- *Corresponding Author:
- R. Madaan
Chitkara College of Pharmacy, Chitkara University, Punjab 140401, India
E-mail: reecha.madan@chitkara.edu.in
| Date of Received | 31 July 2021 |
| Date of Revision | 23 January 2022 |
| Date of Acceptance | 15 December 2022 |
| Indian J Pharm Sci 2022;84(6):1593-1596 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Development of analytical method on high performance thin layer chromatography for estimation of content of marker compound, preferably bioactive constituent, in traditional herb has become a rational approach for its standardization. The literature does not reveal any report on phytochemical investigation to standardize Calotropis gigantea roots on the basis of marker compounds. Non-availability of such study on Calotropis gigantea prompted us to develop high performance thin layer chromatography method for the estimation of triterpenoids (taraxerol and lupeol), which are bioactive compounds of the plant. Numerous solvent systems were tried to resolve these compounds in methanol extract of Calotropis gigantea roots but best resolution of taraxerol and lupeol was obtained using toluene:ethyl acetate:methanol (8:1:1) and toluene:chloroform (3:7), respectively, as solvent systems. The developed thin layer chromatography plates were derivatized by treating with anisaldehyde-sulphuric acid reagent followed by heating. The visible coloured bands of triterpenoids were scanned at 550 nm. The percentage content of taraxerol and lupeol was 0.0086 % and 0.0093 % w/w, respectively, in Calotropis gigantea roots. The consistent and reproducible therapeutic effects of herbal preparations containing Calotropis gigantea roots can be achieved if marker based standardized raw material of the plant is used.
Keywords
Calotropis gigantea, high performance thin layer chromatography, lupeol, taraxerol
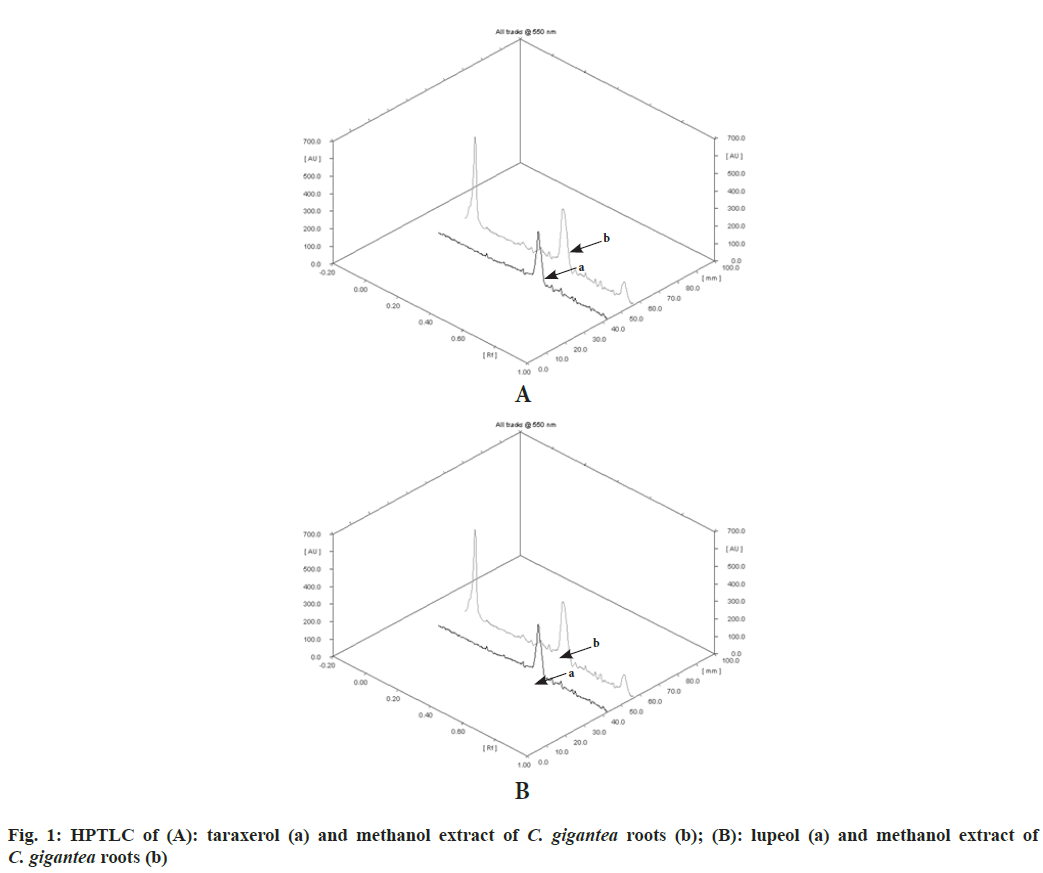
Calotropis gigantea (L.) Dryand (C. gigantea), commonly known as Giant Milkweed, belongs to family Asclepiadaceae. The plant is distributed throughout India, mostly in Andaman Island upto 900 m altitude in hills[1]. The roots are used in traditional Indian systems of medicine for the treatment of various ailments such as mental disorders, malaria and joint pain[2,3]. The plant has been reported to exhibit anxiolytic, analgesic, anticonvulsant, antidiabetic, antibacterial, anti-inflammatory and anticancer activities. A wide range of chemical constituents have been isolated from C. gigantea such as cardenolides, oxypregnane-oligoglycosides, triterpenoids, terpenes, flavonoids, sterols, aromatic product, resin and non-protein amino acid[1,4]. Natural triterpenoids are well known chemical constituents of medicinal plants which possess a wide range of pharmacological activities. Taraxerol and lupeol are main bioactive triterpenoidal constituents reported in C. gigantea roots. The literature reveals a startling fact that no phytochemical investigation has been conducted to develop analytical method for standardizaing C. gigantea roots. Thus, an attempt was made to develop an analytical High Performance Thin Layer Chromatography (HPTLC) method to estimate the content of taraxerol and lupeol. C. gigantea roots were procured from Himalaya Herb Stores, Madhav Nagar, Saharanpur, (Uttar Pradesh), India in September, 2012. Identity of the plant was confirmed by Dr. Sunita Garg, Chief Scientist and Head, Raw Materials Herbarium and Museum, National Institute of Science Communication and Information Resources (NISCAIR), New Delhi (Reference no: NISCAIR/RHMD/Consult/2013/2242/23, dated 21/05/2013). A specimen copy (No. 101) of HPTLC fingerprint profile of plant’s methanol extract was deposited to Museum-cum-Herbarium of Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala. Methanol, toluene, ethyl acetate, chloroform, sulphuric acid, glacial acetic acid (E-Merck Ltd., Mumbai), and anisaldehyde (S.D. Fine Chemicals, Mumbai) were used in present investigations. Accurately weighed 5 mg of marker compound was dissolved in 2 ml methanol. The solution was transferred to a volumetric flask (5 ml) and final volume was made up to 5 ml with methanol. Coarsely powdered roots of C. gigantea (10 g) were exhaustively extracted with methanol in a Soxhlet apparatus. The methanol extract was filtered, concentrated to 10 ml and transferred to a volumetric flask (25 ml). Final volume of methanol extract was adjusted to 25 ml with methanol. Taraxerol stock solution was diluted with methanol to get six dilutions of concentrations - 7.5, 10, 12.5, 15, 17.5 and 20 μg/ml. After loading 10 µl of each dilution in the form of band (1 cm) on precoated Thin layer chromatography (TLC) plate (E Merck, Mumbai, India; 0.2 mm; aluminum base) using CAMAG LINOMAT 5 applicator, the plate was developed in solvent system; toluene:ethyl acetate:methanol (8:1:1) in a CAMAG developing chamber. The developed TLC plate was dried, dipped in CAMAG dipping chamber containing 0.5 % anisaldehyde- sulphuric acid reagent followed by heating at 110° for 2 min in hot air oven and scanned in CAMAG scanner at 550 nm. The Area Under Curve (AUC) of taraxerol peak was recorded in each track. Regression equation was obtained from the standard curve plotted between different concentrations of taraxerol and peak areas. Lupeol stock solution was diluted with methanol to get six dilutions of concentrations (10, 20, 30, 40, 50 and 60 μg/ml). Development of TLC plate of each dilution of lupeol using solvent system; toluene:chloroform (3:7), derivatization and scanning were performed in similar manner as explained above. For estimation of marker compounds in C. gigantea, the test samples of methanol extract (10 µl, each) were loaded on TLC plates using respective solvent systems for taraxerol and lupeol as mentioned above. Development, derivatization and scanning of TLC plates, and recording of peak areas of marker compounds in test samples were performed in similar manner as explained above. The content of each marker compound (in percentage w/w) was calculated by putting values of peak area of each marker compound in their respective regression equations. The compliance of validation parameters of developed HPTLC analytical methods was tested as per International Council for Harmonisation (ICH) guidelines[5]. The bioactive triterpenoids viz., taraxerol and lupeol are main chemical constituents of C. gigantea. An attempt was made to develop TLC of these compounds using same solvent system but lupeol and taraxerol showed best resolution on TLC plate in different solvent systems. Therefore different solvent systems were selected for analysis of these compounds using HPTLC system in plant material. HPTL fingerprint profiles of taraxerol (Rf value: 0.56) and lupeol (Rf value: 0.36) with methanol extract are shown in fig. 1. Standard plots were prepared between different concentrations of taraxerol and lupeol vs. their peak areas after scanning derivatized TLC plates with 0.5 % anisaldehyde-sulphuric acid reagent followed by heating at 110° for 2 min and scanning in TLC scanner at 550 nm. The percentage content of taraxerol and lupeol in C. gigantea roots was found to be 0.0086±0.00003 % and 0.0093±0.00001 % w/w, respectively. The results have been expressed as mean±standard deviation. ICH guidelines have been followed to validate HPTLC methods for analysis of taraxerol and lupeol. The results of validation parameters are shown in Table 1. The ultraviolet spectra and thin layer chromatogram overlays of marker compounds and test sample did not show any interference in quantitative analysis of taraxerol and lupeol. Triterpenoids, particularly taraxerol and lupeol isolated from the plant, have been considered major constituents responsible for most of activities of C. gigantea[6]. Reported pharmacological activities of taraxerol are antitumor, anti-inflammatory, antimicrobial, antioxidant, antiallergic and antiamnesic[7] and of lupeol are antiprotozoal, anti-inflammatory, antimicrobial and anticancer activities[8]. HPTLC technique was used to standardize C. gigantea roots by estimating the contents of bioactive markers, i.e., taraxerol and lupeol. HPTLC technique was selected because of easy method development and validation, high precision, wide applicability and universal acceptability for quantitative determination of particular compounds in complex mixtures of biomolecules[9]. The developed analytical methods were precise and reproducible as validation parameters complied with the prescribed limit. Accuracy studies showed that recovery of taraxerol and lupeol was more than 98 %, inferring that developed methods were accurate. As no deviation was observed in overlay patterns of ultraviolet spectra and thin layer chromatograms of marker compounds and test sample suggest that developed analytical methods were specific. Herbal formulations containing standardized C. gigantea roots and its products may emerge as clinically potential drugs with reproducible therapeutic efficacy and safety.
| Parameter | Taraxerol | Lupeol |
|---|---|---|
| Instrumental precision (% CV, n=7) | 0.86 | 0.90 |
| Repeatability (% CV, n=5) | 0.52 | 0.70 |
| Coefficient of determination (r2) | 0.999 | 0.996 |
| Linearity range (ng) | 75-200 | 100-600 |
| LOD (ng) | 9 | 16 |
| LOQ (ng) | 30 | 49 |
| Intra-day precision (% CV, n=9) | 0.77 | 0.60 |
| Inter-day precision (% CV, n=9) | 0.84 | 0.79 |
| Accuracy (average % recovery± S.D., n=3) | 99.68±0.22 | 99.23±1.45 |
| Specificity | Specific | Specific |
Table 1: Method Validation Parameters of Marker Compounds in HPTLC Analysis
Acknowledgements:
Authors duly acknowledge Prof R.C. Gupta, Coordinator of DBT-IPLS project for providing access to instrumentation facilities at Punjabi University, Patiala.
Conflict of interests:
The authors declared no conflict of interests.
References
- Kumar D, Kumar S. Calotropis gigantea (L.) Dryand-A review update. Indian J Res Pharm Biotechnol 2015;3(3):218.
- Borthakur SK, Nath K, Gogoi P. Herbal remedies of the Nepalese of Assam. Fitoterapia 1996;67(3):231-7.
- Kshirsagar SR, Parabia MH, Reddy MN. Ethnobotany of coastal areas in South Gujarat. Ethnobotany. 2003;15:60-3.
- Kadiyala M, Ponnusankar S, Elango K. Calotropis gigantiea (L.) R. Br (Apocynaceae): A phytochemical and pharmacological review. J Ethnopharmacol 2013;150(1):32-50.
[Crossref] [Google Scholar] [PubMed]
- Randhawa K, Kumar D, Jamwal A, Kumar S. Screening of antidepressant activity and estimation of quercetin from Coccinia indica using TLC densitometry. Pharm Biol 2015;53(12):1867-74.
[Crossref] [Google Scholar] [PubMed]
- Anjaneyulu V, Row LR. The triterpenes of Calotropis gigantea Linn. Curr Sci 1968;37(6):156-7.
- Sharma K, Zafar R. Occurrence of taraxerol and taraxasterol in medicinal plants. Pharmacognosy Rev 2015;9(17):19.
[Crossref] [Google Scholar] [PubMed]
- Gallo MB, Sarachine MJ. Biological activities of lupeol. Int J Biomed Pharm Sci 2009;3(1):46-66.
- Kumar A, Kumar D, Kumar S, Shri R. Comparative evaluation of quercetin content in three varieties of Allium cepa using TLC densitometry. Int J Adv Pharm Biol Chem. 2015;4:612-9.