- *Corresponding Author:
- Devangi Chachad
Department of Botany, Jai Hind College, Mumbai, Maharashtra 400020, India
E-mail: devangi.chachad@jaihindcollege.edu.in
| Date of Received | 03 July 2023 |
| Date of Revision | 11 March 2024 |
| Date of Acceptance | 14 October 2024 |
| Indian J Pharm Sci 2024;86(5):1835-1844 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The presence of various secondary metabolites in the crude plant extracts have always been a source of potential therapeutic activities in animals and humans. The varying phytochemical composition and concentrations are instrumental in deciding and shortlisting raw materials for extractions and isolation of active compounds. Therefore, preliminary phytochemical screening of the plant extracts along with their high-performance thin layer chromatography analysis for classes of compounds become prerequisite for any type of pharmacological study. Detailed profiling of secondary metabolites like cardiac glycosides, triterpenoids and steroids which are found to be instrumental in inducing contraception in male experimental model organisms will help us further understands the mechanism of natural male contraception. In this study, crude seed extracts of Carica papaya Linn., Momordica charantia Linn. and Abrus precatorius Linn in suitable solvents were checked for the high-performance thin layer chromatography profiling of the above-mentioned metabolites. The results obtained were recorded and analysed for repeated authentication of plant parts and standardisation of extract preparation.
Keywords
High-performance thin layer chromatography, cardiac glycosides, triterpenoids, steroids, male contraceptive
The need and interest in identification of the scientific class of compounds present in medicinally important plant materials is increasing worldwide, especially in developing countries where the use of herbal medicines is popular for their basic health needs. Various plant extracts are being used to cure and heal innumerable health disorders and injuries and this empirical knowledge comes from the plants defence system, which generates numerous compounds with diverse molecular structures, in far complex combinations to those derived from synthetic products, so the great interest in the identification and elucidation of new active principles. Preliminary phytochemical screening of the individual extracts of Carica papaya (C. papaya) Linn., Momordica charantia (M. charantia) Linn. and Abrus precatorius (A. precatorius)Linn. in various solvents like water, ethanol, chloroform, hexane, benzene and petroleum ether was carried out. It showed presence of various active phytochemicals like alkaloids, tannins, glycosides especially cardiac glycosides, diterpenes, triterpenoids and steroids. Out of all these phytochemicals found to be present in the different extracts, cardiac glycosides, triterpenoids and steroids are the class of compounds mainly responsible for the antifertility activity[1,2] (Table 1).
Cardiac glycosides are a class of organic compounds which are highly toxic and found in a number of plants, usually consisting of an aglycone (structurally related to steroid hormones) linked to one or more sugar molecules[3].
These compounds are said to increase the output force of the heart and increase its rate of contractions by acting on the cellular sodium-potassium ATPase pump. They are steroidal in nature and are considered important drugs for treatment of heart failure and cardiac rhythm disorders. They also have potential therapeutic use for treating cancer owing to their specific cytotoxicity against cancer cells[4]. Digoxin, a cardiac glycoside isolated from Digitalis sp. was found to cause significant reduction in sperm motility without reducing sperm count as the drug does not affect spermatogenesis and cause damage to the testicular cells[2]. Triterpenoids are cyclised from oxidised squalene precursors by oxidosqualene cyclases, with more than 100 different cyclical triterpene scaffolds[5]. Triterpenoids have recently emerged as a unique metabolite with multifunctional anti-cancer activities as demonstrated by promising research studies along with a low toxicity profile. They are used for various medical purposes like for anti-inflammatory, analgesic, antipyretic, hepatoprotective, cardiotonic, sedative and tonic effects[6]. Many classes of triterpenoids lower cholesterol levels by inhibiting cholesterol synthesis[1]. Thus, it has the potential to regulate the production of hormones that involve cholesterol as a starting molecule. Triterpene, oleanolic acid, isolated from Eugenia jambolana, was found to exhibit antifertility effect on male albino rats without any toxic side effects[7]. Triterpenoids of Nerium oleander were also found to show anti-fertility effect in male albino rats by affecting spermatogenesis[8]. Steroids have the fundamental structure of four carbon rings called the steroid nucleus. The addition of different chemical groups at different positions on the backbone leads to the formation of many different types of steroid compounds including sex hormones progesterone and testosterone, the anti-inflammatory steroids like corticosteroids, cardiac steroids digoxin and digitoxin etc. Plant steroids synthesised by cyclisation of 2,3-epoxysqualene into cycloartenol are further metabolised owing to the enzymatic conversion to produce biologically active steroids. Natural steroids act as regulators of lipid metabolism and influence the production and regulation of the sex hormones in the organism’s body[1]. These plant steroids are found to possess many interesting medicinal and pharmaceutical activities like antitumor, immunosuppressive, hepatoprotective, antibacterial, sex hormones, anthelminthic, cytotoxic and cardiotonic activity[9-11].
Therefore, further studies and confirmation of the presence of those compounds with the help of High- Performance Thin Layer Chromatography (HPTLC) was carried out to prove their presence in the prepared crude drugs which will be administered to the male Wistar, albino rats in pharmacological evaluation.
Materials and Methods
Collection and processing of plant material:
The plant materials were collected from local markets and gardens were authenticated from Blatter Herbarium, St. Xavier’s College, Mumbai. The specimen of C. papaya L. matches with the specimen number KGM- 1079 of Kalpit Mhatre. The specimen of M. charantia L. matches with the specimen number 3800 of S.M. Almeida. The specimen of Abrus precatorius (A. precatorius) L. matches with the specimen number S.H.813 of P.S. Herbert. For phytochemical screening, 4 g of powdered drug was macerated with individual solvents like distilled water, ethanol, benzene, hexane, chloroform and petroleum ether in closed flasks for 24 h with frequent shaking. Those were then filtered using Whatman’s filter paper. All the extracts obtained were tested using standard methodology for the metabolites using respective reagents and treatments[12-19]. 1 g of the dried powder of seeds of C. papaya was extracted in 50 ml of chloroform, 1 g of the dried M. charantia seed powder in 50 ml of benzene and 1g of the seed powder of A. precatorius was extracted in 50 ml of ethanol by cold maceration for 24 hours and then subjected to rotary evaporator for total removal of solvent. An oily thick brown paste was obtained from C. papaya, sticky semi solid dark brown paste was obtained from M. charantia and a blackish brown sticky powder was obtained from A. precatorius which was used for further analysis.
Preliminary phytochemical screening:
Test for tannins: To test for the presence of tannins, 3 ml of extract is combined with different reagents: adding 3 ml of lead acetate (Pb(CH3COO)2) forms a white precipitate, indicating tannins; adding 3 ml of 5% Ferric chloride ( FeCl3 ) (FeCl3) solution results in a blue-black coloration, indicating tannins; and adding 3 ml of potassium dichromate (K2Cr2O7) causes a dark coloration, confirming the presence of tannins.
Test for proteins: To test for the presence of proteins, 3 ml of extract is combined with different reagents: In the biuret test, adding 1 ml of 4 % Sodium hydroxide (NaOH) and 1 ml of 1 % CuSO4 results in violet or pink coloration, indicating proteins. In millon's test, adding 3 ml of millon’s reagent forms a white precipitate that turns brick red upon heating, indicating proteins. In the sulphosalicylic acid test, adding 1 ml of sulphosalicylic acid to 2 ml of extract causes cloudiness, confirming proteins. In the ninhydrin test, adding 1 ml of ninhydrin reagent to 2 ml of extract produces a deep blue or purple coloration, indicating proteins.
Test for reducing sugars: To test for the presence of reducing sugars, 2 ml of extract is combined with different reagents: In Fehling's test, adding a mixture of 1 ml each of Fehling's A and Fehling's B reagents and heating in a boiling water bath for 10 min results in a yellow coloration, indicating reducing sugars. In Benedict’s test, adding 2 ml of Benedict’s solution to the extract and heating in a boiling water bath for 10 min produces yellow, green, or red coloration, confirming the presence of reducing sugars.
Test for carbohydrates: In Molisch’s test for carbohydrates, 2 ml of extract is mixed with 2 drops of Molisch’s reagent, followed by a few drops of concentrated Sulfuric acid (H2SO4). The formation of a violet ring at the interface indicates the presence of carbohydrates.
Test for glucosides: To test for the presence of glucosides, 2 ml of extract is mixed with 2 ml of 1 % aqueous picric acid solution and left aside for 15 min, followed by the addition of 1 ml of aqueous Na2CO3. A red coloration indicates the presence of glucosides.
Test for flavonoids: To test for the presence of flavonoids, 2 ml of extract is combined with different reagents: In the FeCl3 test, adding neutral FeCl3 produces a green coloration, indicating flavonoids. In the Shinoda test, adding 1 ml of Hydrochloric acid (HCl) and a pinch of finely chopped magnesium ribbon to the extract results in a pink, orange, or red coloration, confirming the presence of flavonoids.
Test for triterpenes: To test for the presence of triterpenes, 2 ml of extract is combined with different reagents: In Salkowski’s test, adding concentrated H2SO4 results in a yellow coloration in the lower layer after standing, indicating triterpenes. In the Liebermann- Burchard’s test, adding a few drops of acetic acid and 1 ml of concentrated H2SO4 produces a deep red coloration at the junction of two solvents, confirming triterpenes. In Tschugajen’s test, mixing 2 ml of extract with 5 ml of acetyl chloride and a pinch of Zinc chloride (ZnCl2) then warming in a hot water bath, results in eosin red coloration, indicating triterpenes.
Test for terpenoids: To test for the presence of terpenoids, 2 ml of extract is mixed with a pinch of tin and 1 ml of thionyl chloride (SOCl2) solution. The appearance of violet coloration indicates the presence of terpenoids.
Test for steroids: To test for the presence of steroids, 2 ml of extract is combined with different reagents. In Salkowski’s test, adding concentrated H2SO4 results in the lower layer turning yellow after standing, indicating steroids. In the Liebermann-Burchard’s test, adding a few drops of acetic acid (CH3COOH) and 1 ml of concentrated H2SO4 produces a deep red coloration at the junction of the two solvents, confirming the presence of steroids.
Test for diterpenes: To test for the presence of diterpenes, 2 ml of extract is mixed with 2 ml of copper acetate solution. The formation of a green coloration indicates the presence of diterpenes.
Test for glycosides: To test for the presence of glycosides, 2 ml of extract is combined with different reagents. In the NaOH test, adding 1 ml of water and 1 ml of NaOH solution results in a yellow coloration, indicating glycosides. In Keller-Killiani’s test, adding 1 ml of water, 1 ml of glacial acetic acid, 1 ml of FeCl3 solution, and 0.5 ml of concentrated H2SO4 produces a brown ring at the junction of the two solvents, confirming the presence of glycosides.
Test for Anthraquinones: To test for the presence of anthraquinones, 1 g of powdered drug is boiled in dilute H2SO4 for 3 min and then filtered. The filtrate is divided into three parts, and each part is treated with 2 ml of chloroform, ether, and benzene, respectively. After the organic layer is separated, ammonia is slowly added. The appearance of a pink to red color in the ammoniacal layer indicates the presence of anthraquinones.
Test for quinones: To test for the presence of quinones, 2 ml of extract is mixed with 1 ml of concentrated H2SO4. The appearance of a yellow coloration indicates the presence of quinones.
Test for alkaloids: To test for the presence of alkaloids, 2 ml of extract is mixed with 1 ml of Dragendroff’s reagent. The formation of a reddish-brown precipitate indicates the presence of alkaloids.
Wagner’s test: To test for the presence of alkaloids, 2 ml of extract is mixed with 1 ml of Wagner’s reagent. The formation of a reddish-brown precipitate indicates the presence of alkaloids.
Mayer’s test: To test for the presence of alkaloids, 2 ml of extract is mixed with 1 ml of Mayer’s reagent. The formation of a creamy white precipitate indicates the presence of alkaloids.
Test for phenols: To test for the presence of phenols, 2 ml of extract is mixed with 1 ml of FeCl3 solution. The appearance of a blue or green coloration indicates the presence of phenols.
Test for saponins: To test for the presence of saponins, 2 ml of extract is mixed with 1 ml of water. The formation of foam that persists for more than 10 min after vigorous shaking indicates the presence of saponins.
Test for lactones: To test for the presence of lactones, 2 ml of extract is mixed with a few drops of 10 % NaOH and 1 ml of 0.3 % nitroprusside sodium reagent. The appearance of a dark red coloration indicates the presence of lactones.
Baljet test: To test for the presence of lactones, 2 ml of extract is mixed with 2 ml of methanolic sodium picrate solution and 1 ml of NaOH. The formation of a light wine-red coloration indicates the presence of lactones.
Test for llipids: To test for the presence of lipids, 2 ml of extract is mixed with 2 ml of alcoholic Potassium hydroxide (KOH). The formation of soap indicates the presence of lipids.
Test for coumarins: To test for the presence of coumarins, 2 ml of extract is mixed with 10 % NH4OH solution. The appearance of intense fluorescence indicates the presence of coumarins (Table 1).
Sample preparation for HPTLC analysis:
After total removal of solvent, Chloroform extract of C. papaya, benzene extract of M. charantia and ethanolic extract of A. precatorius were weighed and individual loading samples were prepared by addition of a diluent i.e., chloroform:methanol (1:1) in the concentration of 10 mg/ml. For the prepared combination, the loading samples of the individual extracts were combined and were used as a loading sample for our combination.
Development of HPTLC chromatogram:
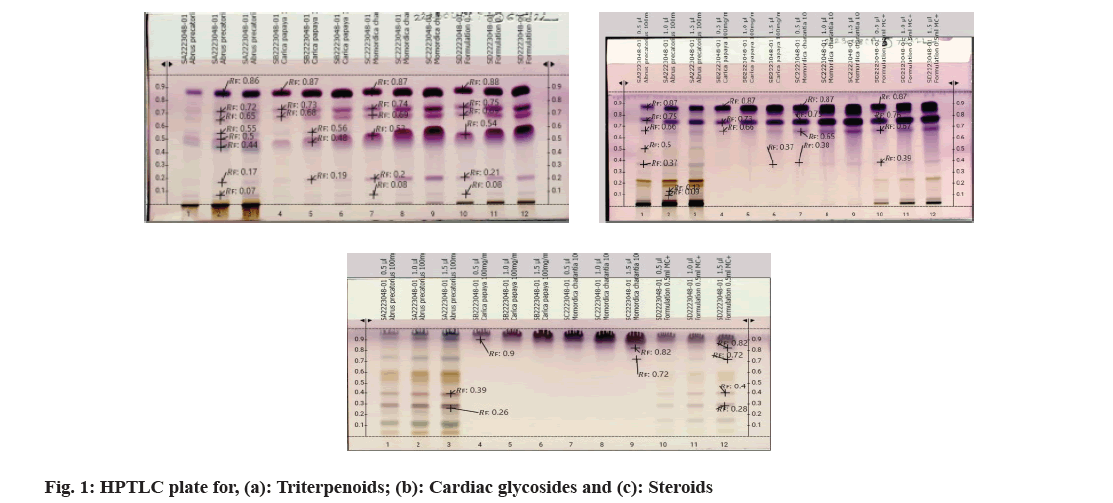
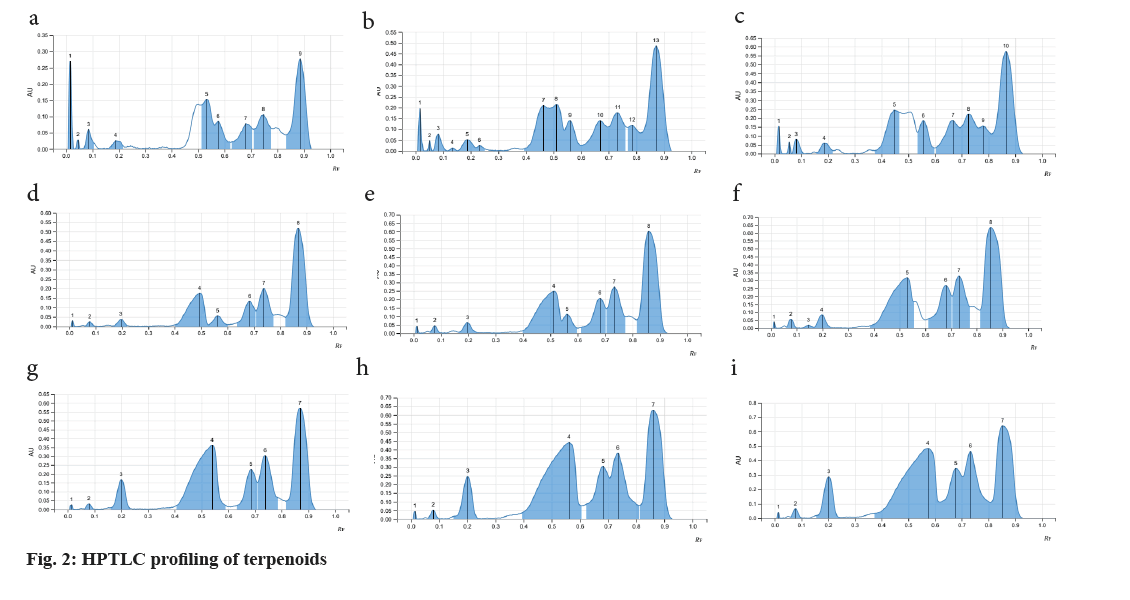
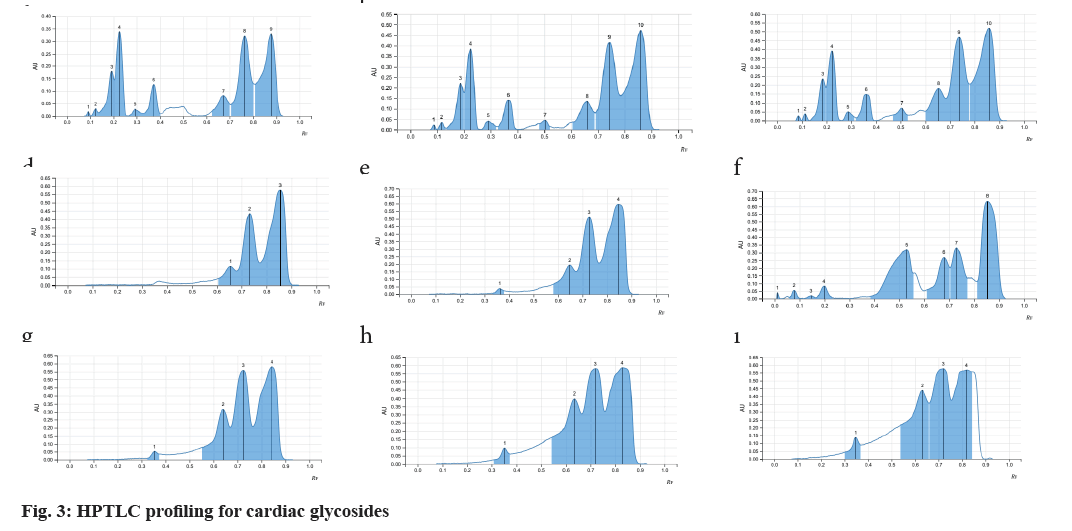
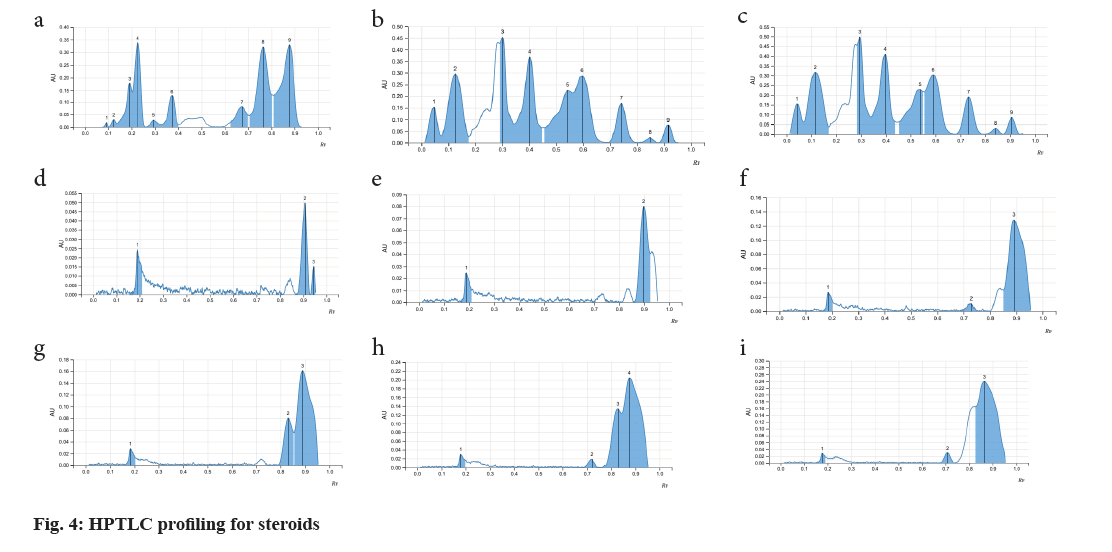
Fine particle size silica gel coated glass plate 5715 (20 cm×20 cm) or Thin-Layer Chromatography (TLC) silica gel 60 F254 aluminium sheets from MERCK™ were selected and were heated at 120° for 15-20 min for removal of adsorbed water if any. The samples were loaded onto the silica plates using CAMAG® automatic TLC sampler in varying concentrations. A CAMAG® Twin trough chamber (TTC) (20 cm×20 cm) was rinsed using methanol and dried completely. For efficient saturation of the chamber, a filter paper (Whatman No. 1) was kept inside the TTC, respective mobile phase was poured in and kept shut for 20 min, undisturbed. Complete saturation of the TTC was done and the loaded silica TLC plate was introduced into the TTC and the mobile phase was let to run up to 115mm. After confirming the proper chromatogram development in a CAMAG® TLC visualizer, spraying of appropriate derivatizing agent was carried out using piezoelectric spray of sample level 4 in CAMAG® derivatizer. The derivatized silica TLC plate was scanned in 254 nm, 366 nm and white light using CAMAG® TLC Scanner, which was attached to data recording software and Retention factor (Rf) values were recorded. The developed spots were viewed as peaks at wavelengths of selected Ultraviolet (UV) regions (fig. 1)[20].
Results and Discussion
As the seeds of C. papaya, M. charantia and A. precatorius are very promising in various pharmacological and clinical studies, a systematic analysis for determination of their standard pharmacognostic and phytochemical characters is of prime importance (Table 1). These characteristics are said to work through specific and non-specific mechanisms. However, extensive and in-depth research is required to evaluate the precise mechanism, active principles, and the safety profile of the plant as a remedy for different health conditions. The phytochemical constitution of the plant drugs is said to be responsible for therapeutic effects. These compounds are the primary and the secondary metabolites, resulting through the plant metabolism. Phytochemical constitution and their concentration differ not only from plant to plant, but also in different parts of the plant. So preliminary screening of phytochemicals in a given plant sample or powdered drug helps us get an idea of the various active constituents present in there and the possible therapeutic uses. Although many solvents were found to extract many of the phytoconstituents, the intensity of positive results and the ability to extract all the bioactive ones responsible for a particular activity varied (Table 2). Therefore, selection of solvent which shows the presence of target phytoconstituents like Cardiac Glycosides, Triterpenoids and Steroids along with higher percentages of extractive value is of prime importance. Complete removal of solvent from the prepared extractive is done to ensure the pharmacological activity is only a result of the phytoconstituents and not the solvent itself. Investigations like these also help us provide alternative sources of herbal drugs and thus reduce the commercial load on particular plant parts. Phytochemical analytical techniques like HPTLC are used for identifying a genuine drug amongst adulterated material and also confirming the presence of the pharmacologically active phytoconstituents. Animal trials carried out by various researchers on Pharmacological activities of the selected seed samples led to shortlisting of solvent used for extraction procedure. The three classes of compounds; cardiac glycosides, triterpenoids and steroids are considered to be active in inducing contraceptive effects in male rats. These compounds directly target either level of cholesterol and other reproductive hormones and metabolites which are responsible for maintaining normal reproductive functions. Thus, the use of HPTLC analysis for identification of class of compounds serve as important tools in determining the identity and purity of these drugs and also for developing reference phytochemical standards (fig. 2-fig. 4).
| No. | Test | C. papaya | M. charantia | A. precatorius | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| w.e. | e.e. | c.e. | p.e. | b.e. | h.e. | w.e. | e.e. | c.e. | p.e. | b.e. | h.e. | w.e. | e.e. | c.e. | p.e. | b.e. | h.e. | ||
| 1 | Test for alkaloids | ||||||||||||||||||
| Dragendroff’s reagent | - | + | - | - | - | - | - | + | - | - | + | - | + | - | + | + | + | - | |
| Wagner’s reagent | - | + | - | - | - | - | - | + | - | - | + | - | + | - | + | + | + | - | |
| Mayer’s reagent | - | + | - | - | - | - | - | + | - | - | + | - | + | - | + | - | - | - | |
| 2 | Test for proteins | ||||||||||||||||||
| Ninhydrin test | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | |
| Millon’s test | - | - | + | - | - | - | - | - | + | - | - | - | + | - | - | + | - | - | |
| Biuret’s test | - | - | + | - | - | - | - | - | + | - | - | - | + | - | - | - | - | - | |
| 3 | Test for tannins | ||||||||||||||||||
| Potassium dichromate test | + | - | - | - | - | - | + | - | + | + | - | - | - | - | - | - | - | - | |
| FeCl3 test | + | - | - | - | - | - | + | - | + | + | - | - | + | - | - | - | - | - | |
| 4 | Test for sugars | ||||||||||||||||||
| Benedict’s test | + | + | + | - | - | - | + | + | + | - | - | - | + | + | - | - | - | - | |
| Fehling’s test | + | + | + | - | - | - | + | + | + | - | - | - | + | + | - | - | - | - | |
| Mollish’s test | + | + | + | + | + | - | + | + | + | + | + | - | + | + | - | - | - | - | |
| 5 | Test for steroids | ||||||||||||||||||
| Salkowski’s test | - | - | + | + | + | + | - | + | + | + | + | + | - | - | - | + | + | + | |
| Liebermann Burchard’s test | - | - | + | + | + | + | - | + | + | + | + | + | - | - | - | + | + | + | |
| 6 | Test for flavonoids | ||||||||||||||||||
| Lead acetate test | - | - | - | - | - | - | - | - | - | - | - | - | + | + | - | - | - | - | |
| 7 | Test for glycosides | ||||||||||||||||||
| NaOH reagent | - | - | + | + | + | + | - | + | + | - | + | + | + | + | - | - | - | - | |
| Kellar Killani’s test | - | - | + | - | - | - | - | - | + | - | + | + | + | + | + | - | - | - | |
| 8 | Test for quinones | ||||||||||||||||||
| Conc. H2SO4 | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | + | + | + | |
| 9 | Test for terpenoids | ||||||||||||||||||
| Tin and thionyl chloride | - | - | - | + | + | - | - | - | - | + | + | - | - | - | + | - | - | - | |
| 10 | Test for diterpenes | ||||||||||||||||||
| Copper acetate test | - | - | - | - | + | - | - | - | - | - | + | - | + | + | - | - | - | + | |
| 11 | Test for saponins | ||||||||||||||||||
| Foam test | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 12 | Test for triterpenoids | ||||||||||||||||||
| Salkowski’s test | - | - | + | + | + | + | - | + | + | + | + | + | - | + | - | - | + | - | |
| Liebermann Burchard’s test | - | - | + | + | + | + | - | + | + | + | + | + | - | - | - | + | + | + | |
| 13 | Test for lipids | ||||||||||||||||||
| Alcoholic KOH test | - | + | + | + | + | + | - | + | + | + | + | + | - | + | + | + | + | + | |
| 14 | Test for coumarins | ||||||||||||||||||
| 10 % NH4OH | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
Note: (+): Present; (-): for absent; w.e.: Aqueous extract, e.e.: Ethanolic extract, c.e.: Chloroform extract, p.e.: Petroleum ether extract, b.e: Benzene extract, h.e: Hexane extract
Table 1: preliminary phytochemical screening for C. Papaya, M. Charantia and A. Precatorius extracts
| S No. | Name of the sample | Rf values | colour | Intensity |
|---|---|---|---|---|
| Terpenoids | ||||
| 1 | Abrus precatorius | 0.07 | Pale violet | Low |
| 0.17 | Pale violet | Low | ||
| 0.44 | blue | Medium | ||
| 0.5 | Pink violet | Medium | ||
| 0.55 | Pale violet | Low | ||
| 0.65 | Pale violet | Low | ||
| 0.72 | violet | Medium | ||
| 0.86 | purple | High | ||
| 2 | Carica papaya | 0.19 | Pale violet | Low |
| 0.48 | purple | High | ||
| 0.56 | Pale violet | Low | ||
| 0.68 | violet | Medium | ||
| 0.73 | violet | Medium | ||
| 0.87 | purple | High | ||
| 3 | Momordica charantia | 0.08 | Pale violet | Low |
| 0.2 | Pink violet | Medium | ||
| 0.53 | violet | Medium | ||
| 0.69 | violet | Medium | ||
| 0.74 | violet | Medium | ||
| 0.87 | purple | High | ||
| Cardiac glycosides | ||||
| 1 | Abrus precatorius | 0.09 | Pale violet | Low |
| 0.13 | Pale violet | Low | ||
| 0.37 | Blue | Medium | ||
| 0.5 | Pale green | Low | ||
| 0.66 | Pale violet | Low | ||
| 0.75 | purple | High | ||
| 0.87 | purple | High | ||
| 2 | Carica papaya | 0.66 | Pale violet | Low |
| 0.73 | Purple | High | ||
| 0.87 | purple | High | ||
| 0.37 | Pale violet | Low | ||
| 3 | Momordica charantia | 0.37 | Pale violet | Low |
| 0.65 | Purple violet | Medium | ||
| 0.75 | purple | High | ||
| 0.87 | purple | High | ||
| Steroids | ||||
| 1 | Abrus precatorius | 0.26 | Pink violet | Medium |
| 0.39 | Pink violet | Medium | ||
| 2 | Carica papaya | 0.9 | Violet | Low |
| 3 | Momordica charantia | 0.72 | Violet | Low |
| 0.82 | Violet | Medium |
Note: Triterpenoids: Mobile phase: 25 ml ethyl acetate:methanol:water v/v/v (100:13.5:10); Observation: Dark blue, blue, blue green or yellow-green fluorescent zones; Cardiac Glycosides: Mobile phase: 20 ml n-Hexane:ethyl acetate v/v (1:1); Observation: Blue-violet, red, red-violet, blue fluorescent zones; Steroids: Mobile phase: 20 ml n-Butanol:Methanol:Water v/v/v (3:1:1) and Observation: Dark Blue, Blue, Blue green or Yellow-green Fluorescent zones
Table 2: Rf values of Triterpenoids , Cardiac glycosides and steroids in extracts
Acknowledgement:
The authors would like to express their gratitude to the Blatter Herbarium, St. Xavier’s College, Mumbai for authentication of the plant samples and also Anchrome Enterprise Ltd., Mulund for assisting in the HPTLC analysis of the sample extracts.
Conflict of interests:
Authors declare that there is no conflict of interest.
References
- Dembitsky VM. In silico prediction of steroids and triterpenoids as potential regulators of lipid metabolism. Mar Drugs 2021;19(11):650.
[Crossref] [Google Scholar] [PubMed]
- Oyedeji KO, Abidoye AO, Shallangwa MM, Obisesan A. Effect of digoxin on reproductive parameters in male Wistar rats. J Pharm Sci 2020;12(9):1242-6.
- Akinmoladun AC, Olaleye MT, Farombi EO. Cardiotoxicity and cardioprotective effects of African medicinal plants. InToxicological survey of African Medicinal plants. Elsevier; 2014 p. 395-421.
- Soto-Blanco B. Herbal glycosides in healthcare. Herbal biomolecules in healthcare applications. Academic Press 2022. p. 239-82.
- Cárdenas PD, Almeida A, Bak S. Evolution of structural diversity of triterpenoids. Front Plant Sci 2019;10:1523.
[Crossref] [Google Scholar] [PubMed]
- Bishayee A, Ahmed S, Brankov N, Perloff M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front Biosci 2011;16:980.
[Crossref] [Google Scholar] [PubMed]
- Rajasekaran M, Bapna JS, Lakshmanan S, Nair AR, Veliath AJ, Panchanadam M. Antifertility effect in male rats of oleanolic acid, a triterpene from Eugenia jambolana flowers. J Ethnopharmacol 1988;24(1):115-21.
[Crossref] [Google Scholar] [PubMed]
- Kumar M. Triterpenoids of Nerium oleander shows antifertility effect in male albino rats. J Adv Lab Res Biol 2019;10(4):104-10.
- Bose BC, Saifi AQ, Vijayvargiya R, Bhagwat AW. Pharmacological study of Carica papaya seeds with special reference to its anthel-mintic action. Indian J Med Sci 1961;15(1):888-92.
[Google Scholar] [PubMed]
- Katiyar D, Singh V, Ali M. Phytochemical and pharmacological profile of Momordica charantia: A review. Biochem Ther Med Plants 2017;12(2):48-50.
- Aswin RK, Tridiganita IS, Arif NM, Gavrila AP, Dina DA, Gabrielle AV. Abrus precatorius: A comprehensive insight into the phytochemical, pharmacological, therapeutic activities and safety. J Drug Deliv Ther 2022;12(1):151-7.
- Brain K, Turner R. Practical evaluation of phytopharmaceuticals. Wright Scientechnica Bristol 1975.
- Farnsworth NR. Biological and phytochemical screening of plants. J Pharm Sci 1966;55(3):225-76.
[Crossref] [Google Scholar] [PubMed]
- Harborne JB. Phytochemical methods, Chapman and Hall, International Edition, Toppan Company Ltd., Japan. 1973.
- Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy, Nirali Prakashan. p. 2005:7-4.
- Paech K, Trace MV. Modern methods of plant analysis. Springer verlag, Berlin. 1955;3(1):477-87.
- Paech K, Trace MV. Modern methods of plant analysis. Heidelberg, Springer verlag, Berlin. 1955;3(2):275-80.
- Paech K, Trace MV. Modern methods of plant analysis. Heidelberg, Springer verlag, Berlin. 1955;3(3):715-6.
- Ramstad E. Modern pharmacognosy. Blakiston division, McGrew-Hill Book Co., New York. 1959.
- Wagner H, Bladt S. Plant drug analysis: A thin layer chromatography atlas. Springer Science and Business Media. 1996.