- *Corresponding Author:
- M. Gandhimathi
Dept. of Pharmaceutical Analysis, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, 395, Sarojini Naidu Street, Coimbatore-641 044, India
E-mail: gands72@yahoo.co.in
| Date of Submission | 8 November 2005 |
| Date of Revision | 22 June 2006 |
| Date of Acceptance | 16 February 2007 |
| Indian J. Pharm. Sci., 2007, 69 (1): 145-147 |
Abstract
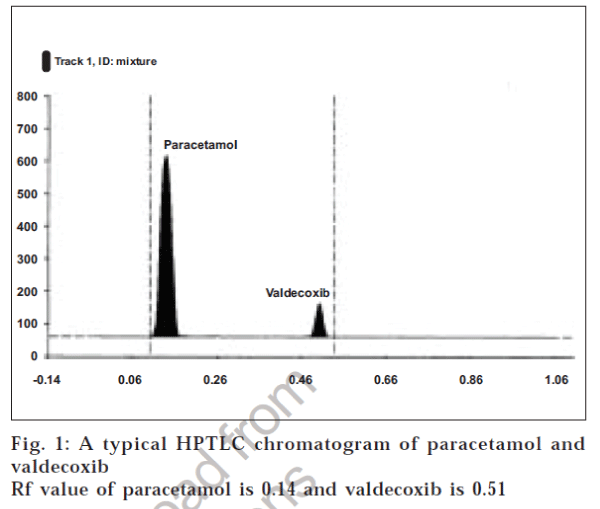
A new simple, sensitive and precise high performance thin layer chromatographic method has been developed for estimation of paracetamol and valdecoxib simultaneously from a combined dosage form. In this method pre coated silica gel 60 GF 254 TLC plate was used as stationary phase and the chromatogram was developed using chloroform: isopropyl alcohol: glacial acetic acid (9.5:1:0.2 v/v/v) as mobile phase. Paracetamol and valdecoxib showed Rf values 0.14±0.01 and 0.51±0.03, respectively. The plate was scanned and quantified at 250 nm using Camag TLC Scanner. The linear concentration was 2.5 to 12.5 µg/spot for paracetamol and 0.1 to 0.5 µg/spot for valdecoxib. The %RSD of intra day variation was 0.18±0.01 for paracetamol and 0.24±0.01 for valdecoxib. For inter day variation the %RSD was 0.03±0.005 and 0.1±0.02 for paracetamol and valdecoxib, respectively.
Paracetamol [1-3] is an analgesic and antipyretic. Valdecoxib is a non-steroidal antiinflammatory drug. Both are in combination used in the treatment of severe pain, rheumatoid arthritis and pyrexia. Determination of paracetamol from formulation [4-11] and from biological fluids [12] has been reported. Most of the methods were HPLC or UV spectroscopic methods for determination of paracetamol either in individual or in combination with other drugs. Valdecoxib has analytical methods for bioequivalence studies [13], metabolite determination [14-16] and estimation of formulation [17]. No method was reported for the estimation of paracetamol and valdecoxib in combined dosage form. The present study describes a simple, sensitive and precise HPTLC method for the estimation of paracetamol and valdecoxib from combined dosage form.
Paracetamol and valdecoxib was received as gift samples from Raptakos and Brett Test Laboratories and Glenmark Pharmaceuticals India Ltd, Mumbai, respectively. Silica gel 60 GF254 TLC plates (20×10) were used as stationary phase. The combined tablet formulation (500 mg paracetamol and 20 mg valdecoxib) was purchased from a local pharmacy.
A Camag HPTLC System comprising of Camag Linomat V semiautomatic sample applicator, Camag TLC scanner, Camag twin-trough chamber (20×10), Camag CATS software, Hamilton syringe (100 μl), and Shimadzu digital electronic balance BL- 220H were used in the study.
Standards of paracetamol (250 mg) and valdecoxib (10 mg) were weighed accurately and transferred to a 100 ml volumetric flask. This was used as standard stock solution. The drugs were dissolved and diluted up to the mark with ethanol. The tablets containing 500 mg of paracetamol and 20 mg of valdecoxib were weighed and finely powdered. The powder equivalent to 10 mg of valdecoxib and 250 mg of paracetamol was transferred to a 100 ml volumetric flask and dissolved with small portion of ethanol and shaken properly. Then the volume was made with the same and the solution was filtered through Whatmann filter paper.
The chromatographic estimations were performed using pre coated silica gel 60 GF254 as stationary phase and mobile phase consisted of chloroform: isopropyl alcohol: glacial acetic acid (9.5:1:0.2 v/v/v). The chamber saturation time was 15 min and the distance of solvent was 82 mm. The detection wavelength was 250 nm.
One to five microlitres of standard stock solution of paracetamol and valdecoxib and aliquot of formulation were spotted on pre coated TLC plates with the help of Linomat V applicator. The plate was developed in 20×10 twin trough chamber up to 82 mm. The plate dried in air after development, scanned with Camag TLC scanner using CATS software incorporating the track optimization option. The amount of paracetamol and valdecoxib present in formulation was estimated by using the calibration curve prepared from standards of paracetamol and valdecoxib. The result of analysis of tablets is shown in Table 1.
| Formulation | Labeled amount (mg/tablet) | Amount found in mg | % label claim *mean ± SD |
|---|---|---|---|
| Paracetamol | 500 | 448.12 | 89.6 ± 0.15 |
| Valdecoxib | 20 | 22.01 | 110 ± 0.20 |
*Average of six determinations. Tablet contains 500 mg of paracetamol and 20 mg of valdecoxib
Table 1: Results of the marketed tablets
The method was validated by establishing linearity, accuracy, inter day and intra day precision, repeatability of measurement of peak area as well as repeatability of sample application. The limit of detection and limit of quantification were also determined.
The linearity of paracetamol and valdecoxib was found to be in the range of 2.5 to 12.5 μg/spot and 0.1 to 0.5 μg/spot, respectively. The correlation coefficient was 0.9280 and 0.9945, respectively, for paracetamol and valdecoxib. The intra day and inter day precision (%RSD) were determined for standard paracetamol and valdecoxib for 6 times on the same day and different days. The intra day and inter day %RSD were found to be less than 2 for both paracetamol and valdecoxib. These values indicate that the method is precise.
Accuracy of the method was evaluated by calculating recovery of paracetamol and valdecoxib by standard addition method. The percentage recovery was found to be 99.2% and 98.6% for paracetamol and valdecoxib respectively, ensuring that the method is accurate.
Different validation parameters for the proposed HPTLC method for determining paracetamol and valdecoxib content were summarized in Table 2 and chromatogram of paracetamol and valdecoxib after separation is shown in fig.1.
| Parameter | Result | |
|---|---|---|
| Paracetamol | Valdecoxib | |
| Linearity range (µg/spot) | 2.5-12.5 | 0.1-0.5 |
| Correlation coefficient (r) | 0.928 | 0.9945 |
| Precision (% RSD) | ||
| Intra day | 0.18 | 0.24 |
| Inter day | 0.03 | 0.109 |
| Repeatability of sample application (%RSD) | 0.47 | 0.343 |
| Repeatability of measurement (%RSD) | 0.01 | 0.151 |
| % Recovery | 99.2 | 98.6 |
| LOD (ng) | 2.5 | 10 |
| LOQ (ng) | 25 | 100 |
Table 2: Validation parameters
The result obtained was in agreement with the labeled value of paracetamol and valdecoxib in dosage form. The results indicate that the proposed HPTLC method was found to be simple, rapid, precise and accurate for assay of paracetamol and valdecoxib in its formulation.
Acknowledgements
The authors thank M/s SNR and sons charitable Trust, Coimbatore for providing facilities to carry out the work.
References
- Indian pharmacopoeia, 4th Edn., The controller of publication, NewDelhi,1996, 554.
- Parfitt, K., Eds., In; Martindale, The Complete Drug Refernce, 32ndEdn, The Pharmaceutical Press, Lonon,1999, 72.2.
- Budavari, S., Eds., In; The Merck Index, 13th Edn., Merck & Co.,Inc., Whitehouse Station, NJ, 2001, 10.
- Halkar, U.P., Kope, P.G. and Rane, S.H., Indian Drugs, 2002, 39, 293.
- Gangwal, S. and Trivedi, P., Indian Drugs, 1999, 36, 683.
- Zarapkar, S.S., Halkar, U.P. and Bhandari, N.P., Indian Drugs, 1999,36, 710.
- Bhatia, M.S., Kashkedikar, S.G. and Chaturvedi, S.C., Indian Drugs, 1997, 34, 149.
- Shrenik, G. and Sharma, A.K., Indian Drugs, 1996, 33, 163.
- Chawla, J.L., Sodhi, R.A. and Sane, R.T., Indian Drugs, 1996, 33, 171.
- Chawla, J.L., Sodhi, R.A. and Sane, R.T., Indian Drugs, 1996, 33,208.
- Bhatia, M.S., Kashedikar, S.G. and Chaturvedi, S.C., Indian Drugs, 1996, 33, 280.
- Chatterjee, S. and Singh, B.P., Indian Herbs, 1996, 33, 355.
- Mandal, U., Jayakumar, M., Ganesan, M., Nandi, S., Pal, T.K.,Chakraborty, M.K., Roy Chowdhary, A. and Chattoraj, T.K., Indian Drugs, 2004, 41, 59.
- Zhang, J.Y., Fast, D.M. and Breau, A.P., J. Pharm. Biomed. Anal., 2003, 33, 61.
- Werner, U., Werner, D., Hinz, B., Lanbrecht, C. and Brune, K., J. Biomed. Chromatogr., 2004, 19, 113.
- Zhang, J.V., Fast, D.M. and Breau, A.P., J. Chromatogr. B Anal. Technol. Biomed. Life Sci., 2003, 785, 123.
- Sutariya, V.B., Rajashree, M., Sankalia, M.G. and Priti, P., Indian J. Pharm. Sci., 2004, 93, 112.