- *Corresponding Author:
- Gino A Kurian
Vascular Biology lab, SASTRA Deemed University, Thanjavur, Tamilnadu 613401, India
E-mail: kurian@scbt.sastra.edu
| Date of Received | 08 August 2021 |
| Date of Revision | 14 September 2022 |
| Date of Acceptance | 02 June 2023 |
| Indian J Pharm Sci 2023;85(3):778-788 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Network pharmacological analysis can be an effective solution for the challenges in drug discovery. Many bioactive-target combinations have been experimentally studied. In the present study, the combination of Resveratrol, Lipoic acid and Coenzyme Q10, having a multifaceted target in cardiac cells was utilized to attenuate ischemia-reperfusion injury in statin-treated rats. Langendorff model of Ischemia-Reperfusion (30 min ischemia+60 min reperfusion) was used in the study where the efficacy of combination drug was compared between normal and statin-treated hearts. The combination drugs were administered as preconditioning agents, prophylactic and therapeutic agents. Cardiac biochemical parameters like haemodynamic, injury, oxidative stress and mitochondrial parameters were assessed. Ischemia Reperfusion imparted significantly higher adverse outcomes in statin-treated rat hearts as compared with the normal heart which was attributed to severe mitochondrial damage. Administration of combination drugs attenuated cardiac injury and mediated the physiological recovery effectively in all Ischemia-Reperfusion challenged experimental groups with varying degrees of efficiency. Preconditioning the isolated rat heart with a combination drug provides the least protection when compared with a therapeutic and prophylactic mode of treatments where the latter render higher protection. Improved mitochondrial activity and reduction in oxidative stress supports the protective ability of combination drug in all experimental groups.
Keywords
Mitochondria, ischemia-reperfusion, statin, combination therapy
Sedentary lifestyles and unhealthy food habits are known risk factors for Cardiovascular Disease (CVD), especially Ischemic Heart Disease (IHD), characterized by mitochondrial dysfunction as the key cellular player[1]. An efficient way of managing the IHD involves early perfusion of the ischemic myocardium using surgical procedures like Coronary Artery Bypass Grafting (CABG) or Percutaneous Coronary Intervention (PCI) procedures which may account for an unavoidable injury termed as Ischemia-Reperfusion (IR) injury. With many risk factors like hypertension, diabetes and atherosclerosis associated with IR pathology, the sensitivity of the heart to withstand the injury varies[2]. In fact, many patients with these co-morbidities are under specific therapeutic regimens that may have drug-induced heart vulnerability towards IR injury. According to American College of Cardiology/American Heart Association Guideline, 2019, statin administration is the first line of treatment for the prevention of atherosclerotic CVD in patients with elevated low-density lipoprotein cholesterol levels (≥190 mg/dl)[3]. Growing pieces of evidence from the literature confirms not only the beneficial effect of statin in decreasing hypercholesterolemia but also acknowledge its ability to reduce cardiac mortality, morbidity, and even the restenosis of patients undergoing PCI[4,5]. Different studies emphasize that statins are equally beneficial in both ischemic and non-IHD to reduce certain cardiovascular complications and thus, their consumption remains peaked as lipid regulators.
In a randomized clinical study by Khush et al.[6] showed that high-dose of atorvastatin (80 mg) is beneficial only to heart failure patients, confirming the usage of a high dose of statin in clinical setting. But another clinical observational study in 7924 hyperlipidemic patients receiving high-dosage statin therapy demonstrated mild to moderate muscular symptoms[7]. In fact, many studies have reported the negative effect of high doses of statin treatment on muscles and may be due to the direct or indirect interactions of drugs with biomolecules that may interfere with the statin metabolism[8]. In alignment with these observations few clinical studies had reported that 80 mg/kg of statin treatment can increase the risk of myopathy, coenzyme Q10 reduction and mitochondrial Deoxyribonucleic Acid (DNA)/nuclear DNA depletion in the skeletal muscles[7-10]. Even the lower dose of 40 mg/kg statin is reported to cause rabidomylosis, high muscle campesterol and increased CK levels[11]. A double-blind clinical trial by Lemos et al.[12] in 2265 patients with simvastatin with a dose of 40 mg for 1 mo and followed by 80 mg/d for 4 mo showed myopathy in a few patients[12].
The dark side of statin treatment at the molecular level is its potential to downregulate transcriptional co-activator peroxisome Proliferator activating receptor Gamma Coactivator 1 (PGC1α) that often imparts decreased mitochondrial biogenesis and its function[13]. Even though statin linked adverse effect is rare in patients, many medical problems like liver or kidney functional impairment, the onset of muscle disease and even diabetes mellitus are reported[14]. In the above cases, impaired mitochondrial function is a common feature and is due to a decrease in Coenzyme Q10 (CoQ10) levels. In agreement with this observation, a clinical study by Stringer et al.[15] and his co-workers had shown a depleted mitochondrial copy in statin-treated patients. In view of the importance of CoQ10 in mitochondrial function, the possible secondary adverse effect of statin reported includes impaired mitochondrial adenosine triphosphate synthesis, deranged fatty acid beta-oxidation, and altered mitochondrial membrane potential that affect the energetics and subsequent initiation of the apoptosis[13].
Various clinical, experimental and epidemiological studies support the role of natural products in the form of nutraceuticals that improve cardiovascular function by preserving mitochondrial functional activity[16]. The growing evidence from the literature suggests that several natural supplements have been used to improve the mitochondrial function either by increasing its number, repairing the damage, or augmenting the removal of damaged mitochondria, thereby maintaining the mitochondrial homeostasis[17]. Recent success in mitochondrial medicine in healthcare suggests that strengthening the mitochondrial network rather than increasing its biomass is an efficient approach to attain the ultimate aim for a healthy mitochondrion. A combination drug offers increased therapeutic efficacy and decreased toxicity is considered to be effective in diseases with multifaceted pathology like in myocardial ischemia reperfusion. Resveratrol modulates Sirtuin 1/3 to improve mitochondrial function and its biogenesis. Lipoic acid influences the ceramide balance of mitochondria and thereby improves the function. On the other hand, CoQ10 restore the electron flux through the redox complex proteins for its function. We hypothesized that the combination of these molecules with different mechanisms will repair/restore the compromised mitochondrial function by statin. In the present study, we evaluate the efficacy of combination drug (Resveratrol, lipoic acid and Coenzyme Q10 (RAC)) in arresting the deteriorating effect of statin on cardiac physiology by administrating the drug as a precondition, prophylactic and therapeutic agent.
Materials and Methods
Animals:
Male wistar rats (200-250 g) used in this study, were housed in the animal facility in polycarbonate cages with 12 h light/dark cycle with free access to water and standard rat chow before the experiment. All procedures during the investigations were following the guidelines approved by the Committee for Control and Supervision of Experiments on Animals (CPCSEA Approval No.468/SASTRA/IAEC/RPP), India.
Identification of mitochondrial-targeted small molecules for formulation:
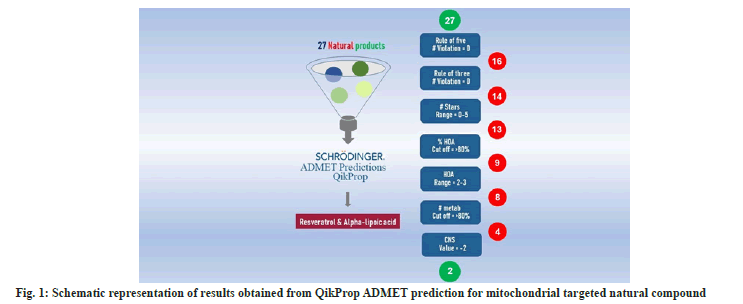
For the combination drug, the potential lead molecules which can modulate mitochondrial function (which plays an important role in oxidative stress, mitochondrial function, and biogenesis) were selected based on published data that were taken through systematic and thorough literature studies. From the identified 40 molecules the initial filtration was done based on the source of the lead molecules. 27 natural molecules were selected and synthetic molecules were eliminated for further studies.
Three-dimensional structural data of filtered leads drawn using ChemAxon MarvinSketch (V17) were used as the input for Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) calculation using Schrödinger Qikprop (Schrödinger, LLC, New York, NY, 2020) module. This process resulted in configurational averages for a number of descriptors resulting an accurate prediction of a molecule’s pharmacologically relevant properties (Table 1). From the experimentally obtained results, molecules were flagged if the value for a utilized descriptor exceeds the range for the experimental training set.
| S. No | Compound | Rule of five | Rule of three | # stars | HOA | % HOA | #metab | CNS |
|---|---|---|---|---|---|---|---|---|
| 1 | Ascorbic acid | 0 | 0 | 1 | 2 | 43.7 | 5 | -2 |
| 2 | Curcumin | 0 | 0 | 0 | 2 | 82.4 | 5 | -2 |
| 3 | Epigallocatechin 3 gallate | 2 | 2 | 5 | 1 | 0.19 | 10 | -2 |
| 4 | Estetrol | 0 | 0 | 0 | 3 | 76.8 | 3 | -2 |
| 5 | Estradiol | 0 | 0 | 0 | 3 | 100 | 6 | -2 |
| 6 | Estriol | 0 | 0 | 0 | 3 | 88.5 | 4 | -2 |
| 7 | Estrone | 0 | 0 | 0 | 3 | 100 | 5 | -2 |
| 8 | Glutathione | 1 | 2 | 2 | 1 | 0 | 7 | -2 |
| 9 | Hydroxytyrosol | 0 | 0 | 0 | 2 | 74.6 | 4 | -1 |
| 10 | Isoflavone | 0 | 0 | 1 | 3 | 100 | 0 | 1 |
| 11 | L Carnitine | 1 | 1 | 2 | 2 | 66 | 3 | -1 |
| 12 | α lipoic Acid | 0 | 0 | 1 | 3 | 84.4 | 1 | -2 |
| 13 | Melatonin | 0 | 0 | 0 | 3 | 92.9 | 2 | 0 |
| 14 | Metformin | 0 | 0 | 1 | 2 | 62.4 | 1 | -2 |
| 15 | Mitochonic acid 5 | 0 | 0 | 0 | 3 | 85.1 | 2 | -1 |
| 16 | Nicotinamide riboside | 1 | 1 | 2 | 2 | 60 | 3 | -1 |
| 17 | Nitric oxide | 0 | 0 | 9 | 2 | 65.1 | 0 | -1 |
| 18 | PQQ | 0 | 1 | 4 | 1 | 0 | 0 | -2 |
| 19 | Quercetin | 0 | 1 | 0 | 2 | 51.8 | 5 | -2 |
| 20 | Resveratrol | 0 | 0 | 0 | 3 | 82.3 | 3 | -2 |
| 21 | Sirolimus | 3 | 1 | 12 | 1 | 66.3 | 13 | -2 |
| 22 | Thyroxine | 1 | 1 | 2 | 2 | 44.7 | 5 | -1 |
| 23 | α-tocopherol | 1 | 1 | 6 | 1 | 100 | 5 | 0 |
| 24 | Tocotrienol | 1 | 2 | 4 | 2 | 64 | 3 | -2 |
| 25 | Triiodothyronine | 1 | 1 | 1 | 2 | 39.5 | 5 | -1 |
| 26 | Triterpenoid | 2 | 2 | 1 | 1 | 42 | 3 | -2 |
| 27 | Flavones | 0 | 0 | 1 | 3 | 100 | 0 | 1 |
Note: Property and recommended values as follows; Rule of five: Maximum 4; Rule of three: maximum 3; #stars: 0-5; Human Oral Absorption (HOA): –1.5-1.5; Percent Human-Oral Absorption (% HOA): >80 % is high <25 % is poor; #metab: 1-8; CNS: -2 (inactive) and 2 (active)
Table 1: Pharmacological relevant molecules and descriptor ranges from QikProp ADME properties.
Experimental groups:
Isolated rat heart protocol was conducted as previously reported using the Lagendorff perfusion system[18]. Rats were randomly assigned into nine groups (n=6). Normal-Rat hearts were isolated and perfused with Krebs-Henseleit (KH) buffer in a Langendorff set-up for 110 min; IR-Normal hearts after 20 min of equilibration subjected to 30 min of global ischemia followed by 60 min of reperfusion; RAC IR the normal heart perfused with the combination drug (RAC) for 10 mins, after 10 min equilibration before the 30 mins of global ischemia and reperfused for 60 mins; Statin Control (S cntrl)-Simvastatin 80 mg/kg pre-treated orally for 14 d, and the heart were isolated at the end of the study subjected, to normal perfusion for 2 h; Statin IR (S IR)- Statin treatment for 14 d followed by subjecting the isolated heart to IR; Statin RAC IR (S RAC IR)-After giving the pre-treatment with statin as described before, the heart is isolated and perfused with RAC in KH buffer for 10 min before subjecting to IR; RAC- rats were fed with RAC combination orally for 14 alternative d and the isolated heart at the end of the study was subjected to normal perfusion for 2 h; RAC Therapeutic (RAC T)- the combination drug RAC was given orally for 14 alternative d after the 14 d of statin induction and the isolated rat hearts were subjected to IR protocol; RAC Prophylactic (RAC P)- treatment of RAC for 14 alternative d along with the 14 d of statin treatment and the isolated rat hearts subjected to IR protocol.
Preparation of RAC:
Drugs were prepared in ethanol/deionised water. For isolated rat heart experiments, drugs were diluted to a final concentration using KH buffer immediately before administration. For oral administration, the final dilutions were made in Deionized water (suspension) and administered to the animal orally. The ethanol concentration infused into the heart was 0.025 % (v/v) which failed to affect the mechanical function of the heart. Resveratrol and Coenzyme Q10 were used as reported previously (10 µM and 20 µM respectively) and the optimal concentration of alpha-lipoic acid was determined experimentally.
The Heart Rate (HR), Left Ventricular End-Diastolic Pressure (LVEDP), Left Ventricular Systolic Pressure (LVSP), Left Ventricular Developed Pressure (LVDP), Rate Pressure Product (RPP=LVDP×HR) and rates of contractility (±dP/dt) were recorded and analysed by data acquisition system (PowerLab; ADInstruments, Australia).
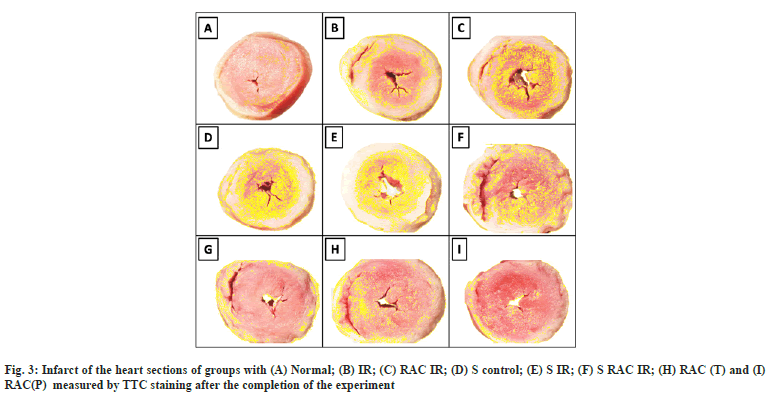
Infarct size assessment by Triphenyl Tetrazolium Chloride (TTC) staining:
Cardiac injury assessment was carried out by TTC Staining method as described elsewhere[19]. Heart sections of 1 mm thickness were made and incubated in a 1.5 % TTC solution for 20 mins at 37°. The TTC negative region (Infarct) was quantified using the Image J analysis tool (NIH-USA) and the images were acquired through a zoom stereo microscope (NIKON-SMZ1270) equipped with a high definition CCD camera (NIKON-DS-Fi2). The percentage infarcted area was calculated by the following equation
Percentage infarcted area=[(Total area-area of infarction)/Total area]×100
Mitochondrial isolation:
Cardiac mitochondria from rat tissue were isolated using differential centrifugation as reported by Bonifacino with minor modifications[20]. The tissue homogenates were prepared in Tris buffer (pH-7.4) and centrifuged at 600 g force for 10 mins maintaining 4°. Later, the supernatant was transferred and centrifuged at 8000 g (4°) for 10 mins and the resulting pellet was spun to 12 000 g (4°) for 10 min to obtain mitochondrial pellet and resuspended in storage buffer (pH-7.4). Protein estimation was done using the BioRad kit method using the instructions provided.
Mitochondrial enzyme activities:
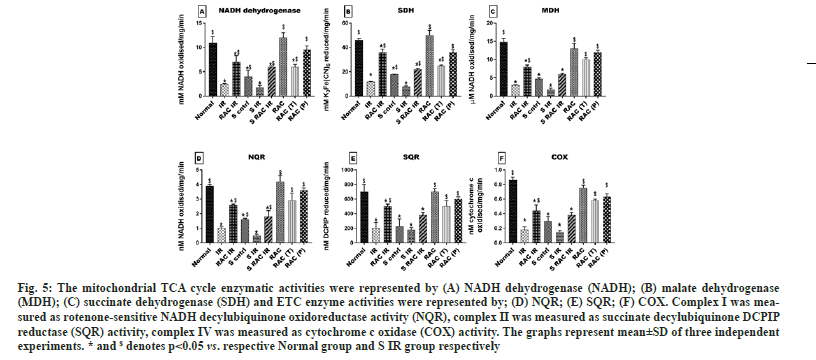
TCA cycle and ETC activity: TCA cycle enzymes such as Malate Dehydrogenase (MDH), Succinate Dehydrogenase (SDH) and Nicotinamide Adenine Dinucleotide (NADH) dehydrogenase were analysed using the previously mentioned methods[21-23]. Mitochondrial Electron Transport Chain (ETC) enzyme activities were measured spectrophotometrically by using specific donor–acceptors. Rotenone sensitive NADH decylubiquinone oxidoreductase (NQR) was used to assess the complex I activity; succinate decylubiquinone DCPIP reductase (SQR) to assess the complex II and cytochrome oxidase (COX) to assess complex IV were measured as per previously described protocols[24].
Mitochondrial antioxidant enzymes and lipid peroxidation:
The antioxidant levels in isolated mitochondria such as reduced Glutathione (GSH), Glutathione Peroxidase (GPx), Glutathione Reductase (GR), and Superoxide Dismutase (SOD) were estimated by the previously reported methods with slight modifications[25,26] respectively. The lipid peroxidation product MDA level, in left ventricular tissue, was evaluated by measuring thiobarbituric acid-reactive substances level as previously reported[27].
Statistical analysis:
All data were reported as mean±SD. Group comparisons were done using one-way Analysis of Variance (ANOVA) with Tukey’s pairwise post-hoc test when appropriate. The hemodynamic performance was analyzed using repeated measure ANOVA. Calculations were performed using GraphPad Prism (GraphPad Software, CA, USA). Statistical significance was denoted when p<0.05.To narrow down the compounds for combination drug and to avoid any unnecessary testing of compounds that will fail later on in in vivo studies we used ADMET prediction. QikProp predicted ADMET descriptors of the 28 natural compounds were depicted in Table 1. The hierarchal filtration criteria followed in this study were depicted in fig. 1. We have used Lipinski’s rule of five as the first level of filtration. Among the selected 27 natural compounds, 16 compounds showed zero violations and were chosen for the next level. Out of the 16 compounds, 14 compounds obeyed the Rule of three with zero violation. QikProp #stars represent the number of property or descriptor values that fall outside the 95 % range of similar values for known drugs. A range of molecular descriptors are used by QikProp for quantifying the value for #stars (MW, dipole, IP, EA, SASA, FOSA, FISA, PISA, WPSA, PSA, volume, #rotor, donorHB, accptHB, glob, QPpolrz, QPlogPC16, QPlogPoct, QPlogPw, QPlogPo/w, logS, QPLogKhsa, QPlogBB, #metabol). Out of 14 molecules, except one, all other molecules were in the range between 0-5 and were chosen for the next level of filtration. Values for Human Oral Absorption (HOA) were determined and among the 13 molecules, 9 molecules that had the values in the acceptable range were chosen. The recommended % HOA value is >80 % for the highest rate of absorption and out of 9, 8 compounds had the value >80 %. Metabolism (#metab) is another important pharmacokinetic parameter. Metabolism is required for drug molecules as it influences the elimination and excretion, but it should not be too rapid. The number of likely metabolic reactions of the compounds should be in the range of 1-8 and we set the cut off as 3 so that the molecules will not undergo rapid metabolism and out of 8, 4 compounds satisfied this criteria. Further, a potential drug molecule should specifically reach the drug’s site of action to avoid any off-target effect. In specific, the drug molecules should not affect the Central Nervous System (CNS). The predicted CNS value should be -2 for inactive and +2 for active. Out of the 4 compounds, only 2 have shown inactive for CNS. Hence resveratrol and ALA were considered for formulation. Depleted CoQ10 level in plasma is one of the characteristic features of statin toxicity and the present study also observed the same. Hence to enhance the efficacy of the combination drug, we added CoQ10 as the third component of the drug combination that substantiates the therapeutic rationale for specific drug development for statin toxicity. The pre-combination study was carried out before the commencement of combination drug administration in rats.
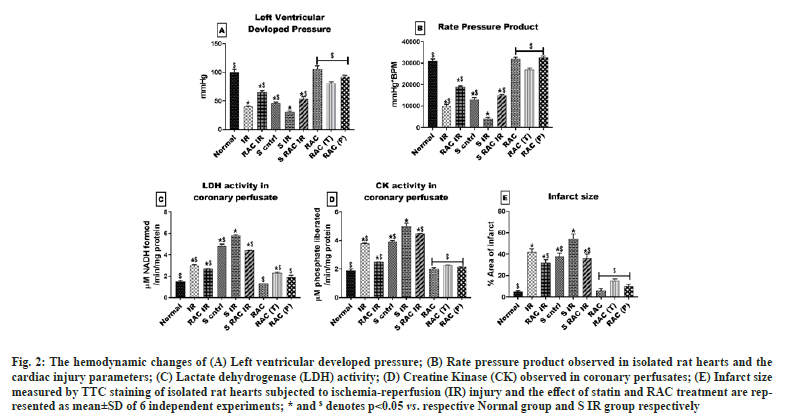
The myocardial protective effect of RAC was evaluated initially by using hemodynamic parameters in the heart after conditioning with RAC. Hemodynamic parameters (LVDP and RPP) have progressively deteriorated over 60 min of reperfusion in statin-treated rat hearts (LVDP 52 %, RPP 58 %) when compared with a normal rat heart. Similarly, a remarkable decrease in the hemodynamics was observed in the IR group of both normal and statin-treated animals (fig. 2A and fig. 2B). However, RAC treatment of isolated rat heart improved the hemodynamics, where the prominent recovery was observed lower in statin-treated rat heart (LVDP 25 %, RPP 11 %) compared with normal rat heart (fig. 2A and fig. 2B). Additionally, we evaluated the myocardial injury using TTC staining. The myocardial infarct size was markedly decreased by treatment with RAC when compared with the IR group in normal (fig. 3), but not as effective as in statin-treated rat heart.
Fig. 2: The hemodynamic changes of (A) Left ventricular developed pressure; (B) Rate pressure product observed in isolated rat hearts and the cardiac injury parameters; (C) Lactate dehydrogenase (LDH) activity; (D) Creatine Kinase (CK) observed in coronary perfusates; (E) Infarct size measured by TTC staining of isolated rat hearts subjected to ischemia-reperfusion (IR) injury and the effect of statin and RAC treatment are represented as mean±SD of 6 independent experiments; * and $ denotes p<0.05 vs. respective Normal group and S IR group respectively.
Once we identified the short-term effect of RAC in an isolated rat heart model, we further measured the long-term impact of the drug by pre-treating the RAC as a prophylactic RAC (P) and therapeutic RAC (T) agent for 10 d. Hemodynamics changes suggest a significant recovery from IR induced alteration in RAC prophylactically administered rat heart than therapeutic treated group. In agreement with these findings, a significant reduction in cardiac injury was observed with rat hearts from the prophylactic group when compared with the therapeutic group (fig. 2). However, improvement in hemodynamic and reduced cardiac injury exhibited by RAC pre-treated rat hearts (prophylactic and therapeutic) from respective IR control was higher than when RAC was administered to the isolated rat heart via KH buffer (fig. 2).
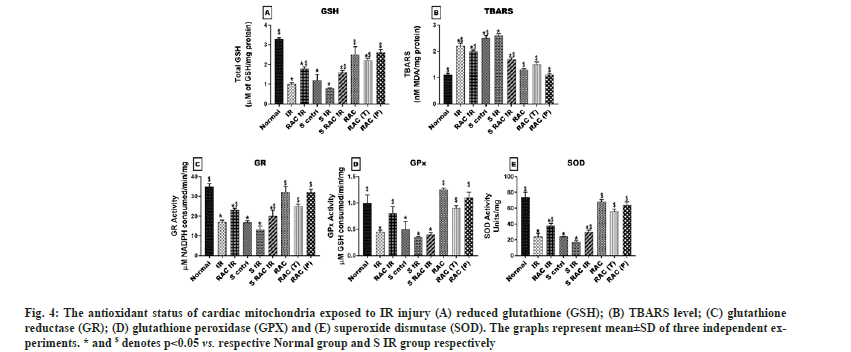
The oxidative stress and mitochondrial dysfunction are the key mediators for IR pathology. Fig. 4 provides the lipid peroxidation and corresponding antioxidant data in rat hearts that underwent different experiments. The baseline oxidative stress experienced by the rat heart treated with statin (measured via increased TBARS 56 % and decreased GSH 62.3 %) was significantly high when compared with normal rat heart. Upon re-perfusion, myocardial tissue from statin-treated animals exhibited high MDA levels (59 %) than normal (50.5 %). A corresponding decline in reduced glutathione content from normal (66.1 %), and statin-treated (73.3 %) myocardium confirms the cellular damage due to ROS. The efficacy of the drug RAC was evaluated in isolated rat hearts for its immediate effect and long-term effect. Administration of RAC to the isolated rat heart via KH buffer reduced the oxidative stress imparted by IR, significantly in both normal (TBARS 25 % and GSH 50 %) and statin (TBARS 11 % and GSH 45 %) treated rat heart from IR control heart. Further, we evaluated the long-term effect of RAC on IR induced oxidative stress by administrating the drug as therapeutic and prophylactic treatment for 14 d. Therapeutic administration of RAC significantly reduced the IR induced oxidative stress and the values were close to the RAC control (TBARS 1.4 ~ 1.235 nM/mg, GSH 2.1~2.6 µM/mg). In fact, higher efficacy of the drug was observed when it was given as a prophylactic agent. TBARS and GSH levels were significantly declined from the IR group and the respective levels were near normal values (TBARS 1.125~1.115 nM/mg, GSH 2.6~3.25 µM/mg).
Fig. 4: The antioxidant status of cardiac mitochondria exposed to IR injury (A) reduced glutathione (GSH); (B) TBARS level; (C) glutathione reductase (GR); (D) glutathione peroxidase (GPX) and (E) superoxide dismutase (SOD). The graphs represent mean±SD of three independent experiments. * and $ denotes p<0.05 vs. respective Normal group and S IR group respectively.
The cellular antioxidant defence network comprises of catalase, GPx, GR and SOD enzymes were found to be impaired in both statin-treated (SOD-77.6 %, GPx-65 %, and GR-75.5 %) heart and normal (SOD-66 %, GPx-52 %, and GR-63 %) heart, when the heart was subjected to IR. Recovery of the antioxidant enzymes by RAC combination was evident and found to be more significant with both prophylactic and therapeutic treatment compared to the RAC in the isolated heart model.
The figure shows the baseline changes in the mitochondrial TCA cycle (fig. 5A-fig. 5C) and ETC activity (fig. 5D-fig. 5F) elucidating the decline in statin-treated hearts (MDH 68 %, SDH 68 % and NADH 63 %) (NQR 60 % SQR 62 %, and COX 56 %) corresponding to the normal level. Induction of IR in both untreated and statin-treated animals has resulted in reduced activity of (MDH 85 % SDH 70 %, and NADH 73 %; NQR 72 % SQR 66 %, and COX 72 %) and (MDH 90 % SDH 88%, and NADH 84%; NQR 84% SQR 75%, and COX 81%) respectively with normal level. However, short-time supplementation of RAC via KH buffer was effective in improvising the mitochondrial function in normal animals (RAC IR) that was not observed in statin-treated animals (S RAC IR) (Except TCA enzymes). Further, administrating RAC as therapeutic and prophylactic treatment did enhance the ETC enzymes and TCA cycle enzymes to significant normal levels. Similar to the previous findings prophylactic mode of treatment proves to be better in efficacy and enhanced the enzyme levels from IR (NQR 70 % SQR 63 % COX 72 %) (MDH 84 % SDH 62.3 % NADH 70 %).
Fig. 5: The mitochondrial TCA cycle enzymatic activities were represented by (A) NADH dehydrogenase (NADH); (B) malate dehydrogenase (MDH); (C) succinate dehydrogenase (SDH) and ETC enzyme activities were represented by; (D) NQR; (E) SQR; (F) COX. Complex I was measured as rotenone-sensitive NADH decylubiquinone oxidoreductase activity (NQR), complex II was measured as succinate decylubiquinone DCPIP reductase (SQR) activity, complex IV was measured as cytochrome c oxidase (COX) activity. The graphs represent mean±SD of three independent experiments. * and $ denotes p<0.05 vs. respective Normal group and S IR group respectively.
Myocardial IR injury is an unavoidable injury occurring in the myocardium during the revascularization procedures. Identifying an effective therapeutic agent that can prevent IR pathology is a great challenge due to its multifactorial reasons and the co-existence of anomalies associated with lifestyle diseases or side effects of drugs consumed[28]. Importantly, the epicentre for IR associated pathological trigger and effector site is confirmed in mitochondria[29]. Even though many monotherapy approaches are found to be successful in preclinical studies, they were not effectively translated to the clinical side. Combination therapy is a promising approach utilized by different investigators to tackle diseases having multifactorial pathological targets, like myocardial IR injury[30,31]. In the present study, we utilized a combination drug (containing Resveratrol, CoQ10, and ALA) to attenuate the IR associated cardiac injury in isolated rat hearts. According to our data, the combination drug, RAC was effective in preventing IR induced myocardial damage in normal rat hearts but its efficacy was low in statin-treated rat hearts. But, when RAC was given as a prophylactic or therapeutic agent for 14 d, the cardioprotection was augmented in statin-treated rat hearts from the IR challenge significantly. Moreover, the RAC could reverse the statin mediated cardiac mitochondrial alterations effectively only with prophylactic and therapeutic treatment, explaining the possible underlying mechanism in the management of statin-treated rat heart to withstand IR injury.
The major advantage of combination therapy is that it can increase the efficiency of the single-pill by acting as a synergetic agent[32]. In fact, it can mitigate the adverse effect of the treatment mainly by providing the feasibility of reducing the dose requirement of each component. The single pill concept was utilised in the management of IHD but without much success due to myotoxicity. A similar finding was reported with a neurological disorder and neurotoxicity was cited as a reason for its failure[33]. This prodrug concept was well studied in models of neurological disorders where it acts as a neuro-stimulant molecule without much success, due to neurotoxicity. Thus, in order to adopt the prodrug concept in cardiovascular pharmacology, direct cardiotoxicity needs to be evaluated. In this regard, we used an isolated rat heart model, an established experimental model to study cardio-toxicity in the absence of neurohormonal axis[34]. The combination drug, RAC comprises of resveratrol, CoQ10, and ALA was developed based on in silico screening. The selection criteria utilized was depended on those established molecules that can preserve the mitochondrial activity as the mitochondrial dysfunction acts as a critical player in IR pathology.
Resveratrol, one of the constituents of RAC, is a well-known polyphenol, reported to have mitochondrial functional modulation via sirtuin apart from having an antioxidant, anti-inflammatory, anti-apoptotic, and anticancer properties. Growing evidence from the literature showed that it can modulate mitochondrial dynamics, induce mitochondrial biogenesis, oxidative phosphorylation, and endogenous antioxidant defences[35]. ALA, the other constituent of RAC, is an essential cofactor for mitochondrial metabolism can increase mitochondrial biogenesis, modulate mitochondrial electron transport chain enzyme activity and can influence mitochondrial dynamics. It acts as a nutrient in improving age-associated mitochondrial and oxidative damage[36]. CoQ10, an important electron carrier of the mitochondrial respiratory chain is also reported to have antioxidant potential[37]. Evidence from the literature suggests that Resveratrol, ALA, and CoQ10 are individually reported to ameliorate myocardial IR injury. Many studies have shown that cardiomyocyte function and survival after IR depends on the quality of the cardiac mitochondria[38]. In the present study, cardiac mitochondrial function was compromised by the treatment of statin and expected to have deteriorated cardiac physiological recovery. Accordingly, we found higher infarct size in statin-treated rat hearts when subjected to IR. The previous study suggests that many successful preclinical drugs fail to render protection if the heart is already having some ailment. Thus, re-evaluating many preclinically proven drugs is under demand for a feasible clinical translation. Evidence from the literature suggests that resveratrol, ALA, and CoQ10 are individually reported to ameliorate myocardial IR injury.
Studies have shown that mitochondrial dysfunction is a critical contributor to the myocardial IR injury[29]. Hence, by subjecting the heart to IR, which has already compromised mitochondrial function (due to statin treatment) may have an adverse IR outcome. In fact, the prevalence of such situations at the bedside is not rare as the majority of the population are under statin regimen for the management of hypercholesteremia, one of the key risk factors of IHD, may undergo revascularization procedures. Tackling IR with suitable pharmacological agents effectively in the clinical side is yet to be accomplished and thus we believe that increasing tolerance to the myocardium to withstand IR injury will be a good option. In this study, we utilized RAC as a prophylactic, therapeutic, and preconditioning agent to improve the cardiac mitochondrial function, which was suppressed by statin treatment and thereby attenuated the IR injury. All modes of treatment do render cardioprotection against IR injury but with varying degrees of efficiency. Preconditioning the heart with RAC before global ischemia did not render prominent protections against IR in statin-treated rat hearts compared to the pre-treatment mode (prophylactic and therapeutic) of RAC administration. Unlike in normal hearts, statin-treated hearts did not show a prominent reversal of mitochondrial dysfunction. Additionally, the present study was conducted in the Langendorff perfusion system, where neurohormonal influence was absent and this model is best suited to study cardiac physiology in terms of contractility and its mechanisms. Administering RAC as a preconditioning agent, we evaluated the direct and immediate impact of RAC on heart challenges to IR. However, co-administration of RAC with statin for 14 d and treatment of RAC for 14 d after 14 d of statin regimen attenuated the IR injury effectively. The significant protection in RAC prophylactic and therapeutic approach against IR may be attributed to the rejuvenation of mitochondria from statin-induced damage, which may be limited or absent in RAC preconditioning. Furthermore, the combination drug will get systematically metabolized and the impact may be attributed to the metabolite or the tolerance induced to the myocardium to withstand IR injury. Thus, the combination drug RAC, apart from its established nutritive value, can also act as a functional food that has a potentially positive effect on health beyond basic nutrition. Moreover, many random control studies are reported in the literature with CoQ10 and resveratrol to claim it as a nutraceutical that can provide a beneficial effect in the management of CVD.
The combination of resveratrol, alpha-lipoic acid, and Coenzyme Q10 was more effective in attenuating myocardial IR injuries in rat heart co-exist with statin-induced alterations. Combination drugs given as prophylactic mode were more effective than therapeutic or as a preconditioning regimen. Mitochondrial rejuvenation or repair might be the underlying mechanism for the protection mediated by the combination drug. The combination was more effective than either single drug in a preconditional setting. RAC components being widely present in the food and food products, they may be used as a supplement or nutraceuticals before revascularization procedures or in statin regimens.
Acknowledgments:
We would like to acknowledge Mr. Karthi Shanmugam, Assistant Professor, Department of Bioinformatics, SASTRA deemed University for providing support with Schrodinger QikProp analysis for ADMET prediction. Priyanka N Prem was supported by a fellowship from Council of Scientific and Industrial Research [09/1095/(0040)/2018-EMR-1].
Author contributions:
Ramalingam C Vedarathinam and Yogeshwari Rajkumar shares equal contribution. Gino A kurian has done conceptualization, methodology, writing-original draft, writing-review and editing and validation. Ramalingam C Vedarathinam did conceptualization, methodology, formal analysis, investigation, writing-original draft, project administration and funding acquisition. Yogeshwari Rajkumar did conceptualization, methodology, formal analysis, investigation, writing-original draft, and funding acquisition. Priya Vetriselvan did formal analysis, investigation, visualisation and funding acquisition. Priyanka N prem did methodology, writing-review and editing and visualisation.
Conflict of interests:
The authors declared no potential conflicts of interest.
References
- Buttar HS, Li T, Ravi N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp Clin Cardiol 2005;10(4):229-49.
[Google Scholar] [PubMed]
- Mahalakshmi A, Kurian GA. Evaluating the impact of diabetes and diabetic cardiomyopathy rat heart on the outcome of ischemia-reperfusion associated oxidative stress. Free Radic Biology Med 2018;118:35-43.
[Crossref] [Google Scholar] [PubMed]
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11):e596-646.
[Crossref] [Google Scholar] [PubMed]
- Ma JM, Jackevicius CA, Genus U, Dzavik V. The use of lipid-lowering therapy for secondary prevention in patients undergoing percutaneous coronary intervention. Can J Cardiol 2006;22(5):419-23.
[Crossref] [Google Scholar] [PubMed]
- Wilt TJ, Bloomfield HE, MacDonald R, Nelson D, Rutks I, Ho M, et al. Effectiveness of statin therapy in adults with coronary heart disease. Archives of internal medicine. 2004 Jul 12;164(13):1427-36.
[Crossref] [Google Scholar] [PubMed]
- Khush KK, Waters DD, Bittner V, Deedwania PC, Kastelein JJ, Lewis SJ, et al. Effect of high-dose atorvastatin on hospitalizations for heart failure: Subgroup analysis of the Treating to New Targets (TNT) study. Circulation 2007;115(5):576-83.
[Crossref] [Google Scholar] [PubMed]
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005;19:403-14.
[Crossref] [Google Scholar] [PubMed]
- Hirota T, Ieiri I. Drug–drug interactions that interfere with statin metabolism. Expert Opin Drug Metabol Toxicol 2015;11(9):1435-47.
[Crossref] [Google Scholar] [PubMed]
- Manoj K, Jain N, Madhu SV. Myopathy in patients taking atorvastatin: A pilot study. Indian J Endocrinol Metab 2017;21(4):504-9.
[Crossref] [Google Scholar] [PubMed]
- Schick BA, Laaksonen R, Frohlich JJ, Päivä H, Lehtimäki T, Humphries KH, et al. Decreased skeletal muscle mitochondrial DNA in patients treated with high‐dose simvastatin. Clin Pharmacol Ther 2007;81(5):650-3.
[Crossref] [Google Scholar] [PubMed]
- Päivä H, Thelen KM, van Coster R, Smet J, de Paepe B, Mattila KM, et al. High‐dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther 2005;78(1):60-8.
[Crossref] [Google Scholar] [PubMed]
- de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase Z of the A to Z trial. JAMA 2004;292(11):1307-16.
[Crossref] [Google Scholar] [PubMed]
- Ramachandran R, Wierzbicki AS. Statins, muscle disease and mitochondria. J Clin Med 2017;6(8):75.
[Crossref] [Google Scholar] [PubMed]
- Magni P, Macchi C, Morlotti B, Sirtori CR, Ruscica M. Risk identification and possible countermeasures for muscle adverse effects during statin therapy. Eur J Intern Med 2015;26(2):82-8.
[Crossref] [Google Scholar] [PubMed]
- Stringer HA, Sohi GK, Maguire JA, Côté HC. Decreased skeletal muscle mitochondrial DNA in patients with statin-induced myopathy. J Neurol Sci 2013;325(1-2):142-7.
[Crossref] [Google Scholar] [PubMed]
- Mashour NH, Lin GI, Frishman WH. Herbal medicine for the treatment of cardiovascular disease: clinical considerations. Arch Intern Med 1998;158(20):2225-34.
[Crossref] [Google Scholar] [PubMed]
- Nicolson GL. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. Integr Med 2014;13(4):35.
[Google Scholar] [PubMed]
- Ravindran S, Boovarahan SR, Shanmugam K, Vedarathinam RC, Kurian GA. Sodium thiosulfate preconditioning ameliorates ischemia/reperfusion injury in rat hearts via reduction of oxidative stress and apoptosis. Cardiovasc Drug Ther 2017;31(5-6):511-24.
[Crossref] [Google Scholar] [PubMed]
- Liu F, Schafer DP, McCullough LD. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods 2009;179(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada KM. Isolation of mitochondria from tissues and cells by differential centrifugation. Curr Protoc Cell Biol 2007;3:1-3.
- Dancey GF, Shapiro BM. The NADH dehydrogenase of the respiratory chain of Escherichia coli. II. Kinetics of the purified enzyme and the effects of antibodies elicited against it on membrane-bound and free enzyme. J Biol Chem 1976;251(19):5921-8.
[Crossref] [Google Scholar] [PubMed]
- Frieden CA, Fernandez-Sousa JO. Kinetic studies on pig heart cytoplasmic malate dehydrogenase. J Biol Chem 1975;250(6):2106-13.
[Crossref] [Google Scholar] [PubMed]
- Slater EC, Bonner WD. The effect of fluoride on succinic oxidase system. Biochem J 1952;52(2):185.
[Crossref] [Google Scholar] [PubMed]
- Frazier AE, Thorburn DR. Biochemical analyses of the electron transport chain complexes by spectrophotometry. Methos Mol Biol 2012:49-62.
[Crossref] [Google Scholar] [PubMed]
- Nandi A, Chatterjee IB. Assay of superoxide dismutase activity in animal tissues. J Biosci 1988;13:305-15.
- Saydam N, Kirb A, Demir Ö, Hazan E, Oto Ö, Saydam O, et al. Determination of glutathione, glutathione reductase, glutathione peroxidase and glutathione S-transferase levels in human lung cancer tissues. Cancer Lett 1997;119(1):13-9.
[Crossref] [Google Scholar] [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
[Crossref] [Google Scholar] [PubMed]
- Schwartz SM, Schwartz HT, Horvath S, Schadt E, Lee SI. A systematic approach to multifactorial cardiovascular disease: Causal analysis. Arterioscler Thromb Vasc Biol 2012;32(12):2821-35.
[Crossref] [Google Scholar] [PubMed]
- Di Lisa F, Bernardi P. Mitochondria and ischemia–reperfusion injury of the heart: Fixing a hole. Cardiovasc Res 2006;70(2):191-9.
[Crossref] [Google Scholar] [PubMed]
- Davidson SM, Ferdinandy P, Andreadou I, Bøtker HE, Heusch G, Ibáñez B, et al. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol 2019;73(1):89-99.
[Crossref] [Google Scholar] [PubMed]
- de Cates AN, Farr MR, Wright N, Jarvis MC, Rees K, Ebrahim S, et al. Fixed‐dose combination therapy for the prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;16(4):CD009868.
[Crossref] [Google Scholar] [PubMed]
- Sonam KS, Guleria S. Synergistic antioxidant activity of natural products. Ann Pharmacol Pharm 2017;2(8):1-6.
- Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, et al. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J 2017;38(13):935-41.
[Crossref] [Google Scholar] [PubMed]
- Lateef R, Al-Masri A, Alyahya A. Langendorff’s isolated perfused rat heart technique: A review. Int J Basic Clin Pharmacol 2015;4(10.18203):2319-003.
- Ferretta A, Gaballo A, Tanzarella P, Piccoli C, Capitanio N, Nico B, et al. Effect of resveratrol on mitochondrial function: implications in parkin-associated familiar Parkinson's disease. Biochim Biophys Acta 2014;1842(7):902-15.
[Crossref] [Google Scholar] [PubMed]
- Liu J. The effects and mechanisms of mitochondrial nutrient α-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: An overview. Neurochem Res 2008;33:194-203.
[Crossref] [Google Scholar] [PubMed]
- Tian G, Sawashita J, Kubo H, Nishio SY, Hashimoto S, Suzuki N, et al. Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxid Redox Signal 2014;20(16):2606-20.
[Crossref] [Google Scholar] [PubMed]
- Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol 2016;13(4):193-209.
[Crossref] [Google Scholar] [PubMed]