- Corresponding Author:
- S. S. Chitlange
Pad. Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Sant Tukaram Nagar, Pimpri, Pune- 411 018. E-mail: Sohanchitlange@rediffmail.com
| Date of Submission | 24 March 2007 |
| Date of Revision | 24 December 2007 |
| Date of Acceptance | 22 June 2008 |
| Indian J Pharm Sci, 2008, 70 (3): 398-400 |
Abstract
High performance thin layer chromatographic method is developed for simultaneous estimation of ibuprofen and pseudoephedrine hydrochloride in tablets. Silica gel 60F 254 plates were used as stationary phase and t.butanol: ethyl acetate: glacial acetic acid: water (7:4:2:2 v/v) as mobile phase. Wavelength selected for analysis was 254 nm. Percent estimation of ibuprofen and pseudoephedrine hydrochloride was found to be 99.56% and 98.77%, respectively. Percent recovery for both the drugs was found in the range of 98.27% to 100.91%, respectively.
Keywords
Ibuprofen, pseudoephedrine hydrochloride, HPTLC, t-butanol, ethyl acetate, glacial acetic acid, water
Ibuprofen (IBU) is a non steroidal antiinflammatory agent with propionic acid group. Chemically it is (RS)-2-(4-isobutyl phenyl) propionic acid. Pseudoephedrine hydrochloride (PEH) is a sympathomimetic agent. Chemically it is (1S,2S)- 2-methyl amino-1-phenyl-1-propanol hydrochloride. Fixed dose combination tablet containing IBU (200 mg) and PEH (30 mg) is available for clinical use. IBU is official in IP, BP and USP. PEH is official in IP, BP, EP and USP. Literature survey revealed spectrophotometric [1], spectrofluorometric [2], HPLC [3,4], GC [5] and HPTLC [6] methods for estimation of IBU alone or in combination with other drugs, in pharmaceutical formulation and biological fluids. PEH is reported to be estimated by non-aqueous titrimetry [7], derivative spectrophotometry [8], HPLC [9,10], capillary electrophoresis [11,12] individually or in combination with other drugs, in pharmaceutical formulation and biological fluids. Spectrophotometric [13,14] methods are reported for simultaneous estimation of these drugs in tablet formulation. In the present work a successful attempt has been made to estimate both these drugs simultaneously by economical and less time consuming HPTLC method.
A Camag-HPTLC system comprising of Linomat- IV automatic sample applicator and Camag TLC scanner 3 with CAT’S version 4.0 software were used for sample application and quantitative evaluation respectively. Samples were applied as bands (band size: 6 mm at 6 mm interval) under a stream of nitrogen on aluminium plates coated with silica gel 60F254 (10×10 cm, Merck) and chromatographed using tertiary butanol:ethyl acetate:glacial acetic acid: water (7:4:2:2 v/v) as mobile phase. Ascending development was performed in a saturated twin-trough TLC chamber. Chromatogram was evaluated by scanning in absorbance/reflectance mode at 254 nm using slit dimensions 4×0.45 mm and quantitation was done by comparing peak height of standard and sample peaks.
Standard solution containing 8 mg/ml of IBU and 1.2 mg/ml of PEH was prepared in methanol. To study the linearity of detector response, the standard stock solutions were appropriately diluted and accurately measured volume ranging from 1 to 10 µl was applied on the TLC plate. The plate was then chromatographed in the selected chromatographic conditions. A calibration graph was constructed by plotting peak height versus concentration.
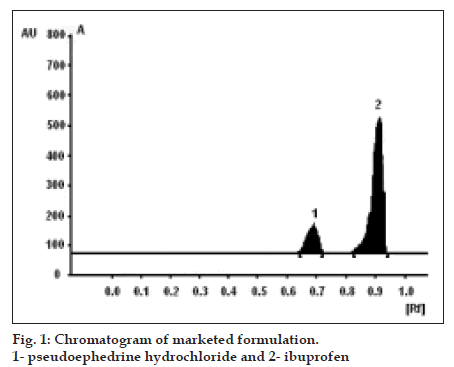
For estimation of IBU and PEH in tablets, an accurately weighed quantity of tablet powder equivalent to 200 mg of IBU was transferred to 25 ml volumetric flask, shaken with 10 ml of methanol for 15 min. and the volume was then adjusted to the mark with methanol. The solution was then filtered through whatman Grade I filter paper and 6 µl of the filtrate (six bands) and standard solution (one band) was applied on the TLC plate and chromatographed. Amount of both the drugs were estimated by comparing the peak height of standard and sample bands. The results are shown in Table 1 and respective densitogram is shown in fig. 1.
| Sample | Label claim (mg/tablet) | % Label claim*, SD, CV | % Recovery*, (mg/tablet) SD, CV | ||
|---|---|---|---|---|---|
| IBU | PEH | IBU | PEH | ||
| Standard laboratory mixture | - | 99.01±0.889 | 99.46±0.731 | - | - |
| 0.030 | 0.024 | ||||
| Arinac tablet | IBU 200 | 99.56±1.668 | 98.77±1.452 | 100.69±1.662 | 101.03±1.854 |
| PEH – 30 | 0.016 | 0.024 | 0.016 | 0.018 | |
*Indicates mean of five observations, ± indicates standard deviation. IBU stands for ibuprofen and PEH denotes pseudoephedrine hydrochloride.
Table 1: Results of estimation in tablet and recovery studies
To study the accuracy of the proposed method recovery studies were carried out using standard addition method. The percent recovery was calculated by using the formula, % recovery= (T-A)/S×100, where T is total amount of drug estimated, A is the amount of drug contributed by tablet powder and S is the amount of pure drug added. Result of recovery studies are shown in Table 1.
The system repeatability was studied by applying five replicate applications of standard solution on TLC plate. The plate was chromatographed and the standard deviation for peak height of IBU and PEH was calculated. The robustness of the method was evaluated by studying analyst-to-analyst, intra and inter day variations.
The selected chromatographic conditions were found to effectively separate IBU (Rf = 0.91) and PEH (Rf = 0.68). The linearity for detector response was observed in the range of 45.6-75.6 µg for IBU (correlation coefficient = 0.9934) and 6.8-11.3 µg for PEH (correlation coefficient = 0.9963). Percent amount of IBU and PEH estimated in the average weight of tablet were found to be 99.56% (standard deviation = ±0.1.668) and 98.77% (standard deviation = ±1.452), respectively. The low values of standard deviation indicate the precision of the method. Percent recovery for IBU and PEH was found to be 100.69% (standard deviation = ±1.662) and 101.03% (standard deviation = ±1.854) indicating that the excipients does not have interference in their estimation. The system repeatability studied by five replicate applications of standard solution. The standard deviations for peak height were found to be ±0.11 for IBU and ±0.06 for PEH. The standard deviation for robustness studies was below 2%.
Based on the above results it can be concluded that the proposed HPTLC method is accurate, precise, specific and reproducible. The method is also economical and less time consuming and can be used for routine analysis of ibuprofen and Pseudoephedrine hydrochloride.
Acknowledgements
The authors are thankful to Head, Department of Pharmaceutical Sciences, Rashtrantsant Tukdoji Maharaj Nagpur University, Nagpur for providing facilities for the research work. The authors are also thankful to Cadila Pharmaceuticals Ltd., Ahmedabad and Universal Lab. Ltd., Nagpur for providing gift samples of the drugs.
References
- Wahbi AA, Hassan E, Hamdy D, Khamis E. Barary M. Spectrophotometric methods for the determination of ibuprofen in tablets. Pak J Pharm Sci 2005;18:1-6.
- Damiani PC, Bearzotti M, Cabazon MA. Spectrofluorometric determination of ibuprofen in pharmaceutical formulation. J Pharm Biomed Anal 2001;25:679-83.
- Farrar H, Letzig L, Gill MJ. Validation of a liquid chromatographic method for the determination of ibuprofen in human plasma. Chromatogr B Analyt Technol Biomed Life Sci 2002;780:341-8.
- Wang P, Qi M, Liu L, Fang L. Determination of ibuprofen in dog plasma by liquid chromatography. J Pharm Biomed Anal
- 2005;38:714-9.
- Paik MJ, Kim KR. Optical purity determination of ibuprofen in tablets by achiral gas chromatography. Arch Pharm Res 2004;27:820-4.
- Save TK, Parmar DV, Devaranjan PV. High Performance thin layer chromatographic determination of ibuprofen in plasma. J Chromatogr B Biomed Sci Appl 1997;690:315-9.
- Indian Pharmacopoeia. Volume II. Govt. of India, New Delhi: Ministry of Health and Family Welfare; 1996. p. 641.
- Mabrouk MM, EI-Fatatry HM, Hammad S, Wahbi AA. Simultaneous determination of loratadine and Pseudoephedrine in pharmaceutical formulation by RP-LC and derivative spectrophotometry. Se Pu 1998;16(4):289-92.
- Ge OH, Zhou Z, Zhi XJ, Wang H. Simultaneous determination of Pseudoephedrine and chlorpheniramine in human plasma by HPLC-UV detection method. Se Pu 2001;19(3):236-8.
- Dinc E, Ozdemir A, Aksoy H, Ustundag O, Baleanu D. Chemometric determination of naproxen sodium and Pseudoephedrine hydrochloride in tablets by HPLC. J Pharm Biomed Anal 2003;33(4):597-604.
- Dong Y, Chen X, Chen Y, Hu Z. Seperation and determination of Pseudoephedrine, dextromethorphan, diphenhydramine and chlorpheniramine in cold medicines by nonaqueous capillary electrophoresis. J Pharm Biomed Anal 2005;39(1-2):285-9.
- Chen H, Chen X, Pu Q, Hu Z, Zhao Z, Hooper M. Seperation and determination of ephedrine and Pseudoephedrine by combination of flow injection with capillary electrophoresis. J Chromatogr Sci 2003;41(1):1-5.
- Palabiyik I. M, Dinc E, Onur F. Simultaneous spectrophotometric determination of Pseudoephedrine hydrochloride and ibuprofen in a pharmaceutical preparation using ratio spectra derivative Spectrophotometry and multivariate calibration techniques. J Pharm Biomed Anal 2004;34:473-83.
- Ivanovic D, Medenica M, Markovic S, Mandic G. Second-derivative spectrophotometric assay of Pseudoephedrine, ibuprofen and loratadine in pharmaceuticals. Arzneimittel Forschung 2000;50:1004-8.