- *Corresponding Author:
- Minghai Wang
Department of Orthopaedic Surgery, Shanghai Fifth People’s Hospital Affiliated to Fudan University, Minhang, Shanghai 200240, China
E-mail: sking1972@163.com
| Date of Received | 15 November 2023 |
| Date of Revision | 12 March 2024 |
| Date of Acceptance | 06 May 2024 |
| Indian J Pharm Sci 2024;86(3):1117-1124 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the mechanism of hsa_circ_0134111 regulating micro RNA-103a/Smad7 axis in chondrocyte degeneration in osteoarthritis. Human chondrocytes (HUM-CO-013) were cultured in vitro and divided into si-control group and si-circ_0134111 group. Chondrocytes were induced by monoiodoacetate to establish osteoarthritis injury model, the apoptosis rate was detected by flow cytometry, the expression of micro RNA-103a and Smad7 messenger RNA in osteoarthritis chondrocytes were detected by quantitative reverse transcription polymerase chain reaction; the targeting relationship between hsa_circ_0134111 and micro RNA-103a was detected by double luciferase report test. The contents of malondialdehyde, L-lactate dehydrogenase and glutathione peroxidase were detected by kit and the expressions of apoptosis-related proteins (B-cell lymphoma 2, B-cell lymphoma 2-associated protein x), collagen II, matrix metalloproteinase 13 and Smad7 in osteoarthritis chondrocytes were detected by Western Blot method. The apoptosis rate of osteoarthritis chondrocytes in si-circ_0134111 group was reduced than si-control group and the micro RNA-103a and Smad7 messenger RNA in osteoarthritis chondrocytes in si-circ_0134111 group were decreased than si-control group. Micro RNA-103a could reduce the activity of wild-type circ0134111 luciferase reporter gene, but had no effect on MUT-circ0134111. The contents of malondialdehyde and L-lactate dehydrogenase in osteoarthritis chondrocytes in si-circ_0134111 group decreased and the content of glutathione peroxidase increased, while the contents of B-cell lymphoma 2 and collagen II in osteoarthritis chondrocytes in si-circ_0134111 group increased, while the contents of B-cell lymphoma 2-associated protein x, matrix metalloproteinase 13 and Smad7 decreased compared with si-control group. Hsa_circ_0134111 can participate in osteoarthritis chondrocyte degeneration through micro RNA-103a/Smad7 axis and has_circ_0134111/micro RNA-103a/Smad7 axis may be a new regulatory mechanism and therapeutic target for osteoarthritis.
Keywords
Osteoarthritis, has_circ_0134111, micro RNA-103a, Smad7, chondrocyte degeneration
Osteoarthritis (OA) is a common joint disease characterized by articular cartilage degeneration, osteophyte growth and arthritis[1]. With the aging of the population, the incidence of OA is increasing, which brings a heavy burden to the society and families. Chondrocytes are the main cell types in articular cartilage and their functional impairment and cell death are important reasons for the progression of OA[2]. Therefore, revealing the molecular mechanism of OA chondrocyte degeneration and finding new therapeutic targets are of great significance for the prevention and treatment of OA. It has been found that circular Ribonucleic Acid (circRNA) and micro RNA (miRNA) play an important role in regulating the occurrence and development of OA chondrocytes and diseases[3]. CircRNA has the characteristics of high stability, long half-life and remarkable regulation. It can regulate the expression of target genes by adsorbing miRNA, thus affecting the function of OA chondrocytes.
It has been found that circ_013411 is differentially expressed in OA chondrocytes, but its specific mechanism is not clear. miRNA is a kind of non-coding RNA, which inhibits the expression of target genes or promotes the degradation of target genes by binding to the 3'-Untranslated Region (3'-UTR) of target genes and then regulates the biological processes such as cell proliferation, differentiation and apoptosis[4]. Smad7 could regulate and inhibit Transforming Growth Factor-Beta (TGF-β) signal pathway, while TGF-β signal pathway plays critical role in OA chondrocyte degeneration. Some studies have found that miR-103a can inhibit Smad7 by binding to the mRNA of Smad7 and then participate in the regulation of TGF-β signal pathway[5]. However, the specific molecular mechanism of OA chondrocyte degeneration is not completely clear. Therefore, the purpose of this study is to reveal the mechanism of hsa_circ_0134111 regulating miR-103a/Smad7 axis in the degeneration of OA chondrocytes and to provide a new theoretical basis and therapeutic strategy for the prevention and treatment of OA. To further clarify the mechanism of has_circ_0134111/miR-103a/Smad7 axis in OA chondrocyte degeneration, and provide a new potential target for the treatment of OA.
Materials and Methods
Research object:
Human chondrocyte (HUM-CO-013) cells were purchased from a typical culture preservation centre in the United States. The cryopreservation tube containing HUM-CO-013 cells was melted in water at 37°. The cell suspension was transferred into the centrifuge tube and 5 min was centrifuged by 1000 r/min at room temperature. The culture medium was added to the precipitated cells at 37° and cultured with 5 % CO2. Discard the old culture medium, add 2 ml preheated Phosphate-Buffered Saline (PBS) washing cells and 0.5 ml trypsin digestion liquid, after complete digestion, add human bone marrow-derived stem cell medium containing 10 % Fetal Bovine Serum (FBS), stop digestion and subculture according to 1:3 proportion.
Cell grouping and treatment:
HUM-CO-013 cells were divided into si-control (si-con) group and si-circ_0134111 group. Lentivirus vector with circ_0134111 knockout was transfected into cells in si-circ_0134111 group, while blank vector was transfected only in si-con group. OA cell model was prepared by culturing in 4 umol/l Monoiodoacetate (MIA) solution for 48 h in both groups.
Experimental materials, reagents and instruments:
HUM-CO-013 cell, FBS, Penicillin-Streptomycin double antibody (PS), MIA inducer (Sigma-Aldrich), aseptic PBS buffer, 2 % agarose gel, 10 % urea, Radioimmunoprecipitation Assay (RIPA) cleavage buffer, Bicinchoninic Acid (BCA) protein quantitative kit, polyacrylamide gel, Electrochemiluminescence (ECL) luminescent solution, B-cell lymphoma 2 (Bcl-2) antibody (ThermoFisherScientific), Bcl-2-associated X protein (Bax) antibody (ThermoFisherScientific), Malondialdehyde (MDA), Lactate Dehydrogenase (LDH), Glutathione Peroxidase (GSH-Px) detection kit, protein electrophoresis buffer (AgilentTechnologies), protein transfer membrane (AgilentTechnologies), protein extract, protein concentration kit, flow cytometry (BeckmanCoulter). USA), Westernblot (Roche, Switzerland), quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) kit (Bio-Rad).
Experimental method:
Flow cytometry: Each group of chondrocytes was washed with precooled PBS, the supernatant was discarded and the cells were resuscitated by adding 5 μ annexin V-Fluorescein Isothiocyante (FITC) to the cell precipitation μ buffer. After incubating with Propidium Iodide (PI) at room temperature for 10 min, the apoptosis rate was determined by FACSCalibur flow cytometry.
RT-PCR: HUM-CO-013 of human chondrocytes in each group was cultured in vitro for 15 d. Total RNA was extracted from chondrocytes of each group by Trizol method. The concentration and purity of RNA were measured by NanoDrop 2000° ultramicro spectrophotometer and stored in an ultra-low temperature refrigerator at 80°. The reverse transcription reaction system includes 5×g DNA buffer 2 μl, 10×King RT buffer 2 μl, fast King RT enzyme mixture 1 μl, FQ-RT primer mixture 2 μl and RNA (2 μg). Using a deionized distilled H2O (ddH2O) supplement system without RNase, the total volume can be up to 20 μl. The reaction conditions are 42° for 15 min and 95° for 3 min. After the synthesized complementary DNA was diluted 20 times, qRT-PCR was carried out, and the operation reaction was described according to the kit. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) was used as the internal reference, and 2-ΔCt was used to calculate the expression level of miR-103a and Smad7 mRNA (Table 1).
| Primer | Sequence (5'-3') |
|---|---|
| miR-103a | F: TCCCCTTGAGTAGAGACCCG |
| R: ACTGAAGGCTCAACAGCTCC | |
| Smad7 | F: GGGTCAGGTGCCTTAGTGAC |
| R: TGTCGATGACACTGACGCAA | |
| GAPDH | F: TGTCGATGACACTGACGCAA |
| R: CCTGGAAGATGGTGATGGGAT |
Table 1: Primer sequence.
Double luciferase report experiment: Circular RNA Interactome prediction showed that there was a targeted relationship between hsa_circ_0134111 and miR-103a. The binding site and mutation site were cloned into pmirGLO vector to obtain wild type vector WT-circ0134111, mutant vector MUT-circ0134111 and amiR-103a co-transferred into chondrocytes with WT-circ0134111 and MUT-circ0134111, respectively. After 48 h of culture, cytopathic cell disease was collected and relative luciferase activity was detected.
Kit detection: The supernatant of cell culture in each group was collected, and the contents of MDA, LDH and GSH-Px were detected according to the instructions of the detection kit.
Western blot: The keratinocytes of psoriasis in each group were collected and lysed by adding RIPA lysate. The protein was quantified according to the working solution prepared by BCA kit. The supernatant was separated by Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis and transferred to Poly Vinylidene Fluoride (PVDF) membrane. 10 ml 5 % skimmed milk powder was sealed at room temperature for 2 h or incubated overnight at 4°. After the PVDF membrane was incubated in an anti-RhoA treatment at 4°, tris-buffered saline, 0.1 % Tween 20 (TBST) washed off the first antibody that binds to the membrane, and the corresponding second antibody was added to incubate at room temperature for 2 h. Coloration by enhanced chemiluminescence.
Statistical method:
Statistical Package for Social Sciences (SPSS) 22.0 software was used for statistical analysis. The measurement data were expressed by mean±standard deviation (x?±s). Pairwise comparison was conducted by least significant difference-t method.
Results and Discussion
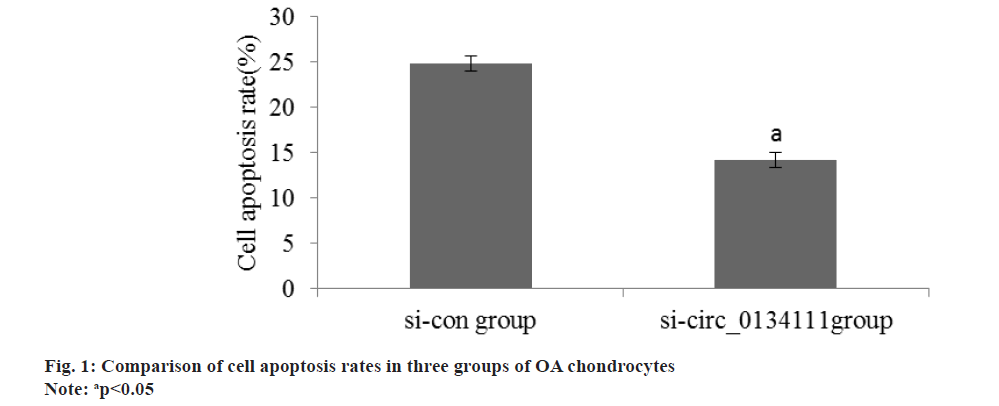
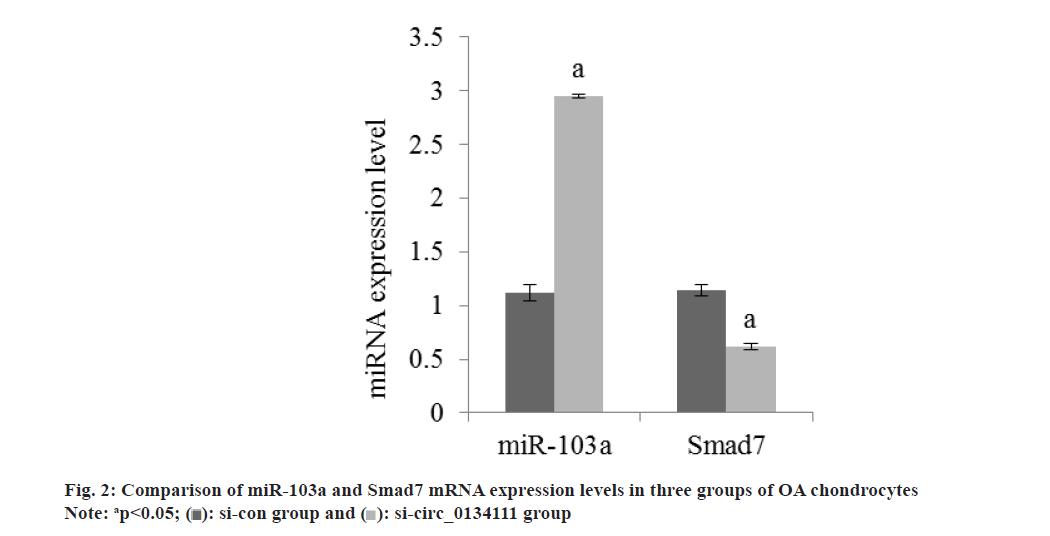
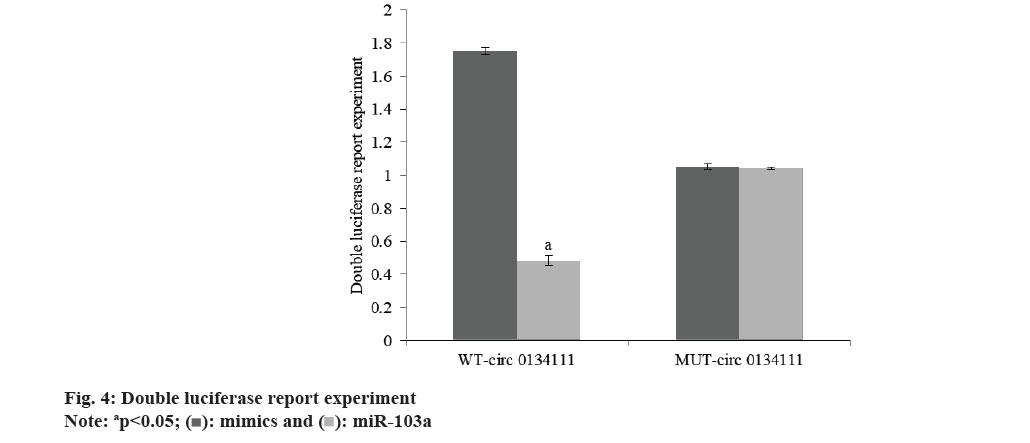
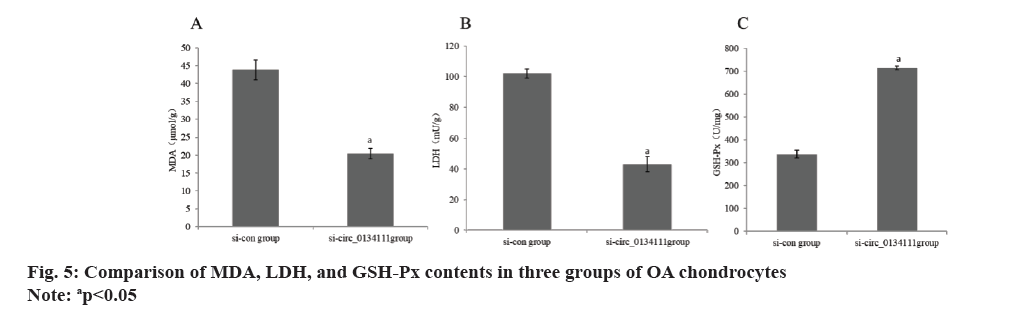
The apoptosis rate of OA chondrocytes in si-circ_0134111 group was reduced than si-con group (Table 2 and fig. 1). The miR-103a and Smad7 mRNA in OA chondrocytes in si-circ_0134111 group were reduced than si-con group (Table 3 and fig. 2). There was a binding site between hsa_circ_0134111 and miR-103a (fig. 3). Double luciferase reporter experiment showed that miR-103a could reduce the activity of wild-type circ0134111 luciferase reporter gene, but had no effect on MUT-circ0134111 (Table 4 and fig. 4). The contents of MDA and LDH in OA chondrocytes in si-circ_0134111 group were decreased while the content of GSH-Px was increased than si-con group (Table 5 and fig. 5).
| Group | Cell apoptosis rate (%) |
|---|---|
| si-con | 24.81±0.83 |
| si-circ_0134111 | 14.23±0.81 |
| t | 24.136 |
| p | <0.001 |
Table 2: Apoptosis rate of OA chondrocytes (x?±s, n=7).
| Group | miR-103a | Smad7 |
|---|---|---|
| si-con | 1.12±0.08 | 1.14±0.05 |
| si-circ_0134111 | 2.95±0.02 | 0.62±0.03 |
| F | 58.714 | 23.594 |
| p | <0.001 | <0.001 |
Table 3: miR-103a and Smad7 mRNA expression in OA chondrocytes (x?±s, n=7).
| Group | WT-circ 0134111 | MUT-circ 0134111 |
|---|---|---|
| mimics | 1.75±0.02 | 1.05±0.02 |
| miR-103a | 0.48±0.03 | 1.04±0.01 |
| t | 93.192 | 1.1832 |
| p | <0.001 | 0.259 |
Table 4: Double luciferase report experiment (x?±s, n=7).
| Group | MDA (μmol/g) | LDH (mU/g) | GSH-Px (U/mg) |
|---|---|---|---|
| si-con | 43.81±2.76 | 102.03±2.95 | 337.13±16.63 |
| si-circ_0134111 | 20.43±1.46 | 43.06±4.86 | 713.29±7.87 |
| t | 19.811 | 27.442 | 54.093 |
| p | <0.001 | <0.001 | <0.001 |
Table 5: Contents of MDA, LDH and GSH-Px in OA chondrocytes (x?±s, n=7).
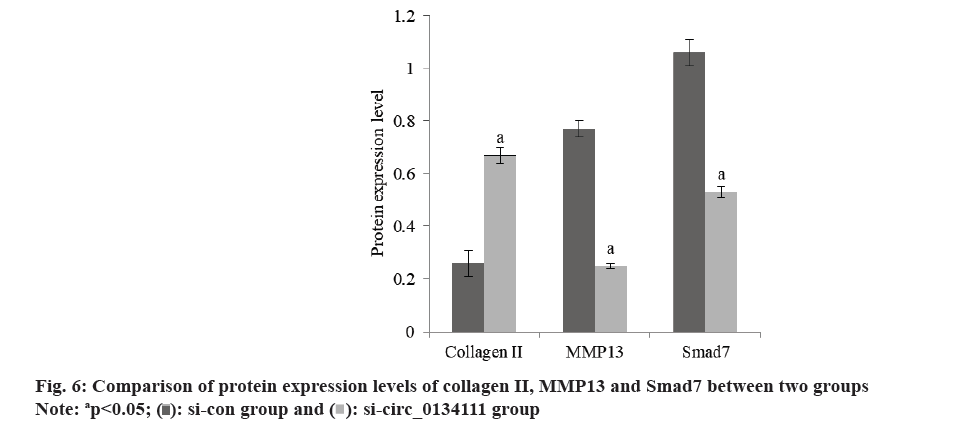
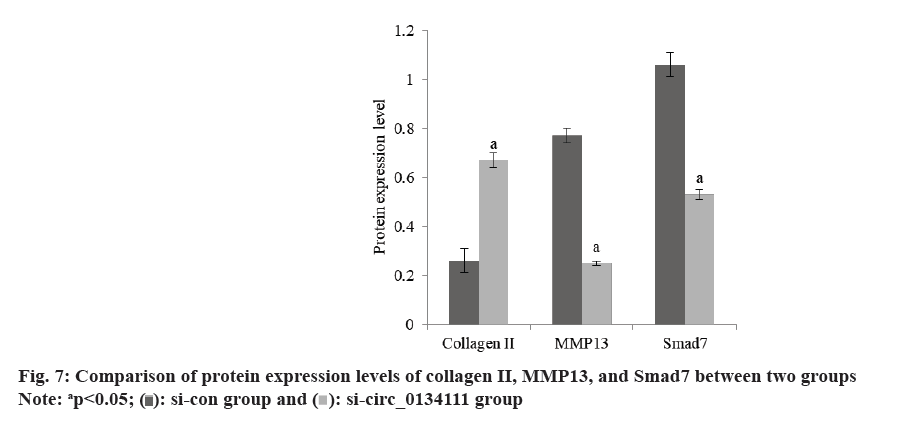
The content of Bcl-2 in OA chondrocytes in si-circ_0134111 group was increased and the content of Bax was decreased than si-con group (Table 6 and fig. 6). The content of Collagen II in OA chondrocytes in si-circ_0134111 group was increased, while the contents of Matrix Metallopeptidase 13 (MMP13) and Smad7 were decreased than xi-con group (Table 7 and fig. 7).
| Group | Bcl-2 | Bax | |
|---|---|---|---|
| si-con | 0.23±0.02 | 0.57±0.04 | |
| si-circ_0134111 | 0.64±0.02 | 0.24±0.03 | |
| t | 38.352 | 17.462 | |
| p | <0.001 | <0.001 | |
Table 6: Expression of Bcl-2 and Bax apoptotic proteins in OA chondrocytes (x?±s, n=7).
| Group | Collagen II | MMP13 | Smad7 |
|---|---|---|---|
| si-con | 0.26±0.05 | 0.77±0.03 | 1.06±0.05 |
| si-circ_0134111 | 0.67±0.03 | 0.25±0.01 | 0.53±0.02 |
| t | 18.603 | 43.506 | 26.039 |
| p | <0.001 | <0.001 | <0.001 |
Table 7: Protein expression of Collagen II, MMP13 and Smad7.
OA is the result of the imbalance between the synthesis and decomposition of articular chondrocytes, extracellular matrix and subchondral bone. The destruction of articular cartilage caused by the decrease of chondrocytes is one of the important reasons[6]. Some studies have found that interleukin-1 inflammatory factors can induce apoptosis of chondrocytes. Chondrocyte degeneration is an important link in the pathogenesis of OA. Abnormal changes in the signaling pathways of TGF-β and Basic Metabolic Panel (BMP) play critical role in the pathogenesis of OA, which leads to the loss of chondrocyte differentiation phenotype and the appearance of terminal hypertrophic chondrocytes during the development of growth plate[7,8]. Some studies have found that circRNA can play an important regulatory role in chondrocyte injury in OA and may be used as a potential target for diagnosis and treatment to promote the degradation of extracellular matrix of chondrocytes[9]. However, the mechanism of some circRNA on chondrocyte injury in OA has not been clarified. si-circ_0134111 is highly expressed in OA chondrocytes, but the mechanism is not clear. Therefore, this study will explore the expression of circ013411 in OA chondrocytes and its mechanism.
The increased expression of miR-103a is related to the release lesion of bone. The relationship between the increased expression of miR-103a and bone degenerative diseases. The high expression of miR-103a was observed in bone marrow mesenchymal stem cells, osteoblasts, chondrocytes and synovial cells. By promoting osteoblast differentiation and inhibiting differentiation into adipocytes, it affects bone cell function[10]. Among them, Smad7 can competitively bind to the receptor with R-Smads, prevent the phosphorylation of R-Smads, thus inhibit the signal transduction effect, promote the synthesis of collagen and matrix proteins in chondrocytes, and help to maintain the structure and function of cartilage tissue[11]. Smad7 is a member of Smads. Smads molecule has two conserved Mad Homology 1 (MH1) domains at the N-terminal of Mad homology domain, which can bind to the CAZAC sequence of DNA[12]. On the other hand, the C-terminal MH2 domain can interact with transcriptional helper repressors or coactivators to regulate the function of Smad. The short junction region between the two domains contains multiple phosphorylation sites, which is a negative regulatory region of Smad and can be inactivated by ERK phosphorylation. Some studies have found that[13], Smad7 can reduce the damage of chondrocytes caused by inflammation by inhibiting the expression and activation of inflammatory factors, thus slowing down the progression of arthritis. The results showed that the expression of miR-103a and Smad7 mRNA in OA chondrocytes in si-circ_0134111 group were reduced than si-con group. It is suggested that the low expression of hsa_circ_0134111 can inhibit the degeneration of arthritic chondrocytes and protect articular cartilage through the miR-103a/Smad7 axis.
GSH-Px belongs to antioxidant enzymes, which can scavenge oxygen free radicals. MDA belongs to the product of lipid membrane oxidation. The activity of LDH increases significantly when chondrocytes are injured. The contents of MDA and LDH in OA chondrocytes in si-circ_0134111 group were reduced than si-con group, while the content of GSH-Px was raised in si-circ_0134111 group. It is suggested that the low expression of hsa_circ_0134111 reduces intracellular oxidative stress and activates the activities of antioxidant enzymes.
Bax belongs to the pro-apoptotic protein family, whereas Bcl-2 belongs to the anti-apoptotic protein family. The increase of its level can activate the mitochondrial pathway and induce cell apoptosis. Collagen II is one of the important causes of extracellular matrix damage. Some studies have shown that oxidative stress injury can activate the expression of apoptotic protein Bax, and the increased expression of Bax can promote the release of CytC from mitochondria and then activate Collagen II to induce apoptosis[14]. MMP13 is one of the important enzymes in the matrix degradation of OA articular cartilage. Zhang et al.[15] found that the expression of MMP13 in OA articular cartilage is more than 40 times that of normal chondrocytes. When cartilage is injured, the expression of MMP13 in chondrocytes increases, which breaks the balance between the production and degradation of Extracellular Matrix (ECM), leads to a large number of ECM degradation, destroys the collagen network and internal environment of chondrocytes, and then leads to the occurrence of OA. The contents of Bcl-2 and Collagen II in OA chondrocytes in si-circ_0134111 group increased, while the contents of Bax and MMP13 decreased. It is suggested that the low expression of hsa_circ_0134111 can increase the levels of Bcl-2 and Bax, activate mitochondria, reduce apoptosis, and decrease the content of MMP13, thus inhibit the progression of OA.
To sum up, hsa_circ_0134111 regulates Smad7 through miR-103a to inhibit the degeneration of arthritis chondrocytes and reduce apoptosis. Has_circ_0134111/miR-103a/Smad7 provides a potential target for new OA treatment strategies.
Author’s contribution:
Peiyan Huang and Qi Chen contributed equally to this work.
Funding:
The work was supported by Minhang District Natural Science Project, (No: 2022MHZ016) and Shanghai Fifth People's Hospital, Fudan University, (No: 2020WYZT06).
Conflict of interest:
The authors declare no conflict of interest.
References
- Xiong Z, Zheng C, Chang Y, Liu K, Shu L, Zhang C. Exploring the pharmacological mechanism of Duhuo Jisheng decoction in treating osteoporosis based on network pharmacology. Evid Based Complement Alternat Med 2021;12(4):245-53.
[Crossref] [Google Scholar] [PubMed]
- Nakamura A, Rampersaud YR, Sundararajan K, Nakamura S, Wu B, Matip E, et al. Zinc finger protein-440 promotes cartilage degenerative mechanisms in human facet and knee osteoarthritis chondrocytes. Osteoarthritis Cartilage 2021;29(3):372-9.
[Crossref] [Google Scholar] [PubMed]
- Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell 2019;179(5):1033-55.
[Crossref] [Google Scholar] [PubMed]
- Liu T, He Z, Li J, Song Y, Yao X, Chen W, et al. Role and mechanism of noncoding RNA in diabetic peripheral neuropathy. Chin J Chem Eng 2024;28(7):1124.
- Li CY, Zhang JR, Hu WN, Li SN. Atrial fibrosis underlying atrial fibrillation. Int J Mol Med 2021;47(3):1.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Zhan P, Wang Q, Zhang M, Huang S, Chen D. Linagliptin ameliorated interleukin-29-induced reduction of extracellular matrix genes through the nuclear factor erythroid 2-related factor 2 (Nrf2)/sry-type high-mobility-group box (SOX)-9 axis in an in vitro study on C-28/I2 chondrocytes. Bioengineered 2022;13(2):3775-84.
- Jeyaraman M, Muthu S, Shehabaz S, Jeyaraman N, Rajendran RL, Hong CM, et al. Current understanding of MSC-derived exosomes in the management of knee osteoarthritis. Exp Cell Res 2022;418(2):113274.
[Crossref] [Google Scholar] [PubMed]
- Cao H, Lu J, Du J, Xia F, Wei S, Liu X, et al. TAK1 inhibition prevents the development of autoimmune diabetes in NOD mice. Sci Rep 2015;5(1):14593.
[Crossref] [Google Scholar] [PubMed]
- Mao X, Cao Y, Guo Z, Wang L, Xiang C. Biological roles and therapeutic potential of circular RNAs in osteoarthritis. Mol Ther Nucleic Acids 2021;24:856-67.
[Crossref] [Google Scholar] [PubMed]
- Chen Q, Bai Y, Liu K, Lin T, Luo X. Osteoblast differentiation after conditional knockout of 3-phosphoinositide-dependent protein kinase-1 gene from bone marrow mesenchymal stem cells. Chin J Chem Eng 2022;26(24):3785.
- Liu D, Ruan M, Tong C, Huang R. Effect of shugan jianpi recipe combined with cross moxibustion on biochemical examination indexes and total score of TCM symptoms in patients with spleen-stomach damp-heat diarrhea irritable bowel syndrome. Comput Math Methods Med 2022;12(13):196-9.
[Crossref] [Google Scholar] [PubMed]
- Yang CC, Liu MJ, Xu ZH, Liu Y, Guo ZH, Li BH, et al. 17β-estradiol inhibits TGF-β-induced collagen gel contraction mediated by human Tenon fibroblasts via Smads and MAPK signaling pathways. Int J Ophthalmol 2023;16(9):1441.
[Crossref] [Google Scholar] [PubMed]
- Chang C, Xu L, Zhang R, Jin Y, Jiang P, Wei K, et al. MicroRNA-mediated epigenetic regulation of rheumatoid arthritis susceptibility and pathogenesis. Front Immunol 2022;13:838884.
[Crossref] [Google Scholar] [PubMed]
- Yao Q, Zhang H, Standish C, Grube J, Mañas A, Xiang J. Expression profile of the proapoptotic protein Bax in the human brain. Histochem Cell Biol 2023;159(2):209-20.
- Zhang Y, Zuo T, McVicar A, Yang HL, Li YP, Chen W. Runx1 is a key regulator of articular cartilage homeostasis by orchestrating YAP, TGFβ, and Wnt signaling in articular cartilage formation and osteoarthritis. Bone Res 2022;10(1):63.
[Crossref] [Google Scholar] [PubMed]
 .
.
 .
.
 .
.
 .
.



