- *Corresponding Author:
- G. A. Kurian
Vascular Biology Lab, SASTRA Deemed University, Thanjavur-613 401, India
E-mail: ginokurian@hotmail.com
| Date of Submission | 14 September 2018 |
| Date of Revision | 29 November 2018 |
| Date of Acceptance | 26 March 2019 |
| Indian J Pharm Sci 2019;81(3):456-463 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Several studies reported the carcinogenic and anticarcinogenic effects of hydrogen sulfide. The present study evaluated the role of mitochondria in mediating the anti/pro-carcinogenic effect of hydrogen sulfide on colon cancer cells as mitochondrial KATP channel and mitochondrial electron transport chain are one of the promising targets for cancer treatment. The colon adenoma cell line and normal small intestinal epithelial cell lines were used to study the antiproliferative effect of hydrogen sulfide in the presence of enzyme inhibitors, mitochondrial KATP channel modulators and in presence of inhibitors of endogenous H2S metabolizing enzymes namely cystathionine-β-synthase and cystathionine-γ-lyase. Antiproliferative effect of hydrogen sulfide was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, crystal violet, sulforhodamine B and lactate dehydrogenase assays with its donor sodium hydrogen sulfide in both HT-29 and IEC-6 cells, where only IEC-6 cells showed significant cytotoxic effect at a concentration of 49.88 µg (IC50) but HT-29 failed to exhibit cytotoxicity with the same H2S concentration. In order to identify the mitochondrial role, several electron transporting chain inhibitors and KATP channel modulators were used, but still H2S could able to enhance the colon adenoma cell line growth indicating mitochondrial in-dependency in the pro-carcinogenic effect. However, anticarcinogenic effect of hydrogen sulfide was observed only when the cells were incubated in the presence of cystathionine-β-synthase and cystathionine-γ-lyase inhibitor, indicating their influential role in determining the exogenous hydrogen sulfide toxicity in colon adenoma cell line cells.
Keywords
Hydrogen sulphide, mitochondria, cystathionine-β-synthase, HT-29, IEC-6
Colorectal cancer is a heterogeneous group of disease, and is one of the leading causes of cancer related deaths in Western countries [1]. A series of genetic changes that controls specific signalling pathway involving the inactivation of tumor suppressor gene and DNA repair gene along with the activation of oncogene is responsible for the pathophysiology [2]. Survival rate statistics in developing countries showed that early detection stage of the cancer can reduce mortality rate, where the disease is highly curable, with five-year survival rates of about 90 % [3]. However, many people delay seeking medical care in the early stage due to their embarrassment of symptoms related to their bowels. Moreover, the risk increases significantly after age 50 and continues to increase with age.
Although targeted therapies have slowly taken over the conventional chemotherapeutic agents, the latter still has its significance in the therapeutic regimen of colon cancer [4]. Targeted therapy with the use of inhibitors of survival pathways, along with immune cells, differentiation agents, and cytotoxic drugs to block the growth of cancer cell by interacting with specific molecules like Wnt regulators, potassium channels are promising, but limitations like resistance to the therapy due to mutations [5] can deteriorate its efficiency. Hence, recently, therapeutic approach that utilizes targeted therapies in combination with conventional chemotherapeutic agents is found to be promising.
Advances in technology to curb cancer identified mitochondrial KATP channel and electron transport chain (ETC) as one of the promising targets [6]. In that aspect, hydrogen sulphide (H2S) is used because it can modulate ETC and mitochondrial KATP channel effectively at lower concentrations [7]. Also, biological interest in understanding the effect of H2S in the management of cancer pathology is gaining interest primarily due to the increased expression of H2S synthesizing enzyme like cystathionine-β-synthase (CBS) in colon and ovarian tumor mass [8]. Few studies have shown that H2S can promote proliferation and migration of human cancer cells (SW 480) by up regulating SIRT 1 [9], and by inhibiting nuclear factor kappa-B signalling [10]. But many other studies have demonstrated that H2S can induce colorectal cancers [11], bring on hyper proliferation in human colon mucosa [12], modulate cell cycle and cause cancer in rat intestinal epithelial cells. But the continuous exposure of H2S to cancer cell decreased its cell survival, induce genomic DNA damage [13] as compared to the non-cancer cells. Some of the H2S releasing vegetables like garlic [14], onion [15], were also reported to have anticancer property. Thus, all the information from the above studies highlights the discrepancies in the mode of action of H2S on colon cancer cells, which needs to be addressed.
Cancer cell metabolism primarily relies on mitochondria for its function and its dysfunction [16] is considered to be one of the hallmarks of the cancer. In the present study, the therapeutic efficacy of H2S in colon cancer cells, HT-29 was evaluated and compared with normal small intestinal epithelial cells, IEC-6 with a notion that H2S can act as either carcinogen or anticancer agent. Further, the role of mitochondria in the ambiguous nature of H2S on cancer cell was investigated by studying the cytotoxic effect in presence of ETC inhibitor, endogenous H2S metabolic enzyme inhibitor and mitochondrial potassium channel inhibitor and opener.
Materials and Methods
The colorectal cancer cell line, HT-29 and small intestinal epithelial cells, IEC-6, obtained from National Center for Cell Science, Pune, India, were cultured in Dulbecco's modified Eagle medium supplemented with 10 % fetal calf serum, 1 % antibiotics/antimycotic (complete medium) and incubated in a 5 % CO2 incubator at 37°. Sodium hydrosulfide (NaHS), sulforhodamine B (SRB), rotenone, diazoxide, propargyl glycine (PAG) and amino oxy acetic acid were purchased from Sigma-Aldrich (St Louis, MO, USA). Fetal calf sera, and antibiotics/antimycotics were obtained from Invitrogen; Thermo Fisher Scientific, Inc. All other reagents were purchased from HiMedia.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay:
The MTT reduction assay protocol of Kupcsik et al. with a slight modification was used to measure cell viability by determining the level of metabolic activity, where blue formazan was formed from MTT by the action of mitochondrial dehydrogenase [17]. Briefly, the HT-29 cells were grown on a 96 well plate with a seeding density of 8000 cells/well and pretreated with various concentrations of NaHS, a donor of H2S for 24 h after allowing the cells to get attached to plate and having a confluence of 75 % cells. At the end of incubation, MTT (5 mg/ml) was added to each well and the formazan product was dissolved in 100 μl of dimethyl sulfoxide after fixing the cell with paraformaldehyde. The absorbance of each well was measured at 570 nm using multimode reader (Biotek, synergy H1, Mumbai).
Crystal violet (CV) assay:
HT-29 cells were grown in a 96 well plate and pretreated with various concentrations of NaHS at their 75 % confluence. The cells were then washed with phosphate-buffered saline (PBS) and fixed with 4 % paraformaldehyde. The cells were then incubated with 0.5 % CV (Sigma-Aldrich Corp., St. Louis, MO, USA) in 30 % methanol for 10 min at room temperature. Excess dye was removed by washing with tap water. The cells were lysed in a 1 % sodium dodecyl sulphate solution. The absorbance of the solution was measured using Biotek multimode reader at a wavelength of 570 nm [18].
SRB assay:
SRB assay protocol as described by Vichai et al. [19] was followed. Briefly, HT-29 cells were seeded in 96 well plate with 7000 cells/well and once it attains 75 % confluency, the cells were washed with PBS and treated with NaHS of different concentrations from 5 μM to 50 mM for 24 h. At the end of incubation, the medium was removed and washed with PBS. The cells were fixed with 10 % trichloroacetic acid, stained with SRB (1 %), solubilized in 10 mM Tris base and measured for the survival of cells using Biotek multimode reader for fluorescence at excitation of 488 nm and emission of 585 nm.
Lactate dehydrogenase (LDH) assay:
The release of LDH from HT-29/IEC-6 cells was measured using a procedure as described previously [20]. After treating the cells with various concentrations of NaHS in a 96 well plate, the supernatant was used to measure the leaked LDH activity. The reaction was initiated by mixing the supernatant with NADH and sodium pyruvate in phosphate buffer. The change in conversion of NADH to NAD was measured at 340 nm.
Clonogenic assay:
HT-29 cells were plated in 24 well plate at a seeding density of 40 000 cells and once the cells are fixed, they were incubated with various concentrations of NaHS for 24 h. After trypsinizing the cells, they were replated in 6 well plate at a seeding density of 20 000 cells and maintained under normal growth condition for 7 to 8 d at 37° in CO2 incubator to form colonies [21]. The colonies were measured via CV assay as described above.
Caspase 3 activity:
Caspase 3 activity was measured using fluorometric assay protocol as described previously [22]. After incubating the cells with NaHS, the cell lysate was prepared and incubated with 10 μl of fluorogenic peptide substrate Ac-DEVD-AMC 37° in the dark for 2 h. The activation of caspase was measured using Biotek Synergy H1 multimode reader at excitation of 390 nm and emission of 500 nm.
Influence of ETC, KATP channel and endogenous H2S on the growth of cells in the presence of H2S:
To study the dependability of H2S over the mitochondrial cellular bio-energetics, rotenone and azide were used to inhibit ETC complexes I and IV. Also, the change in mitochondria membrane permeability pore transition (MPTP), being the key regulator of apoptosis, the effects of H2S were analysed on mitochondrial potassium ATP channel, that regulate the MPTP, by pre-treating it with a channel opener (diazoxide) and a blocker (glibenclamide). In order to distinguish the effect of exogenous and endogenous H2S, DL-PAG was used as an irreversible inhibitor of cystathionine-γ-lyase (CSE) and aminooxyacetic acid (AOA), a CBS inhibitor prior to the H2S treatment.
Statistical analysis:
All data were presented as the mean±SEM for the three independent experiments. A one-way analysis of variance was performed using the prism statistical package (GraphPad Software, USA). P<0.05 was considered statistically significant.
Results and Discussion
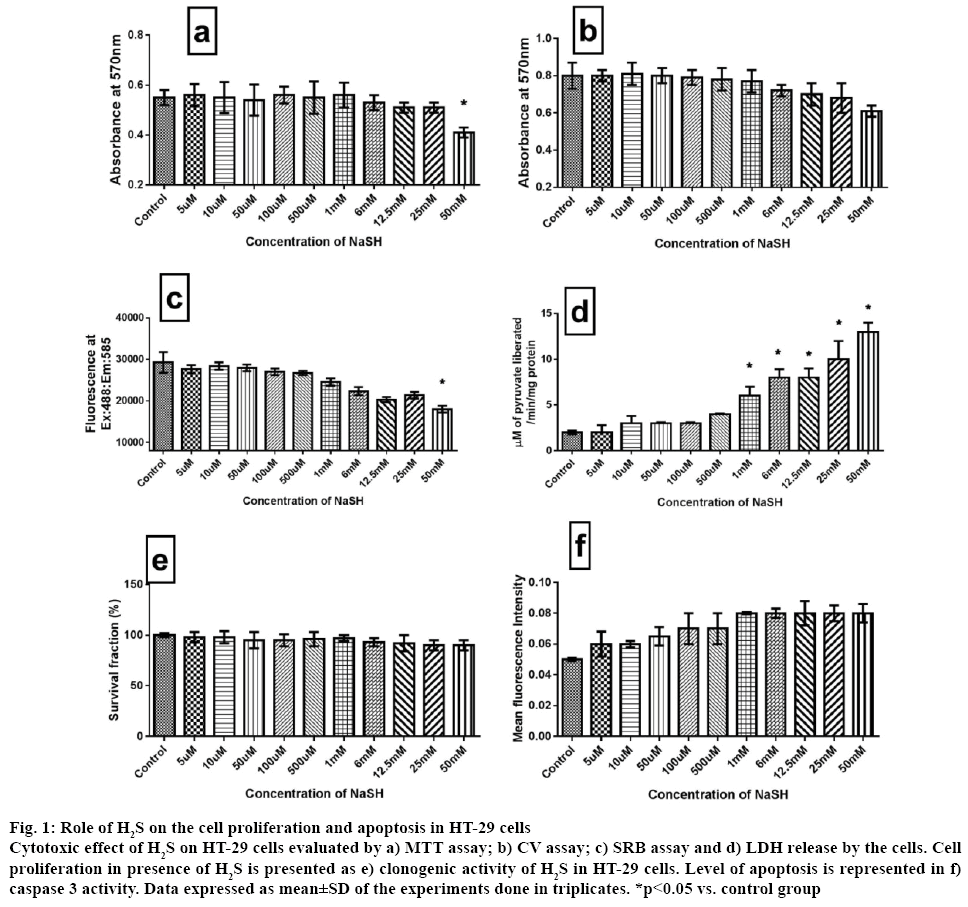
H2S is reported to have both procarcinogenic and anticarcinogenic effect [10]. The procarcinogenic effect was explained through the endogenous H2S action on angiogenesis, cell cycle progression and blockade of apoptosis mechanism [23]. The anticancer effect of H2S was explained as, at high concentration H2S induces cell cycle arrest [24]. Advancements in cancer research identified mitochondrial KATP channel and ETC as one of the promising targets in the cancer treatment. Many studies have shown that cellular physiological H2S can modulate ETC [25] and mitochondrial KATP channel effectively [26]. In the present study, the critical role played by mitochondria in distinguishing the ambiguous impact of H2S on HT-29 cells was addressed. Major findings of the study were, i) unlike in normal colon cells, H2S promoted colon cancer cell proliferation and its action was independent of mitochondria and ii) exogenous H2S-mediated cancer cell growth depended on the level of endogenous H2S. The toxic property of H2S was identified around 300 y ago and recent studies have provided enough evidences for its genotoxicity, mutagenicity and carcinogenicity [13,27]. The effects of different concentration of H2S were examined on the cell viability of HT-29 cells for 24 h by MTT assay, CV assay, SRB assay and LDH activity (figure 1). H2S did not show any inhibition in their growth with different concentrations (5 μM to 50 mM), except 50 mM. All cytotoxicity assays showed similar results suggesting nontoxic nature of H2S in HT-29 colon cancer cells oncogenesis. Furthermore, the clonogenic assay (figure 1) was carried out to assess the cell proliferative potential of H2S and the results suggest no inhibitory action of H2S on HT-29 cell growth. In addition, caspase 3 (an indicator of apoptosis) activity was measured at the end of the experiment and the results suggest relatively no significant increase across the different concentration, supporting the proliferative capacity of H2S (figure 1). Evidence from the literature supported the cell proliferative capacity of H2S in oral cancer cells [28], C6 glioma cells [29] and human colon cancer cell [11]. Results reported by Cai et al. on HCT 116 demonstrated proliferative effects of NaSH up to 1 mM without mentioning its impact on increasing concentrations further [11], which was addressed in the present study.
Figure 1: Role of H2S on the cell proliferation and apoptosis in HT-29 cells
Cytotoxic effect of H2S on HT-29 cells evaluated by a) MTT assay; b) CV assay; c) SRB assay and d) LDH release by the cells. Cell proliferation in presence of H2S is presented as e) clonogenic activity of H2S in HT-29 cells. Level of apoptosis is represented in f) caspase 3 activity. Data expressed as mean±SD of the experiments done in triplicates. *p<0.05 vs. control group
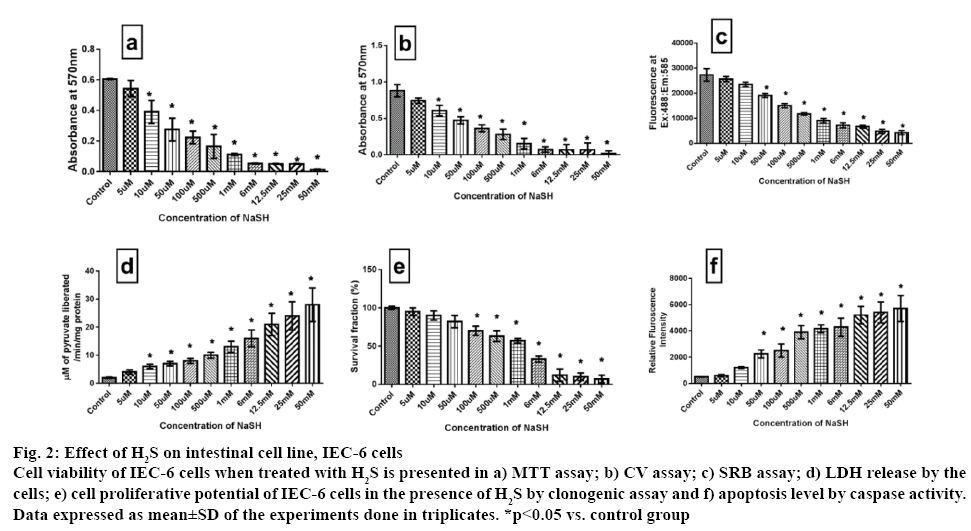
Another cell line, IEC 6 was used to assess the impact of NaSH on the proliferative capacity. Accordingly it was found that H2S (up to 10 μM) did not show any significant inhibitory effect after 24 h exposure (figure 2). But, above those concentrations, H2S inhibited the IEC 6 cell viability in a dose-dependent manner measured via MTT and CV assays. SRB and LDH assay results complemented the above result. Clonogenic assay was used to reconfirm the result and it was found that clonogenic capacity was affected only after 100 μM concentration (figure 2).
Figure 2: Effect of H2S on intestinal cell line, IEC-6 cells
Cell viability of IEC-6 cells when treated with H2S is presented in a) MTT assay; b) CV assay; c) SRB assay; d) LDH release by the cells; e) cell proliferative potential of IEC-6 cells in the presence of H2S by clonogenic assay and f) apoptosis level by caspase activity. Data expressed as mean±SD of the experiments done in triplicates. *p<0.05 vs. control group
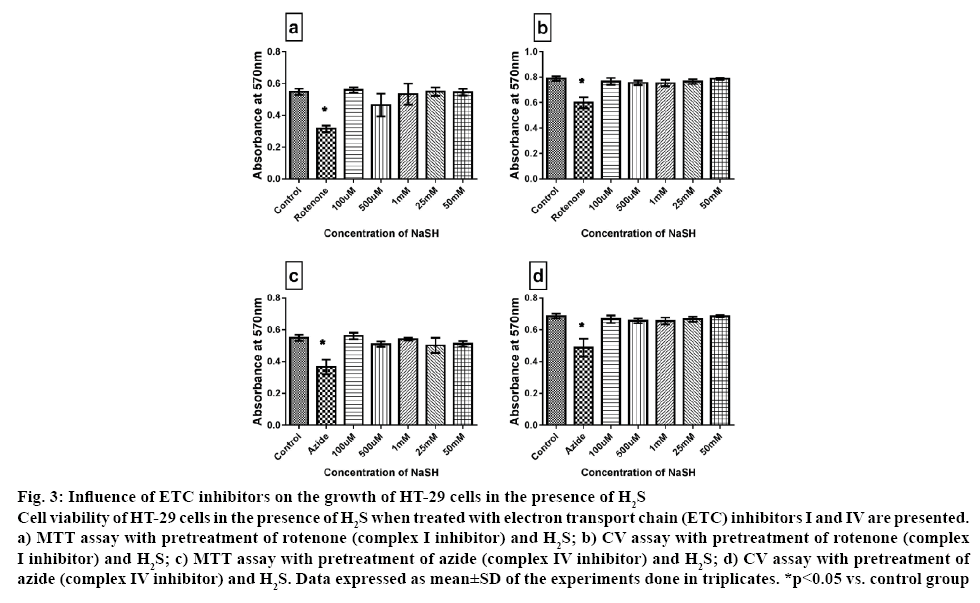
The toxic effect of H2S is well-established and different cell types experienced different toxic level depends on the expression level of its endogenous enzyme like CBS and CSE. Accordingly, it had been generalized that an intracellular concentration ranging between 0.01 to 1 μM is nontoxic; instead it might trigger ATP production via electron supply to complex V. Concentrations 3 to 30 fold higher were considered to be toxic [30]. Hence, in the present investigation whether H2S-mediated cytoprotection of HT-29 cells was dependent on mitochondrial cellular bio-energetics (H2S is reported to modulate mitochondria and control cellular energetics) was studied. It is well-established that the cancer cells have altered respiratory chain complex activities when compared to the normal cell, where the latter is considered to be one of the major sources of reactive oxygen species under pathological conditions. Accordingly, rotenone and azide were used to inhibit ETC complexes I and IV, respectively. The results demonstrated that rotenone and azide had a significant inhibitory effect on cell growth by 40 and 20 %, respectively. According to the early scientific reports, mitochondrial complex I and III are the major source of ROS. Hence by inhibiting complex I and III via rotenone and antimycin, respectively might generate elevated ROS production, as reported by many [31], can kill or reduce the growth of HT-29. However, no similar effects were observed when the cells were incubated with H2S prior to ETC inhibition. The results showed that there was no influence of these ETC enzymes over the cytoprotective effect of H2S on HT-29 cell, measured via MTT and CV assays (figure 3). Based on the above results, it is confirmed that cytoprotective effect of H2S on HT-29 cancer cell was independent of ETC.
Figure 3: Influence of ETC inhibitors on the growth of HT-29 cells in the presence of H2S
Cell viability of HT-29 cells in the presence of H2S when treated with electron transport chain (ETC) inhibitors I and IV are presented. a) MTT assay with pretreatment of rotenone (complex I inhibitor) and H2S; b) CV assay with pretreatment of rotenone (complex I inhibitor) and H2S; c) MTT assay with pretreatment of azide (complex IV inhibitor) and H2S; d) CV assay with pretreatment of azide (complex IV inhibitor) and H2S. Data expressed as mean±SD of the experiments done in triplicates. *p<0.05 vs. control group
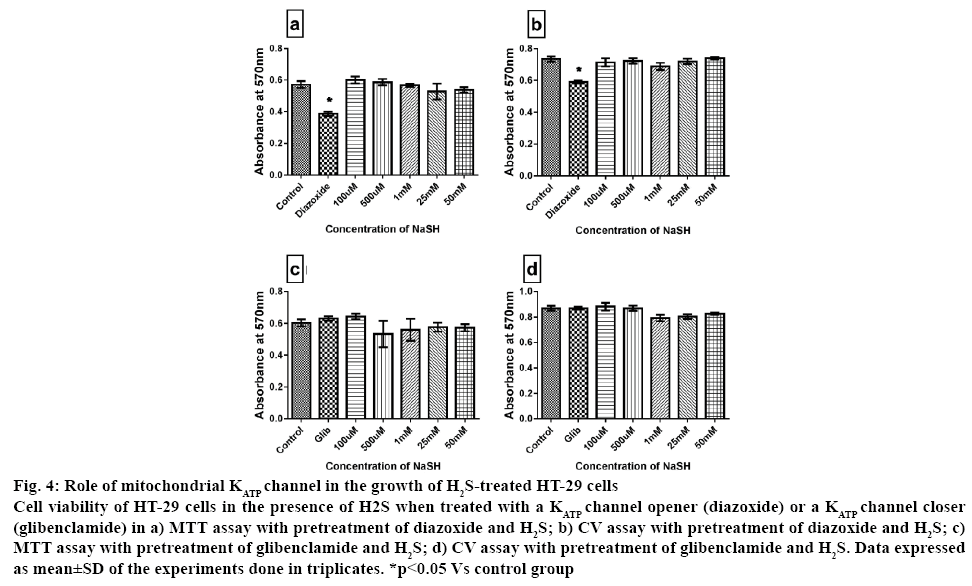
Recent strategy to combat cancer growth included those agents that can specifically compromise the structural and functional integrity of mitochondria, by modulating the respiratory chain, DNA biogenesis, potassium channels, the Bcl-2 protein and the permeability transition pores, provided that these molecules can be selectively delivered to tumor sites [32]. H2S, being metabolized inside mitochondria can influence the MPTP transition that regulate apoptosis via mitochondrial KATP channel. Evading apoptosis is one of the hall marks of cancer cell [33]. The change in mitochondria MPTP is the key regulator of apoptosis and the effects of H2S on mitochondrial potassium ATP channel was analysed, that regulate the MPTP using a channel opener, diazoxide and a channel blocker, glibenclamide. Pre-treatment of cells with diazoxide had an inhibitory effect on cell growth by 40 % whereas glibenclamide had no effect on cell viability as given in figure 4. However, cytotoxicity evaluated in HT-29 in presence of both H2S and mitochondrial KATP channel modulators showed similar insignificant change when compared with the control, indicating that absence of mitochondrial dependency of H2S in promoting the proliferation in HT-29 cells.
Figure 4: Role of mitochondrial KATP channel in the growth of H2S-treated HT-29 cells
Cell viability of HT-29 cells in the presence of H2S when treated with a KATP channel opener (diazoxide) or a KATP channel closer (glibenclamide) in a) MTT assay with pretreatment of diazoxide and H2S; b) CV assay with pretreatment of diazoxide and H2S; c) MTT assay with pretreatment of glibenclamide and H2S; d) CV assay with pretreatment of glibenclamide and H2S. Data expressed as mean±SD of the experiments done in triplicates. *p<0.05 Vs control group
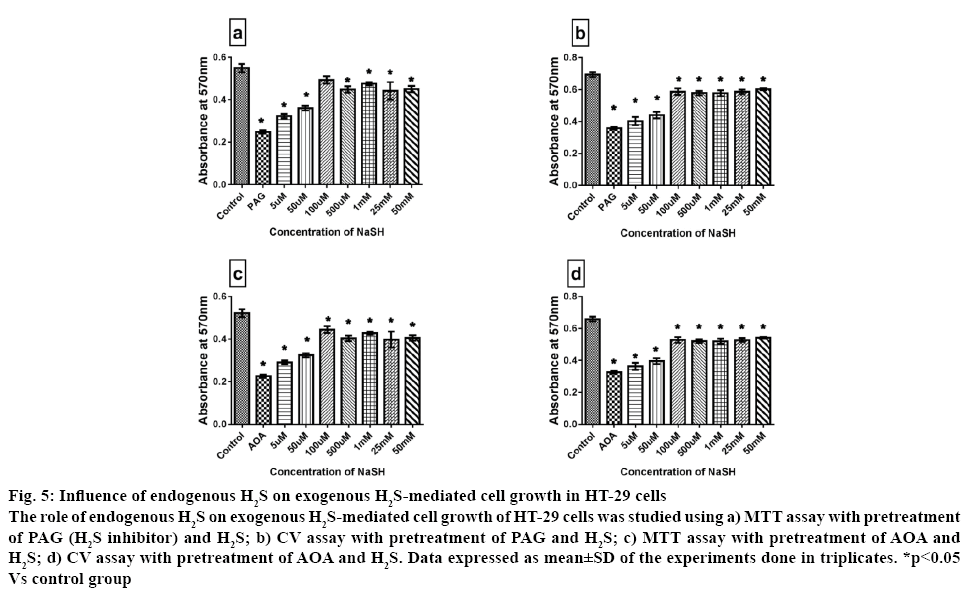
Evidence reported in the literature suggested that H2S can promote cell proliferation via activating COX2/ AKT/ERK1/2 axis [28]. In another study with neuroblast cells (SH-SY5Y), it was reported that H2S could inhibit rotenone-induced cell apoptosis via regulation of mitochondrial KATP channel/p38- and JNK-MAPK pathway [34]. More experimental evidence on the biological effect of H2S suggested that in normal cells, two forms of sulphur store can release H2S that included bound sulfane sulphur (under reducing condition) and acid labile sulphur. Exogenous administration of H2S normally get absorbed into the cell as bound sulfane sulphur and this form can act as the source for sulphur in iron sulphur centre in ETC redox centre [35]. Szabo and associate have shown that CBS derived H2S can stimulate cell proliferation, maintain bioenergetics and promote angiogenesis [12]. The endogenous gasotransmitter H2S is generated from L cysteine via the action of CBS and CSE [36]. In order to distinguish the effect of exogenous and endogenous H2S, PAG was used as an irreversible inhibitor of CSE and AOA, a CBS inhibitor. Administration of the inhibitors induced 60 % reduction in cell viability as shown in figure 5, indicating the impaired utilization of H2S as fuel and the importance of endogenous H2S in the cell survival. However, when the cells were pretreated with higher concentration of exogenous H2S (above 100 μM) for 24 h along with the inhibitors, the cell viability was significantly improved by 45 %, when compared with PAG/AOA control group. In fact, the low dose of H2S administration along with PAG/AOA did not improve the cell viability. This emphasizes the prominent role played by endogenous H2S in colon cancer cell growth or the endogenous H2S enzyme regulation by exogenous H2S administration. In this line of thought, several researchers suggest that PAG/AOA treatment to cancer cell can reduce basal cellular respiration thereby reduce ATP synthesis and attenuated the spare respiratory capacity. It is worth mentioning that H2S mediated two opposing effects on mitochondria, i) H2S suppressed mitochondrial function in mammalian tissue by inhibiting cytochrome c oxidase and ii) H2S acted as an inorganic substrate and electron donor for electron transport system that generate ATP. Thus the variations in H2S function depended on the metabolic status of the cell. Accordingly, high cell death with H2S in presence of PAG/AOA is most likely related to the impaired utilization of H2S as fuel or impaired transporter (malate aspartate shuttle)/transaminase reaction via pyridoxal dependent enzyme by AOA.
Figure 5: Influence of endogenous H2S on exogenous H2S-mediated cell growth in HT-29 cells
The role of endogenous H2S on exogenous H2S-mediated cell growth of HT-29 cells was studied using a) MTT assay with pretreatment of PAG (H2S inhibitor) and H2S; b) CV assay with pretreatment of PAG and H2S; c) MTT assay with pretreatment of AOA and H2S; d) CV assay with pretreatment of AOA and H2S. Data expressed as mean±SD of the experiments done in triplicates. *p<0.05 Vs control group
Based on the above observations it could be concluded that H2S is toxic to normal colon epithelial cells above 10 μM but could be well utilized by colon cancer cell even with 50 mM. The ability of HT-29 to utilize H2S depends on the level of endogenous H2S and independent to electron flux in ETC and change in mitochondrial KATP channel.
Acknowledgements
Authors wish to thank Ms. Shakila Banu for her assistance during the cell culture experiments and Ms. Sri Rahavi Boovarahan for her assistance in manuscript preparation.
Conflict of interest
The authors declare no conflict of interest.
Financial assistance
This study was supported by grants from the SASTRA University; Research and Modernization fund provided to the Principal Investigator Dr. Gino A. Kurian.
References
- Heitman SJ, Ronksley PE, Hilsden RJ, Manns BJ, Rostom A, Hemmelgarn BR. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2009;7(12):1272-8.
- Ziapour P, Ataee R, Shadifar M, Vaillancourt C, Ahmadi A, Jafari-Sabet M, et al. New intracellular and molecular aspects in pathophysiology of colorectal cancer. Gastroenterol Hepatol Bed Bench 2011;4(2):43-52.
- Dulai PS, Sandborn WJ, Gupta S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management. Cancer Prev Res 2016;9(12):887-94.
- Valentini AM, Armentano R, Pirrelli M, Caruso ML. Chemotherapeutic agents for colorectal cancer with a defective mismatch repair system: the state of the art. Cancer Treat Rev 2006;32(8):607-18.
- Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology 2010;138(6):2151-62.
- Leanza L, Zoratti M, Gulbins E, Szabo I. Mitochondrial ion channels as oncological targets. Oncogene 2014;33(49):5569-81.
- Ravindran S, Ansari Banu S, Kurian GA. Hydrogen sulfide preconditioning shows differential protection towards interfibrillar and subsarcolemmal mitochondria from isolated rat heart subjected to revascularization injury. Cardiovasc Pathol 2016;25(4):306-15.
- Bhattacharyya S, Saha S, Giri K, Lanza IR, Nair KS, Jennings NB, et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One 2013;8(11):e79167.
- Hong M, Tang X, He K. Effect of hydrogen sulfide on human colon cancer SW480 cell proliferation and migration in vitro. Nan Fang Yi Ke Da Xue Xue Bao 2014;34(5):699-703.
- Kodela R, Nath N, Chattopadhyay M, Nesbitt DE, Velazquez-Martinez CA, Kashfi K. Hydrogen sulfide-releasing naproxen suppresses colon cancer cell growth and inhibits NF-kappaB signaling. Drug Des Devel Ther 2015;9:4873-82.
- Cai WJ, Wang MJ, Ju LH, Wang C, Zhu YC. Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol Int 2010;34(6):565-72.
- Szabo C, Coletta C, Chao C, Modis K, Szczesny B, Papapetropoulos A, et al. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci U S A 2013;110(30):12474-9.
- Attene-Ramos MS, Wagner ED, Gaskins HR, Plewa MJ. Hydrogen sulfide induces direct radical-associated DNA damage. Mol Cancer Res 2007;5(5):455-9.
- Tsubura A, Lai YC, Kuwata M, Uehara N, Yoshizawa K. Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anticancer Agents Med Chem 2011;11(3):249-53.
- Zhao Y, Fan D, Zheng ZP, Li ET, Chen F, Cheng KW, et al. 8-C-(E-phenylethenyl)quercetin from onion/beef soup induces autophagic cell death in colon cancer cells through ERK activation. Mol Nutr Food Res 2017;61(2):1-10.
- Shapovalov Y, Hoffman D, Zuch D, de Mesy Bentley KL, Eliseev RA. Mitochondrial dysfunction in cancer cells due to aberrant mitochondrial replication. J Biol Chem 2011;286(25):22331-8.
- Kupcsik L. Estimation of cell number based on metabolic activity: the MTT reduction assay. Methods Mol Biol 2011;740:13-9.
- Feoktistova M, Geserick P, Leverkus M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb Protoc 2016;2016(4):pdb.prot087379.
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 2006;1(3):1112-6.
- Chan FK, Moriwaki K, De Rosa MJ. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol Biol 2013;979:65-70.
- Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc 2006;1(5):2315-9.
- Gorman AM, Hirt UA, Zhivotovsky B, Orrenius S, Ceccatelli S. Application of a fluorometric assay to detect caspase activity in thymus tissue undergoing apoptosis in vivo. J Immunol Methods 1999;226(1-2):43-8.
- Wu D, Si W, Wang M, Lv S, Ji A, Li Y. Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide 2015;50:38-45.
- Takeuchi H, Setoguchi T, Machigashira M, Kanbara K, Izumi Y. Hydrogen sulfide inhibits cell proliferation and induces cell cycle arrest via an elevated p21 Cip1 level in Ca9-22 cells. J Periodontal Res 2008;43(1):90-5.
- Szabo C, Ransy C, Modis K, Andriamihaja M, Murghes B, Coletta C, et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol 2014;171(8):2099-122.
- Gade AR, Kang M, Akbarali HI. Hydrogen sulfide as an allosteric modulator of ATP-sensitive potassium channels in colonic inflammation. Mol Pharmacol 2013;83(1):294-306.
- Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskins HR. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res 2006;4(1):9-14.
- Zhang S, Bian H, Li X, Wu H, Bi Q, Yan Y, et al. Hydrogen sulfide promotes cell proliferation of oral cancer through activation of the COX2/AKT/ERK1/2 axis. Oncol Rep 2016;35(5):2825-32.
- Zhen Y, Zhang W, Liu C, He J, Lu Y, Guo R, et al. Exogenous hydrogen sulfide promotes C6 glioma cell growth through activation of the p38 MAPK/ERK1/2-COX-2 pathways. Oncol Rep 2015;34(5):2413-22.
- Jiang J, Chan A, Ali S, Saha A, Haushalter KJ, Lam W-LM, et al. Hydrogen Sulfide--Mechanisms of Toxicity and Development of an Antidote. Sci Rep 2016;6:20831-31.
- Wang L, Duan Q, Wang T, Ahmed M, Zhang N, Li Y, et al. Mitochondrial Respiratory Chain Inhibitors Involved in ROS Production Induced by Acute High Concentrations of Iodide and the Effects of SOD as a Protective Factor. Oxid Med Cell Longev 2015;2015:217670.
- Chhabra A. Mitochondria-centric activation induced cell death of cytolytic T lymphocytes and its implications for cancer immunotherapy. Vaccine 2010;28(29):4566-72.
- Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol 2013;23(12):620-33.
- Hu LF, Lu M, Wu ZY, Wong PT, Bian JS. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol 2009;75(1):27-34.
- Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide 2013;35:5-20.
- Vandiver MS, Snyder SH. Hydrogen sulfide: a gasotransmitter of clinical relevance. J Mol Med 2012;90(3):255-63.