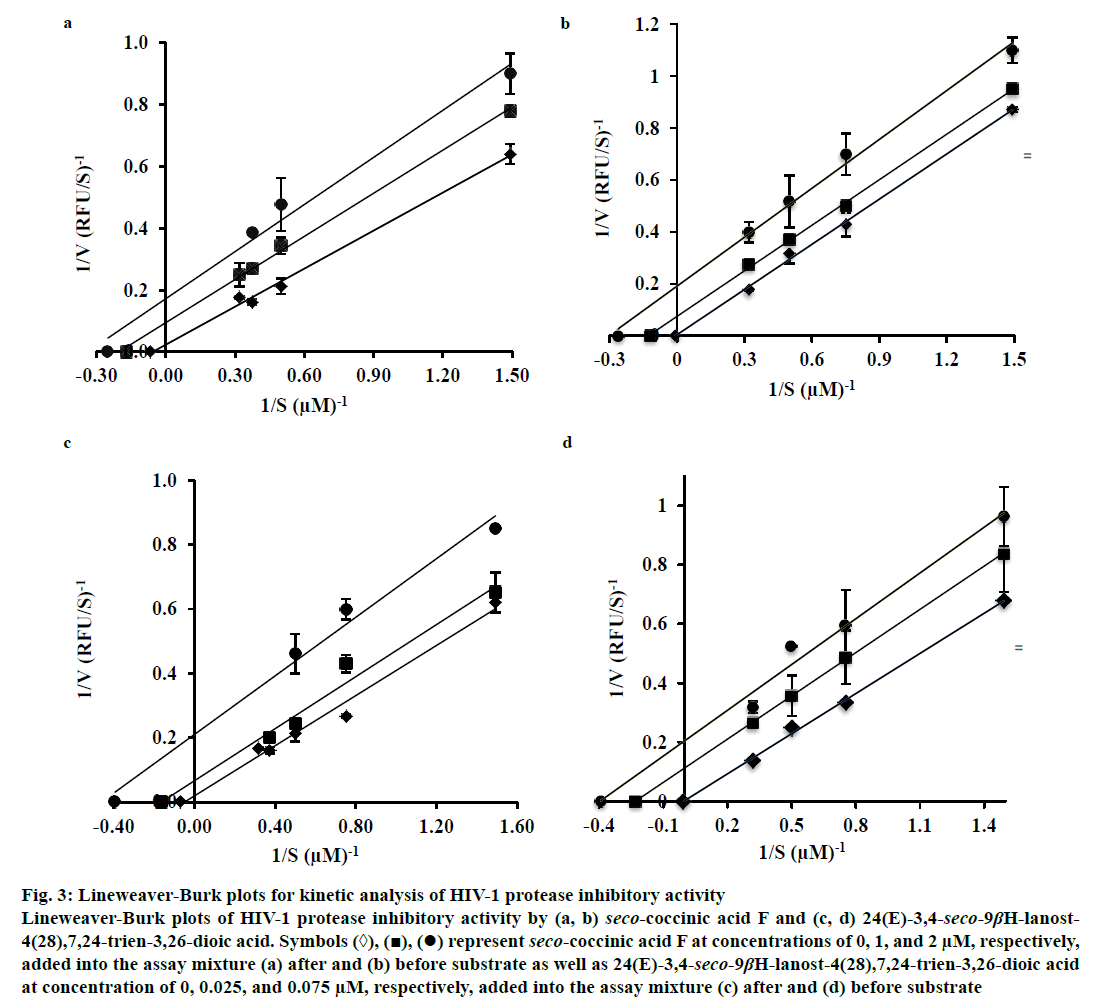
To determine the mechanism by which seco-coccinic acid F and 24(E)-3,4-seco-9βH-lanost-4(28), 7,24-trien-3,26-dioic acid inhibited HIV-1 protease, the changes in the Michaelis-Menten constant (Km) and the maximum reaction velocity (Vmax) at different substrate concentrations were measured in the absence and presence of the inhibitors, using the Lineweaver- Burk Eqn. Seco-coccinic acid F at a final concentrations of 1 and 2 μM was added to the reaction mixtures containing HIV-1 protease either prior to or after the addition of substrate. Similarly, 24(E)-3,4-seco-9βHlanost- 4(28),7,24-trien-3,26-dioic acid was added in the same manner at concentrations of 0.025 and 0.075 μM. As shown in Figure 3, the Vmax and Km values in the absence of the seco-coccinic acid F or 24(E)- 3,4-seco-9βH-lanost-4(28),7,24-trien-3,26-dioic acid were 43.09 RFU/s and 7.71 μM, respectively, and they were higher than values observed in the presence of the inhibitors. The inhibition patterns remained the same whether the inhibitors were added before or after the substrates (Figure 3). Altogether, the data indicated that seco-coccinic acid F and 24(E)-3,4-seco-9βH-lanost- 4(28),7,24-trien-3,26-dioic acid did not bind HIV-1 protease at its active site and the compounds likely inhibited the enzyme in an uncompetitive manner.
Figure 3: Lineweaver-Burk plots for kinetic analysis of HIV-1 protease inhibitory activity
Lineweaver-Burk plots of HIV-1 protease inhibitory activity by (a, b) seco-coccinic acid F and (c, d) 24(E)-3,4-seco-9βH-lanost-
4(28),7,24-trien-3,26-dioic acid. Symbols (◊), (■), (•) represent seco-coccinic acid F at concentrations of 0, 1, and 2 μM, respectively,
added into the assay mixture (a) after and (b) before substrate as well as 24(E)-3,4-seco-9βH-lanost-4(28),7,24-trien-3,26-dioic acid
at concentration of 0, 0.025, and 0.075 μM, respectively, added into the assay mixture (c) after and (d) before substrate
Seco-coccinic acid F was isolated for the first time from the roots of K. coccinea and exhibited anticancer activity by inhibiting the growth of human leukemia HL-60 cells [7,13], whereas 24(E)-3,4-seco-9βH-lanost- 4(28),7,24-trien-3,26-dioic acid was isolated from Abies koreana and exhibited weak activity against human tumor cell lines [14]. Several previous studies reported that many compounds isolated from the Kadsura genus of plants were active against HIV. For example, binankadsurin A, isolated from K. angustifolia, exhibited potent antiHIV activity, with an EC50 value of 3.86 μM [15]; kadsulignan N, isolated from K. coccinea, exhibited strong activity against HIV in vitro, with an IC50 value of 0.0119 μM; and schizarin E, isolated from K. matsudai, demonstrated a strong inhibitory effect against HIV replication in H9 lymphocytes, with an IC50 value of 2.08 μg/ml [16]. Many lanostane triterpenoids were isolated from K. coccinea [5,8,13], but this is the first study to evaluate the inhibitory activity of seco-coccinic acid F and 24(E)- 3,4-seco-9βH-lanost-4(28),7,24-trien-3,26-dioic acid against HIV-1 protease.
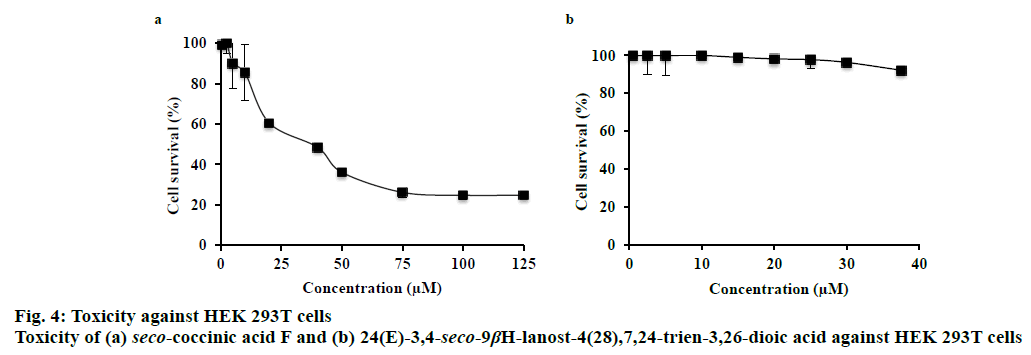
The toxicity of seco-coccinic acid F and 24(E)- 3,4-seco-9βH-lanost-4(28),7,24-trien-3,26-dioic acid against HEK 293T cells was determined at different concentrations, starting from 0.5 μM to the maximum concentration of each compound soluble in DMSO (125 μM in case of seco-coccinic acid F and 37.5 μM in case of 24(E)-3,4-seco-9βH-lanost- 4(28),7,24-trien-3,26-dioic acid). Intrinsic cytotoxicity was represented by the concentration leading to 50 % cell death (IC50), as determined by MTS assay. The results indicated that seco-coccinic acid F killed HEK 293T cells, with an IC50 value of 40 μM (Figure 4a), whereas at the same concentration, 24(E)-3,4-seco- 9βH-lanost-4(28),7,24-trien-3,26-dioic acid did not exhibit any effect on the cells (Figure 4b). As mentioned above, the two seco-lanostane triterpenoids investigated were potent HIV-1 protease inhibitors with IC50 values of 1.0±0.03 and 0.05±0.009 μM, respectively. These concentrations were much lower than the concentrations at which they showed toxicity against HEK 293T cells. Consequently, our data indicated that K. coccinea, a traditional medicinal plant used in Vietnam, and these seco-lanostane triterpenoids were potential candidates for development of antiHIV/AIDS drugs.
This study indicated that two seco-lanostane triterpenoids (seco-coccinic acid F and 24(E)-3,4-seco- 9βH-lanost-4(28),7,24-trien-3,26-dioic acid) isolated from the roots of K. coccinea significantly inhibited HIV-1 protease and showed no toxicity against HEK 293T cells at concentrations effective against HIV-1 protease. These findings support the potential development of these compounds as drugs for the treatment of HIV/AIDS.
Acknowledgements
This research was financially supported by Vietnam National University, Hanoi (project code: KLEPT.14.03).
Conflict of interest
The authors declare no conflicts of interest.
References
- Rose JR, Babe LM, Craik CS. Defining the level of human immunodeficiency virus type 1 (HIV-1) protease activity required for HIV-1 paticle maturation and infectivity. J Virol 1995;69:2751-8.
- Ma C, Nakamura N, Miyashiro H, Hattori M, Shimotohno K. Inhibitory effects of ursolic acid derivatives from Cynomorium songaricum and related triterpenes on human immunodeficiency viral protease. Chem Pharm Bull (Tokyo) 1998;47(2):141-5.
- Yust MDM, Pedroche J, Megias C, Giron-Calle J, Alaiz M, Millán F, et al. Rapeseed protein hydrolysates: a sourse of HIV protease peptide inhibitors. Food Chem 2004;87:387-92.
- Cheenpracha S, Karalai C, Ponglimanont C, Subhadhirasakul S, Tewtrakul S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg Med Chem 2006;14:1710-14.
- Li H, Wang L, Miyata S, Kitanaka S. Kadsuracoccinic acids A-C, ring-A seco-lanostane triterpenes from Kadsura coccineaand their effects on embryonic cell division of Xenopuslaevis. J Nat Prod 2008;71:739-41.
- Nguyen HH, Vu VT, Nguyen MK, Phuong TT. Two triterpenoids and one lignan isolated from the roots of Kadsura coccinea (Lem.) AC Smith collected in Lang Son Province. Pharm J 2014;54:71-75.
- Ban NK, Thanh BV, Kiem PV, Minh CV, Cuong NX, Nhiem NX, et al. Dibenzocyclooctadiene lignans and lanostane derivatives from the roots of Kadsura coccinea and their protective effects on primary rat hepatocyte injury induced by t-butyl hydroperoxide. Planta Med 2009;75:1253-7.
- Wang N, Li Z, Song D, Li W, Fu H, Koike Y, et al. Lanostane-type triterpenoids from the roots of Kadsura coccinea. J Nat Prod 2008;71:990-4.
- Wei Y, Ma CH, Chen DY, Hattori M. Anti-HIV-1 protease triterpenoids from Stauntonia obovatifoliola Hayata subsp. intermedia. Phytochemistry 2008;69:1875-9.
- Nguyen HL, Nguyen TT, Vu QT, Le HT, Pham Y, Trinh PL, et al. An efficient procedure for the expression and purification of HIV-1 protease from inclusion bodies. Protein Expr Purif 2015;116:59-65.
- Lozano-Mena G, Sanchez-Gonzalez M, Juan ME, Planas JM. Maslinic acid, a natural phytoalenxin-type triterpene from olives - a promising nutraceutical? Molecules 2014;19:11538-59.
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 1995;49:57-68.
- Wang N, Li ZL, Song DD, Li W, Pei YH, Jing YK, et al. Five new 3,4-seco-lanostane-type triterpenoids with antiproliferative activity in human leukemia cells isolated from the roots of Kadsura coccinea. Planta Med 2012;78:1661-6.
- Kim HJ, Choi EH, Lee IS. Two lanostane triterpenoids from Abies koreana. Phytochemistry 2004;65:2545-9.
- Gao XM, Pu JX, Huang SX, Yang LM, Huang H, Xiao WL, et al. Lignans from Kadsura angustifolia. J Nat Prod 2004;71:558-63.
- Liu J, Qi Y, Lai H, Zhang J, Jia X, Liu H, et al. Genus Kadsura, a good source with considerable characteristic chemical constituents and potential bioactivities. Phytomedicine 2014;21:1092-07.