- *Corresponding Author:
- Seena T. Pandarekandy
Department of Life Sciences, University of Calicut, Thenjipalam, Malappuram-673 635, India
E-mail: thadathil.s@gmail.com
| Date of Submission | 01 October 2016 |
| Date of Revision | 17 February 2017 |
| Date of Acceptance | 24 July 2017 |
| Indian J Pharm Sci 2017;79(5):768-777 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study is aimed to evaluate the hypoglycaemic effect of glibenclamide monotherapy on streptozotocin-induced diabetic rats and therapeutic impact of this agent on various organs by measuring the oxidative stress biomarker, lipid peroxidation and creatinine after a specific period of oral treatment. Eighteen male Wistar rats were taken of which Twelve were randomly selected and diabetes was induced using streptozotocin (40 mg/kg) intraperitoneally, while 6 rats were vehicle injected. After inducing diabetes, the animals were grouped into healthy normal, diabetic control and diabetic test groups. The diabetic test rats were treated with glibenclamide (10 mg/kg) orally for 10 days continuously. On the 30th day of the experiment, all the animals were sacrificed and their blood and a portion of both the liver and pancreas were taken for biochemical analysis. The experimental parameters such as body weight, fasting blood glucose level, serum creatinine level and tissue lipid peroxidation status using malondialdehyde assay were investigated. All the results of the experimental data were analysed statistically using one-way analysis of variance using the SPSS 16. The current study reveals that glibenclamide treatment has a significant role in lowering the blood glucose but at the same time it increased the oxidative stress, which was reflected in the elevated malondialdehyde activity of both the hepatic and pancreatic tissues.
Keywords
Streptozotocin, diabetes, glibenclamide, pancreas, lipid peroxidation, creatinine
Diabetes mellitus is a multifactorial disease, where complications and treatment of this lifestyle disease is under dispute. Current treatment for this metabolic disorder is focussed only to regulate the blood glucose level when it is abnormally increased. It is carried out either by controlling the mechanism of insulin release from the existing beta cells or via sensitization of insulin receptors. According to the modern system of medicine, the treatment of diabetes mellitus deals with the treatment against the hyperglycaemic condition and the medicines are classified as first generation and second generation medicines on the basis of its pharmacological mode of action.
Diabetes mellitus pathogenesis is always associated with oxidative stress. Uncontrolled hyperglycaemia [1,2] is the exact cause of oxidative stress and creates deleterious effect to various tissues by subsequent production of free radicals, especially the reactive oxygen species (ROS) [3]. The mechanisms involved in increased free radical production in diabetes include auto oxidation of glucose, shifts in redox balances, reduced tissue antioxidant status [4] and reduced activity of tissue antioxidant enzymes [5]. Obi and his associates have suggested that diabetes mellitus is not only the situation of production of free radicals but also associated with elevation in lipid peroxidation (LPO) due to a continuous DNA damage or protein degradation [6]. Moreover, diabetes mellitus is a powerful risk factor for long term damages and dysfunction of organs including heart, blood vessels, nerves, eyes and kidneys [4,7]. The micro and macrovascular complications in the pathology of diabetes lead to characteristic symptoms that are developed in both the types of diabetes (type 1 diabetes mellitus, T1DM and type 2 diabetes mellitus, T2DM).
Kidney is the central organ that plays an important role to remove the metabolic wastes from blood. Diabetes mellitus is a leading cause of kidney damage and develops chronic kidney disease later in life [8]. This is because of haemodynamic changes and further free radical generation in hyperglycaemia [9,10]. Diabetic nephropathy, one of the microvascular complications of diabetes, initiates with microalbuminuria and followed by albuminuria. It leads to structural changes in kidneys including thickening of glomerular basement membrane, microaneurysm and nodule formation and have been observed in both types of diabetes [11]. These changes may adversely affect the normal functions of kidney hence it can be easily evaluated by associated markers. Therefore, this study also attempts to study the kidney function on the basis of metabolic by-product creatinine level and how the organ responds in various metabolic diseases, especially in diabetes mellitus.
External insulin administration is not effective for all kinds of diabetes management. Sometimes, insulin secretion is normal but their response is abnormal. It may be due to insulin resistance and is more common in T2DM than T1DM. This situation is to overcome by drugs, which reduce the blood glucose level and is known as an antidiabetic medicine. The main treatment objectives of oral hypoglycaemic agents are based on the following mechanisms: increase the amount of circulating insulin, increase the sensitivity of target organs like muscle and liver to insulin, decrease the rate of glucose absorption from the gastro intestinal tract and decrease gluconeogenesis [12].
Currently, different classes of oral antidiabetic agents are available in the market. Considering their antidiabetic activity these medicines would be categorized according to insulin sensitization, insulin secretion and extra pancreatic insulin release. Besides these activities, medicines also significantly reduce the glycated hemoglobin level [13]. Glibenclamide is the most frequently prescribed oral hypoglycaemic agent [14]. It is a sulfonylurea compound chemically, 5-chloro-N- [2- [4-cyclohexyl carbamoyl sulfamoyl) phenyl] ethyl]-2-methoxy benzamide [15] (Figure 1), which acts either as pancreatic or extra pancreatic, thereby increasing insulin release from the beta cells [16,17]. The mode of action of glibenclamide in hyperglycaemic conditions is to lower blood glucose via stimulating insulin production from the existing beta cells of pancreas [18]. In addition to this direct action, it also shows extra pancreatic effects [19]. This drug binds to the sulfonylurea receptor 1 (SUR 1) [20], a regulatory subunit of the ATP-sensitive potassium channels (KATP) in the pancreatic beta cells [21,22]. This inhibition causes cell membrane depolarization and opens the voltagedependent calcium channels. It increases intracellular calcium concentration in beta cells and subsequently stimulates the release of insulin [23]. Thus, the single medicine glibenclamide stimulates insulin secretion and reduces hepatic glucose production [24,25] in clinical diabetes. A study has shown that sulfonylureas stimulated insulin secretion from the existing pancreatic beta cells in streptozotocin (STZ) diabetic rats [18]. Moreover, this compound exhibits neuroprotective action in the neurons of hippocampal areas of brain [26].
Fortunately, this well-known medicine acts against hyperglycaemic condition during the course of the treatment. Otherwise diabetes may progress to other major complications including angiopathy, neuropathy, retinopathy, nephropathy etc. Sometimes, oral hypoglycaemic agents lead to drug related side effects along with their pharmacological properties [27-29]. A study has reported that metabolism of most of the oral hypoglycaemic agents take place in the organs like liver, kidney, gastrointestinal tract etc. But, the metabolism is incomplete due to lack of tolerance of the organs to different drug doses. Conversely, nonmetabolized content of the hypoglycaemic agent, which accumulates in the above organs worsened their normal functions [8].
Hypoglycaemia is the most common side effect of glibenclamide treatment [30] along with a greater risk of cardiovascular diseases [31]. Since, glibenclamide is a benzamide moiety that inhibits activity of the KATP channel of cardiac, skeletal and smooth muscles. Nagashima et al., clarify the mechanism of inhibition via mitochondrial inner membrane channel or mito KATP that plays a significant role in cardio protection [32]. From the above reviews it is clear that the availability of numerous hypoglycaemic agents used for diabetesinduced complications, is one of the leading causes of mortality. The present study focussed to evaluate hypoglycaemic effect of glibenclamide and the comparison of oxidative stress of various tissues of glibenclamide treated diabetes-induced rat groups with untreated diabetic rat groups on the basis of hepatic and pancreatic tissue LPO and serum creatinine.
Materials and Methods
The chemicals chosen were of high quality analytical grade reagents. The chemicals, drugs and glucose monitoring unit were purchased from Sigma Aldrich Co., USA, Sanofi India Ltd., Mumbai and Morepen Laboratories Ltd., New Delhi, India, respectively. The orogastric tube was used to force feed medicines or drinking water for test animals and purchased from a medical shop.
Experimental animals
Eighteen male Wistar albino strain rats of the same age group (60 d) and weighing about 200 g were used for the present study. All the studies were conducted strictly in accordance with the approved guidelines of the Institutional Animal Ethics Committee regulated by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The rats were maintained in the animal house of the Department of Life Sciences (Reg. # 426/02/CPCSEA). The animals were kept under standard conditions maintained at a temperature of 23-25°, fed with standard food pellets and water ad libitum throughout the experimental period.
Experimental protocol
The duration of the experiment was 30 d and the experimental set up was subdivided into 3 intervals, which were disease induction period, treatment period and post treatment period. Body weight and fasting blood glucose levels were checked before induction of diabetes. Blood glucose level was measured by animal tail vein using Dr. Morepen® GlucoOne blood glucose monitoring system.
Diabetes induction period
From the 18 rats, 12 rats were randomly selected and injected with STZ at a dosage of 40 mg/kg (the injection was freshly prepared by STZ mixed with 0.1 M of cold citrate buffer, pH 4.5) and the remaining animals were injected with citrate buffer without STZ. All the test animals were orally administered with 5% glucose solution for adjusting their blood glucose level. Later, the animals were kept under regular observation under standard conditions. On the 5th d of STZ injection, animals’ fasting blood glucose level was checked. Again all the test animals were kept under observation for the stabilization of hyperglycaemic condition in diabetes-induced animals. On the 10th d of STZ injection, fasting blood glucose level was again checked. Blood glucose level above ≥200 mg/dl in STZ injected animals were considered to be diabetic. Then the animals were grouped into 3 with 6 animals in each group and were named as group I (healthy normal) group II (diabetic control) and group III (diabetic test).
Treatment period
On the 10th d of the experiment after the confirmation of diabetic status in STZ treated rats, diabetic test groups (group III) were orally treated with glibenclamide (10 mg/kg) continuously for 10 d. At the same time group I and II rats were orally treated with drinking water for the same period. On the 20th d of the experiment, glibenclamide treated rats showed a reduced fasting blood glucose level and the treatment was stopped for a five days (20-25th d). After that there was an elevated blood glucose level in the glibenclamide treated groups when they were kept untreated. Again, the treatment was started and continued for the next 5 d (25th and 30th d).
Post treatment period
On the 30th d of the experiment, fasting blood glucose level and body weight were checked. Then the rats were anaesthetized using chloroform, cardiac puncture was made and blood sample collected for estimating the level of serum creatinine. After blood collection, the liver and pancreatic tissues were removed immediately and washed with ice cold saline for malondialdehyde (MDA) estimation in diabetic and glibenclamide treated diabetic groups.
Biochemical analysis
Creatinine estimation was followed by Jaffe’s method [33,34]. All the reagents and the samples were brought to a reaction temperature of 37°. A coloured creatinine compound (orange colour) produced in an alkaline media [34] was spectrophotometrically measured at 520 nm using JASCO V-630 spectrophotometer (serial No. C395561148), Japan.
Tissue LPO
A portion of both the liver and pancreas (10% w/v) were washed in ice cold saline, blotted, separately weighed and homogenized with chilled tris-HCl buffer (0.1 M at pH=7.5) using a homogenizer for MDA estimation. A small quantity of the above two homogenates were separately mixed with 10% trichloroacetic acid (TCA), shaken well, centrifuged and the precipitate was used to estimate the total protein in the tissues.
LPO product MDA formation was assayed by thiobarbituric reactive substance formation method [35]. The TBA-MDA chromophore was taken as an index of LPO, which gives a pink colour, which was measured at 535 nm and the results were expressed in nanomoles of MDA per mg protein. Protein content in tissue samples were estimated by the method of Lowry [36] using bovine serum albumin as a standard protein.
Statistical analysis
The results of experimental parameters were expressed as mean±standard error of mean (SEM). Statistical significance of the result was analysed by one-way analysis of variance (ANOVA), followed by Fisher's least significant difference (LSD) post hoc test for multiple comparison using Statistical Packages for Social Sciences (SPSS) version 16. Statistical level of significance was set at P<0.01.
Results and Discussion
The study was compared with various parameters including body weight, fasting blood glucose level, serum creatinine, LPO product MDA level in liver and pancreas of healthy, diabetic untreated and diabetic with glibenclamide treated groups. The results of the present study were mentioned in the following table and figures.
Table 1 showed the mean values of body weight (g) of healthy and hyperglycaemia-induced rats. Hyperglycaemia is the main cause of significant weight loss in STZ injected groups. From the above table, it showed rapid increase of body weight in healthy normal groups as age advanced. But, this effect was not observed in the STZ treated groups. The study reveals that after the 10th d (after becoming hyperglycaemic) onwards there was a significant reduction in the body weight of STZ injected groups when compared to healthy normal groups. The reversing effect of body weight was not observed in glibenclamide treated groups even after a continuous 10 d of treatment.
| Group | Bodyweight (g) expressed in mean±SEM | ||||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 5 | Day 10 | Day 15 | Day 20 | Day 25 | Day 30 | |
| I (n=6) |
202.67±02.85 | 202.67±02.85 | 214.00±06.75 | 228.67±09.20 | 241.33±09.99 | 249.33±07.84 | 251.33±08.48 |
| II (n=6) |
201.00±04.66 | 201.00±04.66 | 176.00±11.07** | 172.00±11.45** | 166.00±10.81** | 167.00±11.04** | 162.00±03.05** |
| III (n=6) | 198.00±06.08 | 198.00±06.08 | 184.00±09.06** | 188.00±08.51^^ | 171.33±05.97^^ | 170.67±08.43^^ | 170.00±09.89^^ |
Group I: healthy normal, group II: diabetic control and group III: diabetic test. Values are given in mean±SEM for standard error of mean and ‘n’ for number of animals. P-value less than 0.01 P<0.01 statistically significant between the groups and are represented as *comparison between healthy normal and diabetic control groups; ^comparison between healthy normal and diabetic test groups; **or ^^ significant at 0.01 (1%) level
Table 1: Comparison of body weight of various groups
A study strongly supported the present data that the body weight of glibenclamide treated diabetic rats showed a significant reduction when compared with the healthy groups [37]. Hyperglycaemia-induced groups showed a rapid reduction in the body weight which was similar to the finding reported by Gandhi and Sasikumar [38]. Table 1 showed that there was a statistically significant difference between the healthy normal and the diabetic control (P<0.01**) and healthy normal with glibenclamide treated diabetic test group at 1% (P<0.01^^) level. The result indicates that weight loss in diabetes was due to the excessive degradation of proteins in the major organs. However, the present study found that there was no weight gain after the treatment with glibenclamide, though a study suggests that glibenclamide treatment significantly increased the body weight of STZ-induced diabetic rats [2].
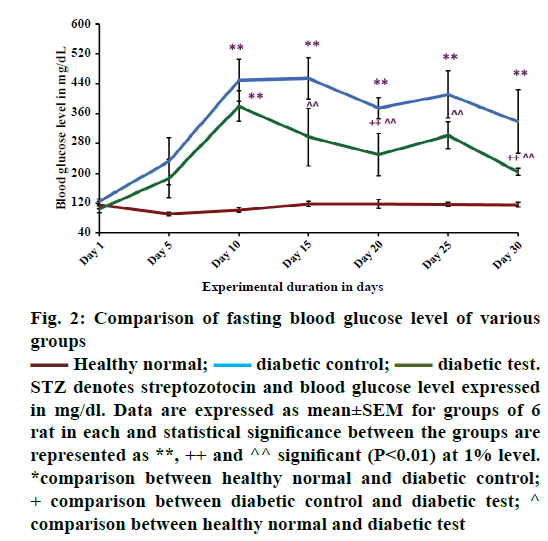
Figure 2 showed the comparison of fasting blood glucose level in healthy normal, diabetic control and diabetic test groups. From the results it was observed that there was a significant increase in the fasting blood glucose level in hyperglycaemia-induced condition. The study reveals that, STZ with this particular dose of 40 mg/kg body weight, may be effective for inducing diabetes in male Wistar albino strain rats. On the 5th d of STZ injection, there was a tendency for a rapid increase of fasting blood glucose level when compared with the healthy groups (healthy normal). This finding was similar to that reported by Waer and Helmy [39].
Figure 2: Comparison of fasting blood glucose level of various
groups
 Healthy normal;
Healthy normal;  diabetic control;
diabetic control;  diabetic test.
STZ denotes streptozotocin and blood glucose level expressed
in mg/dl. Data are expressed as mean±SEM for groups of 6
rat in each and statistical significance between the groups are
represented as **, ++ and ^^ significant (P<0.01) at 1% level.
*comparison between healthy normal and diabetic control;
+ comparison between diabetic control and diabetic test; ^
comparison between healthy normal and diabetic test
diabetic test.
STZ denotes streptozotocin and blood glucose level expressed
in mg/dl. Data are expressed as mean±SEM for groups of 6
rat in each and statistical significance between the groups are
represented as **, ++ and ^^ significant (P<0.01) at 1% level.
*comparison between healthy normal and diabetic control;
+ comparison between diabetic control and diabetic test; ^
comparison between healthy normal and diabetic test
Glibenclamide is an effective drug to facilitate insulin release [40] from the beta cells. This sulfonylurea compound also shows extra pancreatic effect during long term administration and a greater risk of hypoglycaemia [41]. It is an oral hypoglycaemic agent to treat T2DM [42].
The report of the WHO expert committee 2011 says that it is used as one of the oral antidiabetic medicines under the second generation series of sulfonylureas [43]. Studies suggest that glibenclamide shows insulin dependent blood glucose lowering effect [21] especially in STZ-induced type 1 diabetic model [18]. This may be due to the insulin stimulating effect of this medicine in STZ-induced diabetes or clinical diabetes. Study suggests that glibenclamide exerts hypoglycaemic action by stimulating insulin release simultaneously with inhibition of glucagon secretion [44]. From the Figure 2, on the 10th d of the experiment the mean value of blood glucose level of diabetic control (n=6) and diabetic test (n=6) were 450±57.12 and 380±41.32 mg/dl, respectively signifying that the animals were diabetic. During the treatment period there was a gradual reduction in fasting blood glucose level of glibenclamide treated groups (diabetic test 249±56.43 mg/dl) when compared with the diabetic untreated groups (diabetic control 374±28.60 mg/dl). But, fasting blood glucose level was not reduced to normal level as in healthy groups (118±12.04 mg/dl).
In healthy groups, the fasting blood glucose level was always at a normal range. The significance of the present result was analysed using post hoc test, where there was a significant difference at 1% level between (P<0.01**) the healthy normal group and diabetic control group, diabetic untreated group (diabetic control) and diabetic test group (P<0.01++) and healthy normal group and diabetic test group (P<0.01^^). The present study showed that the oral hypoglycaemic agent glibenclamide was not effective for short course diabetic treatment. The result proposed that a combination therapy was more effective for improving glycaemic control in STZ-induced diabetics. A study has shown that, combination medicines metformin and glibenclamide were used to adjust blood glucose level in type 2 diabetic cases [45]. Monotherapy stabilizes the blood glucose level, but it does not prevent the complications of diabetes [46]. Thus, combination therapy is required as a better option for the successful management of type 2 diabetes [47]. From the above data, it is clear that medicines of different classes and therapies are effective in combination for the management of diabetes. However, majority of diabetic population face treatment related complications and no one got completely recovered from this noncommunicable disease.
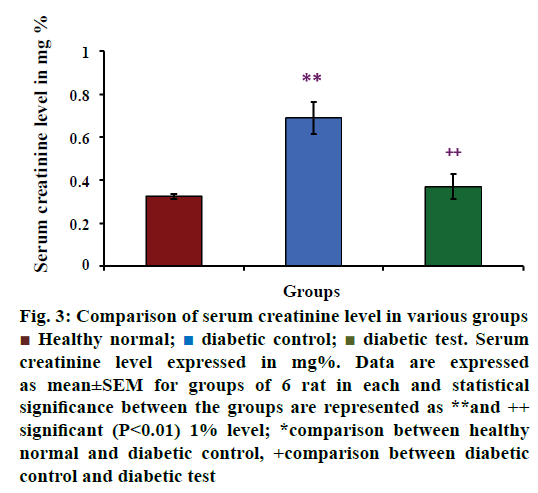
Serum creatinine level in healthy normal, diabetic control and diabetic test groups were depicted in Figure 3. The mean value of serum concentration was increased in diabetes-induced groups than the healthy normal groups. The mean values of serum creatinine level in various groups were 0.325±0.013 mg% for healthy groups (n=6), 0.690±0.074 mg% for diabetic control groups (n=6) and 0.370±0.059 mg% for diabetic test or glibenclamide treated groups (n=6). From this value, it is clear that serum creatinine concentration was increased in hyperglycaemia- induced rats. The result of the present study was statistically analysed using multiple comparison test, which showed that there was a significant difference between the healthy normal group and diabetic control group (P<0.01**) and diabetic control group and diabetic test group (P<0.01++), at 1% level.
Figure 3: Comparison of serum creatinine level in various groups
 Healthy normal;
Healthy normal;  diabetic control;
diabetic control;  diabetic test. Serum
creatinine level expressed in mg%. Data are expressed
as mean±SEM for groups of 6 rat in each and statistical
significance between the groups are represented as **and ++
significant (P<0.01) 1% level; *comparison between healthy
normal and diabetic control, +comparison between diabetic
control and diabetic test
diabetic test. Serum
creatinine level expressed in mg%. Data are expressed
as mean±SEM for groups of 6 rat in each and statistical
significance between the groups are represented as **and ++
significant (P<0.01) 1% level; *comparison between healthy
normal and diabetic control, +comparison between diabetic
control and diabetic test
Diabetes mellitus is an underlying cause of chronic kidney disease [48]. It develops certain degree of damage and lead to abnormal kidney function. However, kidney impairment may also be associated with its recognised risk factors such as hypertension, altered metabolism, alcoholism and smoking. A study has been reported that end-stage kidney disease was observed particularly in type 2 diabetic patients and requires dialysis for effective management of kidney function simultaneously with antidiabetic therapies [49]. Sometimes antidiabetic medicines cause rare side effects including renal failure, hepatic dysfunction or tissue ischemia [50]. A study reported that impaired renal function is one of the consequences of glucose lowering agents [8]. It indicates that oral antidiabetic medicines have both hypoglycaemic and deleterious effect simultaneously. It may develop kidney damage progressively and lead to kidney failure.
Kidney function can be easily assessed by the level of significant markers such as creatinine, urea, uric acid and certain electrolytes [51]. Gayathri and Kannabiran reported that plasma levels of urea, uric acid and creatinine increased in STZ-injected animals [52] and it may be disturbing the renal function. The present study also agreed with an earlier study that STZ-induced diabetic animals showed a significant elevation in serum creatinine [53,54]. This significant variation in creatinine concentration in diabetic condition may be due to the altered function of kidneys to remove waste products [55].
Creatinine is a metabolic by product and its concentration is increased in the blood indicating kidney dysfunction [56]. From the Figure 3, diabetes test groups showed that there was no significant elevation in serum creatinine concentration when compared with the diabetic control groups, but the level was found to be slightly increased in diabetes test than the non-diabetic groups. This reveals that hypoglycaemic action of glibenclamide sometimes may be a reason for the concomitant side effects. According to the present result it may be due to the loss of kidney function to remove the accumulated metabolites and lack of adaptation of the body to the medicines of different doses.
Several studies have been reported that oxidative stress is connected with the depletion of free radical scavengers and excessive generation of free radicals [57-59] in experimental diabetes. STZ, the drug which is given intraperitoneally at a particular dose can effectively produce hyperglycaemia [60] in experimental animals. The diabetogenic property of this drug can be ensured by the observable symptoms such as polyphagia, polydipsia and polyuria with weight loss after its administration. Study revealed that STZ inhibited insulin production via molecular mechanisms, because STZ is a source of free radicals that can also contribute to DNA damage and subsequent degeneration of beta cells after its administration in animals [61].
LPO is an important biomarker of oxidative stress and can be easily measured. Other markers are of different molecular species formed inside the body, which are highly reactive, short lived and are difficult to quantify directly as it is very low in concentration [62]. LPO is a free radical mediated complex reaction producing a variety of highly reactive aldehyde compounds such as MDA and 4-hydroxynonenal (HNE) [63]. Saravanan and Ponmurugan reported that chronic complications of diabetes are also associated with LPO status of target tissues [64]. The present study investigated on both the hepatic and pancreatic LPO status by measuring MDA levels. Padalkar et al. [65] suggested that increased tissues MDA is a sign of oxidative stress in degenerative diseases such as diabetes mellitus and chronic kidney diseases. But, this study did not reveal the kidney impairment on the basis of LPO level.
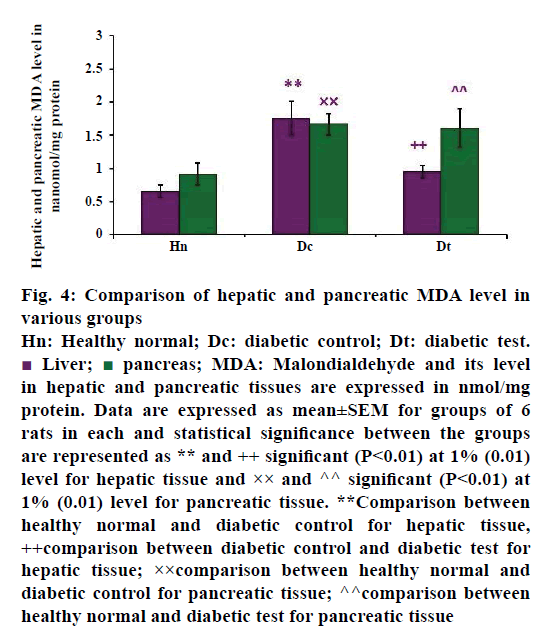
A significant increase of MDA activity was observed in the liver and pancreas of STZ-injected diabetic untreated groups, as shown in Figure 4. The mean value of hepatic MDA activity of healthy normal (n=6), diabetic control (n=6) and diabetic test groups (n=6) were 0.657±0.092, 1.763±0.252 and 0.955±0.090 nmol/mg protein, respectively. The present study revealed that increased hepatic MDA activity in diabetic untreated group may be reflecting the complications of oxidative damage. When diabetic rats were treated with glibenclamide, there was a reduction in the hepatic MDA than in the diabetic control group. This finding was in concomitant with the study of Obi et al. [6] which reports that glibenclamide has some protective role against the oxidative damage in hyperglycaemic conditions. The data was analysed by post hoc test, the P-value was less than 0.01, hence the result was statistically significant at 1% level between the healthy groups and diabetic control group (P<0.01**) and diabetic control group with diabetic test group (P<0.01++).
Figure 4: Comparison of hepatic and pancreatic MDA level in
various groups
Hn: Healthy normal; Dc: diabetic control; Dt: diabetic test. Liver;
Liver;  pancreas; MDA: Malondialdehyde and its level
in hepatic and pancreatic tissues are expressed in nmol/mg
protein. Data are expressed as mean±SEM for groups of 6
rats in each and statistical significance between the groups
are represented as ** and ++ significant (P<0.01) at 1% (0.01)
level for hepatic tissue and ×× and ^^ significant (P<0.01) at
1% (0.01) level for pancreatic tissue. **Comparison between
healthy normal and diabetic control for hepatic tissue,
++comparison between diabetic control and diabetic test for
hepatic tissue; ××comparison between healthy normal and
diabetic control for pancreatic tissue; ^^comparison between
healthy normal and diabetic test for pancreatic tissue
pancreas; MDA: Malondialdehyde and its level
in hepatic and pancreatic tissues are expressed in nmol/mg
protein. Data are expressed as mean±SEM for groups of 6
rats in each and statistical significance between the groups
are represented as ** and ++ significant (P<0.01) at 1% (0.01)
level for hepatic tissue and ×× and ^^ significant (P<0.01) at
1% (0.01) level for pancreatic tissue. **Comparison between
healthy normal and diabetic control for hepatic tissue,
++comparison between diabetic control and diabetic test for
hepatic tissue; ××comparison between healthy normal and
diabetic control for pancreatic tissue; ^^comparison between
healthy normal and diabetic test for pancreatic tissue
Pancreas is the organ that regulates blood glucose level to a normal range. The beta cells neither produce enough insulin nor act on the insulin receptors leading to hyperglycaemia. Thus complete loss of beta cell function or beta cell apoptosis is the main source of free radical production in the pancreatic tissue. These changes can be easily identified by the level of LPO of the pancreas. The Figure 4 also showed the pancreatic MDA activity of healthy normal group, diabetic untreated and diabetic treated groups. The values were expressed as mean±SEM. The mean value was higher in diabetic control (n=6), which was 1.667±0.165 nmol/mg protein when compared with the diabetic test group. The mean value of pancreatic MDA level in glibenclamide treated diabetes-induced test group (n=6) is 1.611±0.288 nmol/mg protein. When compared with the healthy groups (n=6), there was a reduced pancreatic MDA (0.919±0.166 nmol/mg protein) activity. The result also showed that there was a significant difference between healthy normal group and diabetic control group (P<0.01××) and healthy normal group with diabetic test group (P<0.01^^), at 1% level.
From the data, it was clear that in healthy groups, the MDA level was not exceeding 1.0 nmol/mg protein. This reveals the insufficiency of antioxidants for the defence mechanisms, which was the hall mark of LPO status of STZ-injected groups. LPO alters the membrane lipids or membrane bound proteins especially unsaturated fatty acids [7,66,67] leading to the generation of highly reactive compound MDA [68]. In the present study, significant increase of hepatic and pancreatic tissue MDA level in STZ-injected groups may be the reason for the altered metabolism. These metabolic alterations may occur by either the inducing agent or the oral hypoglycaemic agent in the respective groups. The study also revealed that LPO reaction is tissue specific and was increased during the progression of the disease. It is reported in a study of Sellamuthu and others [69] that the tissue LPO status was used as a method of measuring the progress of diabetes. The lipid peroxide level of diabetic test group was elevated and near to the value of diabetic control group. It agreed with the study of STZ-induced diabetic model with the effect of glibenclamide treatment and other oral hypoglycaemic agents [59] on tissue MDA level.
An earlier study agreed with the present result that the STZ diabetic rats treated glibenclamide alone showed slightly but insignificant reduction in MDA, while those treated with combination with other agents [70]. Therefore, this study showed that tissue MDA level did not reduce to normal values (values of healthy groups) during the glibenclamide monotherapy. This finding was also agreed with the study of Abdulkadir and Thanoon, they reported that no significant decrease in serum MDA level in type II diabetic patients after a short course treatment with glibenclamide [71]. While a study suggests that glibenclamide with other combination has a tendency to reduce MDA level in Parkinson’sinduced animal model [72]. In the present study, MDA level was elevated suggesting that glibenclamide monotherapy has no favourable effect to restore the antioxidants in diabetes-induced diseased conditions but is more prominent in combination therapy [73]. It reveals that oxidative damage is still obvious, may be due to the reduced antioxidant potential of this antidiabetic medicine.
From the present investigation, it is clear that oxidative stress is a remarkable feature of diabetes. The above results of the present study conclude that, oral hypoglycaemic agent glibenclamide has some potential role against hyperglycaemic condition. That may be linked with direct insulin like activity of this medicine. The hypoglycaemic effect with some other remarkable changes is also observed in the major organs such as liver, pancreas and kidneys of the experimental groups. The study proves that glibenclamide has no capacity to restore the original body weight, which is clear from the liver and pancreatic LPO status of glibenclamide treated rat groups.
Diabetes is a situation of altered antioxidant status that may be creating an increased LPO in various tissues. Therefore, medicines for diabetic therapy should not only focus on hypoglycaemia alone, but also be considerate on other pharmacological aspects for the overall management of diabetes like antihyperlipidemic, reduced lipid peroxidative effect and restoration of antioxidant levels. This oral hypoglycaemic agent cannot properly work against the oxidative stress in diabetic conditions. It was reflected in the MDA activity of both the liver and pancreas of STZ-injected groups, especially in the pancreatic tissue of diabetic test groups. Hence, the present study proposes an oxidative stress and related complications in STZ-induced diabetes, which sustained even after the treatment with glibenclamide. This irreversible effect of oxidative stress during glibenclamide treatment may be completely or partially associated with reducing the activities of free radical scavengers. Further detailed biochemical, molecular and histopathological studies are needed to elucidate the mechanism of antidiabetic property of glibenclamide and other allopathic medicines.
Acknowledgements
Authors thank the Department of Life Sciences, University of Calicut for providing financial support of this research work (University Fellowship Order No. CDC/A2/1351/09).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Financial support and sponsorship
Nil.
References
- Rehman A, Nourooz-Zadeh J, Moller W, Tritschler H, Pereira P, Halliwell B. Increased oxidative damage to all DNA bases in patients with type - II diabetes mellitus. FEBS Lett 1999;448:120-2.
- Kumar V, Ahmed D, Gupta PS, Anwar F, Mujeeb M. Anti-diabetic, anti-oxidant and anti-hyperlipidemic activities of Melastoma malabathricum Linn. leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med 2013;13:01-19.
- Ahmadi R, Pishghadam S, Mollaamine F, Monfared MRZ. Comparing the effects of ginger and glibenclamide on dihydroxybenzoic metabolites produced in STZ-induced diabetic rats. Int J Endocrinol Metab 2013;11:01-05.
- Matough FA, Budin SB, Hamid ZA, Alwahaini N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J 2012;12:05-18.
- Bisht S, Sisodia SS. Diabetes, dyslipidemia, antioxidant and status of oxidative stress. Int J Res Ayurveda Pharm 2010;1:33-42.
- Obi BC, Okoye TC, Okpashi VE, Igwe CN, Alumanah EO. Comparative study of the antioxidant effects of metformin, glibenclamide and repaglinide in alloxan-induced diabetic rats. J Diabetes Res 2016;2016:01-05.
- Sireesha K, Sailaja RP. Oxidative stress and diabetes: An overview. Asian J Pharm Clin Res 2015;8:15-9.
- Arnouts P, Bolignano D, Nistor I, Bilo H, Gnudi L, Heaf J, van Biesen W. Glucose-lowering drugs in patients with chronic kidney disease: A narrative review on pharmacokinetic properties. Nephrol Dial Transplant 2014;29:1284-300.
- Prabhu KS, Lobo R, Shirwaikar A. Antidiabetic properties of the alcoholic extract of Sphaeranthus indicus in streptozotocin-nicotinamide induced diabetic rats. J Pharm Pharmacol 2008;60:909-16.
- Shokeen P, Anand P, Murali YK, Tandon V. Antidiabetic activity of 50% ethanolic extract of Ricinus communis and its purified fractions. Food Chem Toxicol 2008;46:3458-66.
- Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008;26:77-82.
- Devani U, Pandita N, Kachwala Y. Evaluation of inhibitory activity of Vitex negundo and Terminalia chebula by alpha amylase inhibition assay in management of diabetes. Asian J Plant Sci Res 2013;3:06-14.
- Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C Levels: A systematic review and meta-analysis. Diabetes Care 2010;33:1859-64.
- Nathan D, Buse J, Davidson M, Ferrannini E, Holman R, Sherwin R, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: A consensus algorithm for the initiation and adjustment of therapy. Diabetologia 2009;52:17-30.
- Budavari S. The Merck Index, an Encyclopaedia of Chemicals, Drugs and Biologicals. 11th ed. Rahway, New Jersey: Merk & Co; 1989. p. 703.
- Schmid-Antomarchi H, De Weille J, Fosset M, Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem 1987;262:15840-44.
- Luzi L, Pozza G. Glibenclamide: An old drug with a novel mechanism of action? Acta Diabetol 1997;34:239-44.
- Rajasekaran S, Sivagnanam K, Subramanian S: Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol Rep 2005;57:90-96.
- Tsiani E, Ramlal T, Leiter LA, Klip A, Fantus IG. Stimulation of glucose uptake and increased plasma membrane content of glucose uptake and increased plasma membrane content of glucose transporters in L6 skeletal muscle cells by the sulfonylureas gliclazide and glyburide. Endocrinology 1995;136:2505-12.
- Kassem SA, Raz I. Is there evidence that oral hypoglycaemic agents reduce cardiovascular morbidity or mortality? No. Diabetes Care 2009;32:S337-41.
- Sokolovska J, Isajevs S, Sugoka O, Sharipova J, Paramonova N, Isajeva D, et al. Comparison of the effects of glibenclamide on metabolic parameters, GLUT1 expression, and liver injury in rats with severe and mild streptozotocin-induced diabetes mellitus. Medicina (Kaunas) 2012;48:532-43.
- Sharma N, Kar A. Combined effects Gymnema sylvestre and glibenclamide on alloxan induced diabetic mice. Int J Appl Pharm 2014;6:11-4.
- Naidoo P, Rambiritch V, Butkow N, Saman S. Optimal utilisation of sulphonylureas in resource constrained settings. Cardiovasc J Afr 2014;25:83-5.
- Rendell M. The role of sulfonylureas in the management of type 2 diabetes. Drugs 2004;64:1339-58.
- Nazaroglu NK, Dincel AS, Altan N. The effects of sulfonylurea glyburide on superoxide dismutase, catalase and glutathione peroxidase activities in the brain tissue of streptozotocin-induced diabetic rat. J Diabetes Complications 2009;23:209-13.
- Kim CH, Park SH, Sim YB, Kim SS, Kim SJ, Lim SM, et al. Effect of tolbutamide, glyburide and glipizide administered supraspinally on CA3 hippocampal neuronal cell death and hyperglycemia induced by kainic acid in mice. Brain Res2014;1564:33-40.
- Olsson J, Lindberg G, Gottsater M, Lindwall K, Sjöstrand A, Tisell A, et al. Increased mortality in type II diabetic patients using sulphonylurea and metformin in combination: A population-based observational study. Diabetologia 2000;43:558-60.
- Simpson SH, Majumdar SR, Tsuyuki RT, Eurich DT, Johnson JA. Dose-response relation between sulfonylurea drugs and mortality in type 2 diabetes mellitus: A population-based cohort study. CMAJ 2006;174:169-74.
- Radhika B, Rahul B, Mahendra KS, Vipin S. Therapeutic approaches for the treatment of diabetes mellitus. Int J Pharm 2012;2:794-800.
- Sola D, Rossi L, Schianca GPC, Maffioli P, Bigliocca M, Mella R, Corlianò F, Fra GP, Bartoli E, Derosa G. Sulfonylureas and their use in clinical practice. Arch Med Sci 2015;11:840-8.
- Abdelmoneim AS, Hasenbank SE, Seubert JM, Brocks DR, Light PE, Simpson SH. Variations in tissue selectivity amongst insulin secretagogues: a systematic review. Diabetes Obes Metab 2012;14:130-8.
- Nagashima K, Takahashi A, Ikeda H, Hamasaki A, Kuwamura N, Yamada Y, et al. Sulfonylurea and non-sulfonylurea hypoglycemic agents: Pharmachological properties and tissue selectivity. Diabetes Res Clin Pract 2004;66S:S75-S8.
- Roscoe MH. The estimation of creatinine in serum. J Clin Pathol 1953;6:201-7.
- Bonsnes R, Taussky H. On the colorimetric determination of creatinine by the Jaffe reaction. J Biol Chem 1945;158:581-91.
- Kumar V, Lemos M, Sharma M, Shriram V. Antioxidant and DNA damage protecting activities of Eulophia nuda Lindl. Free Radic Antioxidants 2013;3:55-60.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75.
- Elmali E, Altan N, Bukan N. Effect of the sulphonylurea glibenclamide on liver and kidney antioxidant enzymes in streptozotocin-induced diabetic rats. Drug R D 2004;5:203-8.
- Gandhi GR, Sasikumar P. Antidiabetic effect of Merremia emarginata Burm. F. in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 2012;2:281-86.
- Waer HF, Helmy SA. Cytological and histochemical studies in rat liver and pancreas during progression of streptozotocin-induced diabetes and possible protection of certain natural antioxidants. J Nutr Food Sci 2012;2:01-07.
- Candasamy M, Murthy TG, Gubiyappa KS, Chellappan DK, Gupta G. Alteration of glucose lowering effect of glibenclamide on single and multiple treatments with fenofibrate in experimental rats and rabbit models. J Basic Clin Pharm 2014;5:62-7.
- Sarkar A, Tiwari A, Bhasin PS, Mitra M. Pharmacological and Pharmaceutical Profile of Gliclazide: A review. J App Pharm Sci 2011;01:11-9.
- Li Y, Wei Y, Zhang F, Wang D, Wu X. Changes in the pharmacokinetics of glibenclamide in rats with streptozotocin-induced diabetes mellitus. Acta Pharm Sin B 2012;2:198-204.
- http://apps.who.int/iris/bitstream/10665/44771/1/WHO_TRS_965_eng.pdf.
- Avizeh R, Najafzadeh H, Pourmahdi M, Mirzaee M. Effect of glibenclamide and fruit extract of Zizyphus spina-christi on alloxan-induced diabetic dogs. Int J Appl Res Vet Med 2010;8:109-13.
- Tosi F, Muggeo M, Brun E, Spiazzi G, Perobelli L, Zanolin E, et al. Combination treatment with metformin and glibenclamide versus single-drug therapies in type 2 diabetes mellitus: A randomized, double-blind, comparative study. Metabolism 2003;52:862-7.
- González-Ortiz M, Guerrero-Romero JF, Violante-Ortiz R, Wacher-Rodarte N, Martínez-Abundis E, Aguilar-Salinas C, et al. Efficacy of glimepiride/metformin combination versus glibenclamide/metformin in patients with uncontrolled type 2 diabetes mellitus. J Diabetes Complications 2009;23:376-9.
- Derosa G, Sibilla S. Optimizing combination treatment in the management of type 2 diabetes. Vasc Health Risk Manag 2007;3:665-71.
- Betonico CC, Titan SM, Correa-Giannella ML, Nery M, Queiroz M. Management of diabetes mellitus in individuals with chronic kidney disease: Therapeutic perspectives and glycemic control. Clinics (Sao Paulo) 2016;71:47-53.
- Akbar DH, Hagras MM, Amin HA, Khorshid OA. Comparison between the effect of glibenclamide and captopril on experimentally induced diabetic nephropathy in rats. J Renin Angiotensin Aldosterone Syst 2012;14:103-15.
- Yale JF. Oral antihyperglycaemic agents and renal disease: New agents, new concepts. J Am Soc Nephrol 2005; 16:S7-10.
- Enogieru AB, Momodu OI, Omoruyi SI, Om'iniabohs FAE. Changes in biochemical markers of kidney function and antioxidant status of diabetic rats treated with aqueous leaf extracts of Ficus exasperata (Vahl). Afr J Biomed Res 2015;18:61-67.
- Gayathri M, Kannabiran K. 2-Hydroxy 4-methoxy benzoic acid isolated from roots of Hemidesmus indicus ameliorates liver, kidney and pancreas injury due to streptozotocin-induced diabetes in rats. Indian J Exp Biol 2010;48:159-64.
- Patel SS, Shah RS, Goyal RK. Antihyperglycaemic, anti-hyperlipidemic and antioxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin- induced diabetic rats. Indian J Exp Biol 2009;47:564-70.
- Sreekutty MS, Mini S. Ensete superbum ameliorates renal dysfunction in experimental diabetes mellitus. Iran J Basic Med Sci 2016;19:111‐18.
- Enechi OC, Oluka IH, Ugwu OPC. Acute toxicity, lipid peroxidation and ameliorative properties of Alstonia boonei ethanol leaf extract on the kidney markers of alloxan-induced diabetic rats. Afr J Biotechnol 2014;13(5):678-82.
- Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj MHG, Ouhtit A. In vivo evidence of hepato and reno-protective effects of garlic oil against sodium nitrite-induced oxidative stress. Int J Biol Sci 2009;5:249-55.
- Bonnefont D-Rousselot, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab 2000;26:163-76.
- Turk HM, Sevine A, Camci C, Cigli A, Buyukberber S, Savli H, Bayraktar N. Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetol 2002;39:117-22.
- Erejuwa OO, Sulaiman SA, Wahab MSA, Sirajudeen KNS, Md Salleh MS, Gurtu S. Antioxidant protective effect of glibenclamide and metformin in combination with honey in pancreas of streptozotocin-induced diabetic rats. Int J Mol Sci 2010;11:2056-66.
- Nagarchi K, Ahmed S, Sabus A, Saheb SH. Effect of streptozotocin on glucose levels in albino Wister rats. J Pharm Sci Res 2015;7:67-9.
- King AJF. The use of animal models in diabetes research. Br J Pharmacol 2012;166:877-94.
- Pryor WA, Godber SS. Non-invasive measures of oxidative stress status in humans. Free Radic Biol Med 1991;10:177-84.
- Ramana KV, Srivastava S, Singhal SS. Lipid peroxidation products in human health and disease 2014. Oxid Med Cell Longev 2014;2014:01-03.
- Saravanan G, Ponmurugan P. Ameliorative potential of S-allyl cysteine on oxidative stress in STZ-induced diabetic rats. Chem Biol Interact 2011;189:100-6.
- Padalkar RK, Shinde AV, Patil SM. Lipid profile, serum malondialdehyde, superoxide dismutase in chronic kidney diseases and type 2 diabetes mellitus. Biomed Res 2012;23:207-10.
- Rukmini MS, Benedicta D’Souza, Vivian D’Souza. Superoxide dismutase and catalase activities and their correlation with malondialdehyde in schizophrenic patients. Indian J Clin Biochem 2004;19:114-18.
- Sivajothi V, Dey A, Jayakar B, Rajkapoor B. Antihyperglycaemic, antihyperlipidemic and antioxidant effect of Phyllanthus rheedii on streptozotocin-induced diabetic rats. Iran J Pharm Res 2008;7:53-59.
- Pandarekandy ST, Kannamparathazhathethil SG, Abdulrahiman R, Shahal A, Subrahmanyan SP, Edakkot S. A prospective study to evaluate daily moderate consumption of ethanol on oxidative stress markers of diabetes induced WistarStrain albino rats. Br J Pharm Res 2015;8:1-11.
- Sellamuthu PS, Arulselvan P, Kamalraj S, Fakurazi S, Kandasamy M. Protective nature of mangiferin on oxidative stress and antioxidant status in tissues of streptozotocin-induced diabetic Rats. ISRN Pharmacol 2013;2013:1-11.
- Erejuwa OO, Sulaiman SA, Wahab MS, Sirajudeen KNS, Salleh MS, Gurtu S. Effect of glibenclamide alone versus glibenclamide and honey on oxidative stress in pancreas of streptozotocin-induced diabetic rats. Int J Appl Res Nat Prod 2011;4:1-10.
- Abdulkadir AAA, Thanoon IAJ. Comparative effects of glibenclamide and metformin on C-reactive protein and oxiant/antioxidant status in patients with type II diabetes mellitus. SQU Med J 2012;12:55-61.
- Sarukhan M, Yazdi HH, Piri H, Rastgoo N. Synergistic antiparkinsonian effect of flunarizine, glibenclamide and B vitamins in a Rate 6-Hydroxydopamine Model; The Role of malondialdehyde. Biotech Health Sci 2014;01:07.
- Erejuwa OO, Sulaiman SA, Ab Wahab MS, Salam SKN, Md Salleh MS, Gurtu S. Comparison of antioxidant effects of honey, glibenclamide, metformin and their combinations in the kidneys of streptozotocin-induced diabetic rats. Int J Mol Sci 2011;12:829-43.