- *Corresponding Author:
- C. K. Zhu Department of Gynecologic Oncology, Women’s Reproductive Health Laboratory of Zhejiang Province, Women’s Hospital,Zhejiang University School of Medicine, Hangzhou 310006, People’s Republic of China E-mail: zhuchangkun@ zju.edu.cn
| This article was originally published in a special issue, “Therapeutic Perspectives in Biomedical Research and Pharmaceutical Sciences and their Nursing Methods” |
| Indian J Pharm Sci 2021:83(4)Spl issue “27-33” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Here, the carcinogenicity between the wild type and mutant human papillomavirus 58 E7 in cervical cancer was compared both in vitro and in vivo. Keratinocyte cell line HaCaT and human papillomavirus negative cervical cancer cell line C33A cells stably expressing wild type human papillomavirus 58 E7 (WT) or human papillomavirus 58 E7 variant (T20I/G63S, MT) were constructed by using lentivirus system. Through a series of phenotypic tests, such as wound healing assay, Transwell experiment, 3-(4,5-dimethyl- 2-thia-zolyl)-2,5-diphenyl-2-H-tetrazolium bromide method, flow cytometry, colony formation assay, we initially explored the carcinogenicity differences of human papillomavirus 58 E7 variants in vitro. The role of two types of human papillomavirus 58 E7 on cervical cancer cell growth in vivo was examined using nude mouse xenograft model and the terminal deoxynucleotidyl transferase dUTP nick end labeling staining was performed to measure the cell apoptosis in tumors. Our results showed that both keratinocyte cell line HaCaT and human papillomavirus negative cervical cancer cell line C33A cells with overexpressed variant human papillomavirus 58 E7 (T20I/G63S, MT) presented stronger migration and invasion potential and stronger proliferation and colony formation activity and much low cell apoptosis rates compared with the corresponding wild type group cells. The results from animal experiment revealed that xenografts from human papillomavirus negative cervical cancer cell line C33A cells overexpressed variant human papillomavirus 58 E7 (T20I/G63S, MT) grow much quickly and less cells were under apoptosis. We identified the human papillomavirus 58 E7 (T20I/G63S, MT) variants which possessed higher carcinogenicity on cervix than the wild type one.
Keywords
Cervical cancer, human papillomavirus, human papillomavirus 58 E7, T20I/G63S variant, oncogenicity
Persistent infection by certain types of human papillomaviruses (HPVs) is recognized as a necessary cause for cervical cancer, which is the fourth most common cancer in women worldwide and one of the leading causes of death among women in developing countries [1]. HPV16 and 18 are the most common cancer-associated types, accounting for about 70 % of all cervical cancers worldwide and the proportion is quite consistent throughout the world, whereas the proportion for human papillomavirus 58 (HPV58) infections is for 0-3 % [2]. However, accumulated studies have shown that HPV58 are relatively more frequent among HPV-positive Chinese and other Asian women and there is 11.5-28 % of prevalence across the full spectrum of cervical neoplasia in these women and it plays a special role in East Asia [3-9].
Chan et al. meta-analysis has demonstrated that the attribution of HPV58 to cervical cancer was 3.7‐ fold higher in East Asia than elsewhere, even after adjustment for bystander effect in multiple‐type infections. This meta-analysis also verified that the attribution rate of HPV58 was ranged from 6.8 % to 9.9 % among cervical cancer and cervical intraepithelial neoplasia (CIN) in Chinese and other Asian women [10]. Our previous epidemiologic study has demonstrated that the infection rate of HPV58 is higher than that of HPV18 (19.1 % vs. 5.4 %) among CIN and the same with that of HPV18 (9.4 %) among cervical cancer in women of Zhejiang province, East China [11]. However, the reason for such ethnic or geographical specificity in the disease burden associated with HPV58 remains unknown.
In addition to host genetic background, oncogenic risk of HPV variant may play an indispensable role. Our previous study has demonstrated that the presence of C632T (T20I)/G760A (G63S) variants in E7 showed a positive association with the severity of neoplasia (ptrend<0.05, χ2 test for trend) [12]. An epidemiological study by Chan et al. on 1924 Hong Kong women identified that HPV58E7 T20I/G63S variant conferred an odds ratio of 26.8 for CIN III or Squamous Cell Carcinoma (SCC) [13].
Another study by Law et al. has successfully identified the HPV58E7 (T20I/G63S) variant as a highest carcinogenic one than prototype and other HPV58 variants. But for phenotype assay in this study, only soft agar colony formation assay using transient ectopic expression cell lines was performed. More cellular phenotype experiments using stably transfected cells both in vitro and in vivo is more convincing [14,15]. Therefore, this study aimed to investigate the oncogenic properties of HPV58E7 prototype and the HPV58E7 T20I/G63S variant, using a series of phenotypic and molecular assays on keratinocyte cell line (HaCaT) and HPV negative cervical cancer cell line (C33A) with ectopic stable expression of different types of HPV58E7. Our findings can further help to improve HPV surveillance and therapeutic design in East Asia women, especially in Chinese.
Materials and Methods
Cell culture and stably transfected cell line construction:
HaCaT and C33A cells were purchased from the American Type Culture Collection (ATCC Manassas, VA, USA) and maintained in Dulbecco’s modified eagle medium (DMEM), supplemented with 10 % Fetal bovine serum (FBS) in a 37° humidified incubator containing 5 % CO2. The logarithmic growth phase cells were used for follow-up experiments.
HPV58E7 prototype and variant were obtained by gene synthesis (Suzhou AiKangDe biological technology co., LTD) and sub cloned into the Lenti-EF1a-CMV-EGFP-puro stable expression vector by digestion of the restriction endonuclease enzyme EcoRI and Bacillus amyloliquefaciens (Bam HI). HaCaT and C33A cells were individually infected by lentivirus and selected by puromycin incubation and the expression of HPV58E7 was determined using western blot assay.
Transwell assay for cell migration and invasion:
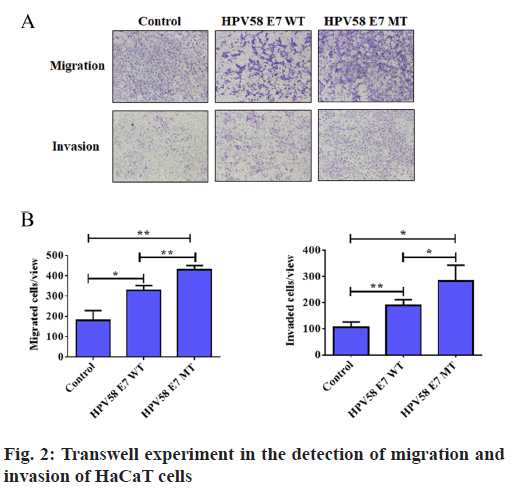
Transwell experiments were performed to detect the cell migration and invasion potential. Briefly, transwell chambers (8 μm pore, Corning Life Science) with or without matrigel (BD Biosciences) were used to investigate invasion and migration respectively. 3×104 cells in 100 μl serum-free medium were placed into the chambers without matrigel for migration assay or with matrigel for invasion assay. After that, these chambers were then put in 24-well plate wells filled with 600 μl of medium containing 20 % FBS as an attractant. After 24 h incubation, the transwells were then fixed with iced methanol for 30 min and stained with 0.1 % crystal violet for 30 min at room temperature. Then, the residual cells in the upper sides of membrane were removed using a swab and the migrated or invaded cells on the underside of the membrane were counted in three independent areas under microscope.
Wound healing assay for cell migration:
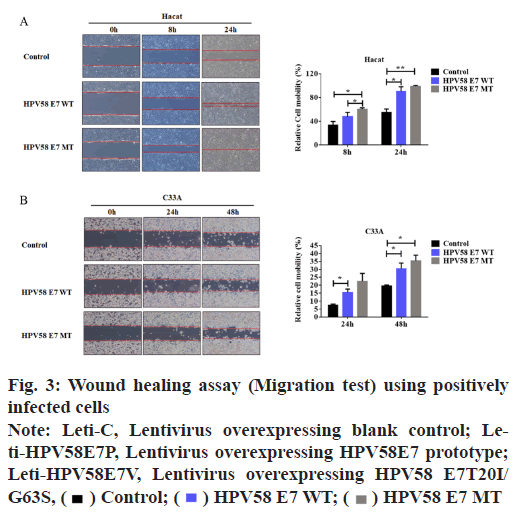
A wound healing experiment was carried out to determine the cell migration potential. Briefly, logarithmic growth phase cells were seeded in six well plate and allowed to grow to 100 % confluence and a wound for each well were obtained with a 200 μl sterile tip (time 0 h) and then the wound healing process was monitored. The healing process was observed by microscopy and shot using Leica Application Suite measuring the “healing area” at different time. Recorded the scratch distance and calculate the relative migration rate.
Colony formation assay:
The colony formation assay was performed to detect the cell colony grow potential. Briefly, 800 cells were seeded in the 6-well plate and allowed to incubate for about 2 w. After that, cells were washed with phosphate-buffered saline (PBS) buffer and the colonies were fixed with methanol and stained with crystal violet solution both for 30 min at room temperature and the number of colonies (>50 cells) was counted.
Fluorescence-activated cell sorting (FACS) assay for cell apoptosis:
Cell apoptosis analysis was carried out by flow cytometry (Fluorescence-activated cell sorting (FACS)). Cells (1×106 per well) were harvested and washed twice with PBS buffer. Then, cells were stained with fluorescein isothiocyanate (FITC) labeled Annexin V and propidium iodide (PI) and analyzed by flow cytometry (FASCanto, Becton–Dickinson). Data acquisition and analysis were performed using software MinWID 2.9 (Becton–Dickinson). The percentage of apoptotic cell death was defined as the sum of early (Annexin V-positive and PI-negative cells) and later (Annexin-V positive and PI-positive cells) apoptosis. Quantification of fluorescence was analyzed by flow cytometry (BD Company).
Cell proliferation assay in vitro:
Cell proliferative activity was determined using the 3-(4,5-dimethyl-2-thia-zolyl)-2,5-diphenyl-2-Htetrazolium bromide (MTT) method (Sigma). HaCaT and C33A cells, stably transfected with HPV58E7 (T20I)/(G63S), HPV58E7 or negative control were seeded in 96-well plates at a density of 8×103 cells per well in 100 μl of growth medium and cultured for 24 h respectively. Subsequently, 50 μl of MTT solution (1 mg/ml) was added to each well. After 3 h incubation at 37°, the cells were disrupted in 150 μl of dimethyl sulfoxide (DMSO, Sigma-Aldrich) and the absorbance was measured at 570 nm on a microplate reader (Olympus).
Subcutaneous nude mouse xenografts:
4 w old nude female mice were purchased from Shanghai Slake Laboratory Animal Co. Ltd (Shanghai, China). The mice were maintained in the feeding environment with a temperature controlled at 18°-26°; and a relative humidity at 40 %-70 % and were fed with sterilized food and autoclaved water, according to the experimental animal guidelines. All animal procedures were approved by the Committee on Animal Experimentation of Zhejiang University (Hangzhou, China) and the procedures complied with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Eighteen mice were randomly divided into three groups and one group contains eight mice. Under sterile conditions, a total of 0.2 ml (2.5×107 cells/ ml) C33A cells stably expressed HPV58E7 prototype or HPV58E7 T20I/G63S variant or the empty vector control were injected subcutaneously into the flanks of the nude mouse with a 1 ml sterile syringe. Mouse weight and tumor diameters were measured every 4 d and the tumor volume (mm3) was calculated as follows: V=length×width×width×1/2. 16 d after injection, mice were sacrificed and the tumors were resected and preserved in liquid nitrogen or soaked in paraffin for further study.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining:
Here, cell apoptosis of transplanted tumor tissue was determined by TUNEL experiment. Tumor tissues are fixed with formaldehyde and then permeabilized with ethanol. Tumors were embedded in paraffin and cut into 5 μm sections. After de-paraffination with two, 10 min washes in xylene and re-hydration in graded ethanol, sections were subjected to TUNEL staining. 100 μl proteinase k solution at concentration of 20 μg/ml was added drop wise to each sample and make each sample completely covered and incubated at room temperature for 20 min. Dilute 5× Equilibration Buffer with deionized water at a ratio of 1:5. Add 100 μl of 1×Equilibration Buffer and make each sample completely covered and incubate at room temperature for 10-30 min. Following the above fixation and washing, incorporation of biotinylated-dUTP (Alexa Fluor 488-12-dUTP Labeling Mix, Shanghai Xusheng Biotechnology Co., Ltd.) on to the 3’ ends of fragmented Deoxyribonucleic acid (DNA) is carried out in the reaction containing Terminal deoxynucleotidyl transferase (TdT) enzyme. 4′,6-diamidino-2-phenylindole (DAPI) was used in nucleus staining.
Statistical analysis:
All experiments were conducted in triplicate wells/ dishes for at least three independent times. Statistics were assessed using software packages SPSS version (SPSS 24.0 for IBM). Data were expressed as mean±standard error (SE)/standard deviation (SD). Effects of HPV58E7 variations were compared against prototype and each other by one‐way analysis of variance (ANOVA). A p-value of <0.05 was considered to be statistically significant. Independent sample t-tests were done for statistical analyses. Probability values of p< 0.05 were considered statistically significant. Experiments were done in triplicate and data are expressed as mean±SE.
Results and Discussion
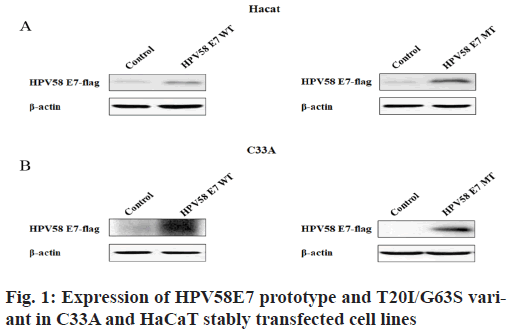
Construction of stably transfected cell lines carrying HPV58E7 prototype or HPV58 E7 T20I/G63S variant is done. In our previous epidemiological study, we found that HPV58E7 T20I/G63S variant was more widely distributed in high grade cervical lesions and cervical cancer tissues relative to normal or low-grade cervical lesions (31.7 % vs. 17.6 %) in the population of Zhejiang province by sequencing of the clinical specimens of different cervical lesions positive for HPV58. Therefore, in order to verify whether this variant has higher carcinogenicity, stably transfected C33A and HaCaT overexpressed HPV58E7 T20I/ G63S were constructed using lentivirus system to further study the carcinogenic function of this variant in vitro and in vivo. The expression efficiency of both HPV58E7 prototype and T20I/G63S variant was verified by western blot experiment. As shown in fig. 1, both HPV58E7 prototype and T20I/G63S variant were successfully overexpressed in corresponding C33A and HaCaT stably transfected cell lines. The western blot experiments showed that the stable expression of lentivirus E7 prototype and E7 (T20I/G63S) vectors were successfully constructed.
HPV58E7 (T20I/G63S) variant confers higher migration, invasion and proliferation potential in stably transfected cell lines. Firstly, we compared the migration and invasion ability of HPV58E7 variants and prototype in the stably transfected cell lines by using Transwell assay. Our data indicated that HaCaT cells expressing the HPV58E7 variants exhibited strongest migration and invasion potential than the cells expressing the prototypes one and the control cells (fig. 2). The lentivirus transfected with HPV58E7T20I/ G63S variants further promoted cell migration and invasion after mutation. An independent sample t test analysis was used, where p<0.05. However, C33A cells surprisingly shown an extremely weak migration and invasion potential as no cell could transfer through the transwell membrane. To further confirm the role of HPV58E7 variants in cervical cancer cell metastasis, a wound healing assay was performed. A migration test was carried out using positively infected cells after scratch test. Through the migration test, it was found that the cells which infected by HPV58E7 had higher migration rate and further promotes cell migration after mutation (p<0.05). The results revealed that cell migration and invasion ability of both HaCaT (fig. 3A) and C33A (fig. 3B) cells stably overexpressing We further verified the proliferation ability of the HPV58E7 T20I/G63S variant and E7 prototype through the MTT experiment. The results revealed that both HaCaT and C33A cells transfected with HPV58E7 variant showed the strongest cell proliferation ability than stable transfected cells expressing prototype and control (fig. 4A and fig. 4B) using independent sample t test analysis, where p<0.05.
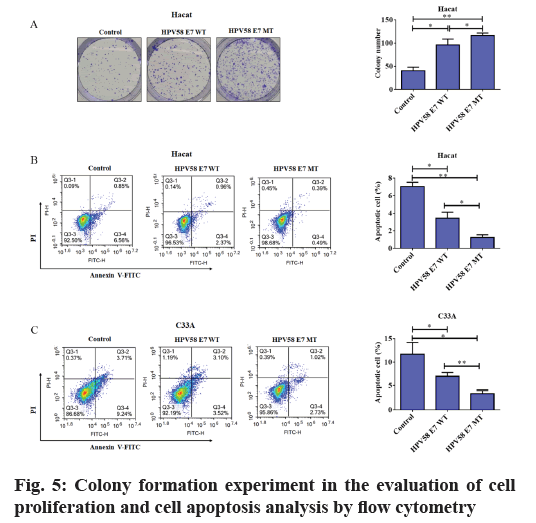
To date, there are few studies on the transformation ability of HPV58E7. Chan’s research showed that HPV58E7 T20I/G63S variant has stronger ability to induce anchorage‐independent growth of the mouse embryonic fibroblasts (NIH/3T3) with the transient transfection of HPV58E7 prototype and variants and has higher transformation ability. However, this study is only about the transient transfection of HPV58E7 and there is little data of HPV58E7 in human keratinocytes till now. Therefore, the colony formation experiment was performed using the stably transfected HaCaT and C33A cell lines and we found that the transfection of HPV58E7 prototype significantly increased the cloning ability of HaCaT cells and HPV58E7 T20I/G63S variant further promoted the colony formation ability (fig. 5A). This experiment has been repeated three times. Using independent sample t test analysis, where p<0.05. Unfortunately, the colony formation potential of C33A cells was also disappointing and no data was obtained in this study (data not shown).
Furthermore, we also detected the apoptosis of stably transfected cells lines with different HPV58E7 types by flow cytometry. As shown in fig. 5B and fig. 5C, the apoptosis rates of both HaCaT (fig. 5B) and C33A (fig. 5C) cells stably transfected with HPV58E7 prototype was lower than that of the control cells. But the apoptosis rates of the cells transfected with HPV58E7 T20I/G63S variant decreased more obviously. Using independent sample t test analysis, where p<0.05 showed significant statistical difference. Taken together, HPV58E7 T20I/ G63S variant not only promotes HaCaT and C33A cells growth but also impairs the cell apoptosis.
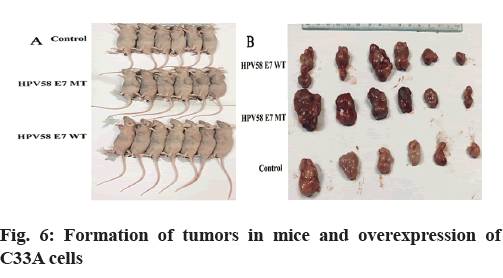
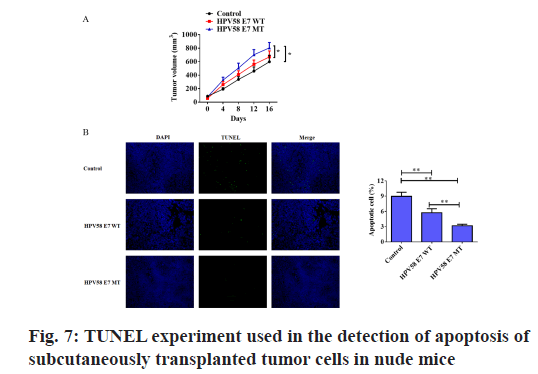
HPV58E7 T20I/G63S variant promotes tumor growth and suppresses the apoptosis in vivo. In order to further verify the carcinogenicity between HPV58E7 prototype and E7 T20I/G63S variant, athymic mice were subcutaneously inoculated with 5×106 C33A stably transfected cells. Tumors formed in nude mice. C33A cells stably over-expressing or empty vector were injected into the flanks of nude mice (n=18) and the mice were sacrificed after 16 d (fig. 6A). The volume of the tumors from the mice injected with HPV58E7 prototype or E7T20I/G63S overexpressing C33A cells was larger than that in those injected with control cells (fig. 6B). Our results found that the volume of tumors derived from C33A cells stably expressed HPV58E7 T20I/G63S variant increased more quickly compared to the wild type and the control group, while the volume of tumor derived from C33A cells stably expressed HPV58E7 prototype increased more quickly than the control group (fig. 6A and fig. 6B).
We transplanted C33A containing HPV58E7 prototype or E7T20I/G63S into nude mouse skin respectively. The TUNEL staining experiment was also performed to detect the apoptosis of subcutaneously transplanted tumor cells in nude mice. As shown in fig. 7, the percentage of apoptotic cells in the HPV58E7 T20I/ G63S variant group was significantly lower than that in the HPV58E7 prototype group and the control group. Using independent sample t test analysis, where p<0.05.
While HPV16 and 18 remain to be the most important HPV types contributing to majority of cervical cancer cases world-widely, HPV58 has a unique carcinogenic role in East Asia including the Chinese population. Our previous epidemiological studies have shown that HPV58E7 T20I/G63S variant was more highly distributed in high-grade cervical lesions or cervical cancer tissues in the population of southeastern China [12]. A meta-analysis of 219 published articles from 1994 to 2012 by Chan found that the attribution of HPV58 to cervical cancer was 3.7-fold higher in East Asia than elsewhere. Chan also conducted an epidemiological study of 1924 Hong Kong women and identified that the HPV58E7 (T20I/G63S) variant conferring an odds ratio of 26.8 for CIN III or SCC compared to other variants [13].
Previous research on HPV variation has focused on the most popular HPV16 type. In this regard, many studies have shown that the epidemiologically high-risk HPV16 E6 Asian American (AA) variant has a higher carcinogenic potential and is different from other variants and its overexpression is sufficient to immortalize and transform human primary keratinocytes without E7 [16]. Compared with HPV16, there are few researches on the biology of HPV58. One study on HPV58 variation was to compare the transcriptional activity of HPV58 variant on long control regions. It was found that the substitution of guanine at nucleotide position 7788 with an adenine residue enhances the promoter activity of the E6/E7 transcript. In addition, Chan has studied the carcinogenicity of HPV58E7 prototype and its variants through a series of phenotypes and molecular analysis in vitro. Their research has shown that the E7 (T20I/ G63S) variant can increase the immortalization of primary murine epithelial cells and increase anchored independent cell growth compared to the prototype and other variants. The E7 (T20I/G63S) variant has a similar ability to degrade retinoblastoma protein (pRb) as the HPV58E7 prototype.
But to our best knowledge, there is no research on human keratinocytes physiologically related and there is also no report about the function of HPV58E7 (T20I/ G63S) variant in cervical cancer cells using stably transfected cell lines. Our current study is the first to describe and compare the carcinogenic properties of HPV58E7 prototype and E7T20I/G63S variant in HaCaT and C33A. Our findings indicate that stronger migration, invasion and colony formation ability and lower cell apoptosis of HaCaT cells stably expressed HPV58E7 (T20I/G63S) was observed compared to that of cells expressed the HPV58E7 prototype. For C33A cell line, due to its weak migration and colony formation potential, only wound healing assay and FACS for apoptosis detection was performed. But similar results were observed. More importantly, the data from the animal experiment also supported that HPV58E7 (T20I/ G63S) could promote tumor growth and inhibit cell apoptosis in xenograft derived C33A stably transfected cells. All these findings are consistent with our previous epidemiological date. That is, HPV58E7 (T20I/G63S) has a stronger cancer risk correlation than the prototype.
In summary, our study determined the HPV58E7T20I/ G63S variant possessed a stronger carcinogenic potential than the HPV58E7 prototype. HPV58E7T20I/ G63S variant has a higher carcinogenic potential, indicating that their carriers may have a more malignant phenotype and may have poorer disease outcomes. Therefore, it is recommended to carry out more indepth cancer surveillance for carriers of E7 (T20I/ G63S) variant to detect diseases earlier.
Acknowledgements:
This research was supported in part by the Natural Science Fund of Zhejiang Province, China (No. LQ17H160004), the Natural Science Fund of Zhejiang Province, China (No. LY21H160032) and by the Natural Science Fund of Zhejiang Province, China (No. LY19H160038).
Conflicts of interest:
The authors declared no conflict of interest.
References
- Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002;2(5):342-50.
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst 1995;87(11):796-802.
- Li LK, Dai M, Clifford GM, Yao WQ, Arslan A, Li N, et al. Human papillomavirus infection in Shenyang City, People's Republic of China: a population-based study. Br J Cancer 2006;95(11):1593-7.
- Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 2003;88(1):63-73.
- De Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010;11(11):1048-56.
- Li N, Franceschi S, Howell‐Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 2011;128(4):927-35.
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high‐grade cervical lesions: a meta‐analysis update. Int J Cancer 2007;121(3):621-32.
- Huang S, Afonina I, Miller BA, Beckmann AM. Human papillomavirus types 52 and 58 are prevalent in cervical cancers from Chinese women. Int J Cancer 1997;70(4):408-11.
- Song L, Lyu Y, Ding L, Li X, Gao W, Wang M, et al. Prevalence and Genotype Distribution of High-Risk Human Papillomavirus Infection in Women with Abnormal Cervical Cytology: A Population-Based Study in Shanxi Province, China. Cancer Manag Res 2020;12:12583-91.
- Chan PK, Ho WC, Chan MC, Wong MC, Yeung AC, Chor JS, et al. Meta-analysis on prevalence and attribution of human papillomavirus types 52 and 58 in cervical neoplasia worldwide. PLoS One 2014;9(9):e107573.
- Hong D, Ye F, Chen H, Lu W, Cheng Q, Hu Y, et al. Distribution of human papillomavirus genotypes in the patients with cervical carcinoma and its precursors in Zhejiang Province, China. Int J Gynecol Cancer 2008;18(1):104-9.
- Ding T, Wang X, Ye F, Cheng X, Ma D, Lu W, et al. Distribution of human papillomavirus 58 and 52 E6/E7 variants in cervical neoplasia in Chinese women. Gynecol Oncol 2010;119(3):436-43.
- Chan PK, Lam CW, Cheung TH, Li WW, Lo KW, Chan MY, et al. Association of human papillomavirus type 58 variant with the risk of cervical cancer. J Natl Cancer Inst 2002;94(16):1249-53.
- Law PT, Boon SS, Hu C, Lung RW, Cheung GP, Ho WC, et al. Oncogenic comparison of human papillomavirus type 58 E7 variants. J Cell Mol Med 2019;23(2):1517-27.
- Boon SS, Xia C, Lim JY, Chen Z, Law PT, Yeung AC, et al. Human papillomavirus 58 E7 T20I/G63S variant isolated from an East Asian population possesses high oncogenicity. J Virol 2020;94(8):e00090-20.
- Niccoli S, Abraham S, Richard C, Zehbe I. The Asian-American E6 variant protein of human papillomavirus 16 alone is sufficient to promote immortalization, transformation and migration of primary human foreskin keratinocytes. J Virol 2012;86(22):12384-96.
 Control;
Control; HPV58 E7 WT;
HPV58 E7 WT; HPV58 E7 MT
HPV58 E7 MT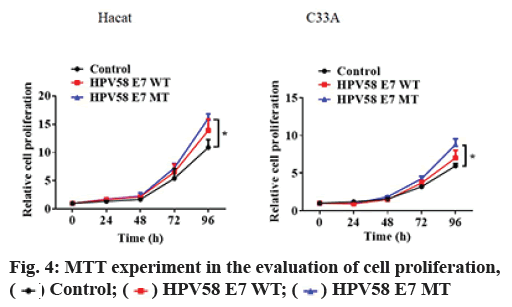
 Control;
Control; HPV58 E7 WT;
HPV58 E7 WT; HPV58 E7 MT
HPV58 E7 MT