- *Corresponding Author:
- Zhi Wang
Department of Oncology, Jiang Xi Pingxiang People’s Hospital, Pingxiang, Jiangxi 337000, China
E-mail: Wangzhi0399@126.com
| Date of Submission | 15 November 2022 |
| Date of Revision | 28 May 2023 |
| Date of Acceptance | 08 November 2023 |
| Indian J Pharm Sci 2023;85(6):1640-1647 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the anti-cancer effect and mechanism of hederagenin on laryngeal squamous cell carcinoma cells. Laryngeal squamous cell carcinoma cells TU177 were divided into control group, hederagenin group (5, 10, 20 μM hederagenin), anti-microRNA-negative control group (transfected with anti-microRNA-negative control), anti-microRNA-1269 group (transfected with anti-microRNA-1269), hederagenin+microRNA-negative control group (transfected with microRNA-negative control, 20 μM hederagenin), hederagenin+microRNA-1269 group (transfected with microRNA-1269 mimics, 20 μM hederagenin). We used cell counting kit-8 and a plate replicating experiment to determine TU177 cell proliferation; the wound recuperation test to examine TU177 cell migration; the Transwell assay to identify TU177 cell assault; the Western blotting method to examine N and E-cadherin protein communication; and reverse transcription-quantitative polymerase chain reaction to evaluate microRNA-1269 expressing themselves. Compared with the control group, the inhibition rate (14.81±1.26) %, (32.94±3.22) %, (57.74±4.29) % vs. (0.00±0.00) % and E-cadherin protein expression of TU177 cells in the hederagenin (5, 10, 20 μM) group were notably increased (p<0.05), the number of clone formation, the invasion number (85.88±7.36) individuals, (70.67±5.37) individuals, (52.23±5.05) individuals vs. (119.34±12.89) individuals and the scratch healing rate [(56.91±4.85) %, (38.93±3.31) %, (24.22±2.19) % vs. (72.16±5.66) %], N-cadherin protein expression and microRNA-1269 expression [(0.77±0.06), (0.58±0.05), (0.38±0.04) vs. (1.00±0.00)] were notably reduced (p<0.05). Inhibition of cells was much higher in the anti-microRNA-negative control group [(48.98±4.62) % vs. (5.89±0.48) %], E-cadherin protein expression of TU177 in anti-microRNA-1269 group were notably increased (p<0.05), the number of clone formation, the invasion number [(61.36±5.13) individual vs. (118.02±11.84) individual], and the scratch healing rate (33.28±3.02) % vs. (73.11±6.39) %, N-cadherin protein expression were notably reduced (p<0.05). The rate of inhibition was much lower in the hederagenin+microRNA-negative control group (19.62±1.16) % vs. (58.35±4.72) % and E-cadherin protein expression of TU177 in the hederagenin+microRNA-1269 group were notably reduced (p<0.05), the number of clone formation, the invasion number [(91.94±7.83) individuals vs. (50.74±5.01) individuals], and the scratch healing rate (58.02±4.36) % vs. (23.07±3.02) %, N-cadherin protein expression were notably increased (p<0.05). Hederagenin has anti-proliferation, anti-migration and anti-invasion impacts on TU177 laryngeal squamous cell carcinoma cells by repressing microRNA-1269 expression.
Keywords
Hederagenin, microRNA-1269, laryngeal squamous cell carcinoma, proliferation, migration, invasion
The most prevalent kind of head and neck cancer that develops through the laryngeal mucosal epithelium includes laryngeal squamous cell carcinoma. Laryngeal carcinoma with squamous cells is mostly treated surgically, however owing to the disease’s high incidence of local penetration and posterior lymph node metastases, patient 5 y survival rates are very poor[1]. In order to effectively treat laryngeal squamous cell carcinoma, it is necessary to understand the molecular pathway that drives its growth. Hederagenin, a triterpenoid isolated from Chinese ivy (Hedera helix L.) leaves, has shown strong anti-tumor activity in research conducted in vivo and in vitro studies. Hederagenin promotes apoptosis in cervical and colorectal cancer cells via the mitochondriamediated intrinsic apoptotic pathway, according to studies[2,3]. In addition, hederagenin also inhibits colon cancer cell proliferation, and invasion properties[4]. However, there are limited reports on the anti-cancer effect of hederagenin in laryngeal squamous cell carcinoma. MicroRNAs (miRNA) are short non-coding Ribonucleic Acid (RNA) molecules that play roles in cell development, differentiation, cycle regulation, regulation of apoptosis, and may control the growth of tumors by acting as tumor suppressors or cancer-causing genes. It has been reported that miR-1269 is upregulated in gastric cancer and is involved in regulating gastric cancer cell proliferation and apoptosis[5]. Increased miR-1269 production in primary tumors that have been surgically removed is strongly correlated with malignancy recurrence and metastasis[6]. A previous pre-experiment found that hederagenin treatment significantly decreased miR-1269 expression levels in laryngeal squamous cell carcinoma cells. Therefore, in order to offer an empirical foundation for the utilization of hederagenin for the prevention and therapy of laryngeal carcinoma with squamous cell carcinoma, this research hypothesized the fact that hederagenin has anti-cancer properties and that its mechanism of action corresponds to the inhibition of miR-1269 expression, and then verified this hypothesis using in vitro cell investigations.
Materials and Methods
Materials:
Human laryngeal squamous cell carcinoma cells TU177 were purchased from Shanghai Yagi Biotechnological Co., Ltd.; hederagenin (Lot No. 111733-201205, purity 98.3 %) was purchased from the National Institute for Food and Drug Control; miR-1269 inhibitor (anti-miR-1269), inhibitor negative sequence (anti-miR-Negative Control (NC)), miR-1269 mimics (mimics), mimic control sequence (miR-NC), miRNA fluorescent quantitative Polymerase Chain Reaction (qPCR) kit (dye method), Bicinchoninic Acid (BCA) protein assay kit were purchased from Sangon Biotech (Shanghai); Transwell chambers were purchased from BD, United Statas of America (USA); mouse monoclonal antibody to E-cadherin (sc-71008), mouse monoclonal antibody to N-cadherin (sc-8424), and mouse monoclonal antibody Santa Cruz, USA, provided the antibody to Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (sc-47724); a company in USA, supplied Lipofectamine 2000; Abcam, USA, supplied the goat anti-mouse Immunoglobulin G (IgG) secondary antibody (ab6789); Beijing Biolab Biological Co., Ltd., supplied the Cell Counting Kit-8 (CCK-8), the miRNA reconstitution kit, and the TRIzol reagent.
Methods:
In vitro culture: At 80 % convergence, transmitted 1:3, as well as having the media replaced once every 2–3 d, TU177 cells were grown in Dulbecco's Modified Eagle Medium (DMEM) medium coupled with 10 % human bovine serum, 1 % penicillin dual antibody, and 37° in an incubator with humidification holding 5 % carbon dioxide, 95 % air and 0.25 % protease.
Grouping and treatment: The TU177 cells were grown in a growth medium containing 0, 5, 10 and 20 μM hederagenin[7] and sequentially labeled as Control (CON) group, Low Hederagenin group (L-Hed group), Middle Hederagenin group (M-Hed group) and High Hederagenin group (H-Hed group); 1×105 TU177 cells were seeded in 24-well plates and transfected with 20 pmol of anti-miR-1269, anti-miR-NC, miR-1269 mimic and miR-NC using Lipofectamine 2000 at 50 % confluence. After 48 h, miR-1269 expression in TU177 cells of each group was measured by Reverse Transcriptionquantitative Polymerase Chain Reaction (RTqPCR) to verify the transfection efficacy. AntimiR- NC transgenic TU177 cells and anti-miR-1269 transgenic TU177 cells were referred to as the anti-miR-NC group and the anti-miR-1269 group, respectively; hederagenin (20 M) culture media was incubated with the transfection TU177 cells with miR-NC and miR-1269 mimics, sequentially denoted as hederagenin+miR-NC (Hed+miR-NC), hederagenin+miR-1269 (Hed+miR-1269) group.
CCK-8 assay: For TU177 cell proliferation; TU177 cells in each group were collected. Every single well was treated with 10 l of CCK-8 reagent after 48 h of incubation. A microplate reader detected the absorbance of each well incubated for 2 h (A)
Inhibition rate (%)=(1-Aexperimental group/Acontrol group)×100 %
Plate cloning experiment to detect clone formation: TU177 cells in each group were suspended in fresh medium. Seed 6-well plates at a density of 200 cells per well for each group and gently swirl the plates to disperse the cells evenly. Incubate for 2-3 w and stop growing when macroscopic clones appear and stained using 0.1 % violet crystals and 4 % paraformaldehyde. The number of clones (>50 cells) was counted microscopically.
Transwell test: TU177 cells Matrigel was used to cover the inside of the Transwell. Single-cell suspensions (1×105 cells/ml) of TU177 cells were grown in serum-free media from each group. 200 ml of cell suspension were added to the top chamber of the Transwell, and 500 ml of culture media were poured to the bottom chamber. After 24 h, the cells were gathered and photographed underneath a magnifying glass following a staining with 0.1 % crystal violet dye and a 20 min treatment with 4 % paraformaldehyde.
Migration test for scratch mending: In 6-well plates, TU177 cells being plated (1×104/well). With 200 μl pipette tip to scratch the monolayer of cells, they were subsequently immersed in serum-free media for 24 h after being rinsed with Phosphate Buffered Saline (PBS). Photographs were taken under a microscope, and the scratch distance was measured by using Image.
Scratch healing rate (%)=(Initial scratch distance-24 h scratch distance)/initial scratch distance×100 %
Western blot assay for protein: N-cadherin and E-cadherin expression 1.2.2 TU177 cells from each group were lysed using Radioimmunoprecipitation Assay (RIPA), after centrifuging at 12 000 r/min for 5 min at 4°, the supernatant was gathered. The BCA kit was used to determine protein concentration. Subsequently, 30 μg protein samples from each group were subjected to Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) with voltage 100 V and electrophoresis time 110 min; the transmembrane was completed in a wet transmembrane apparatus with the following parameters; current 300 mA, time 15 min. NC membranes were treated with autoantibodies against E-cadherin after being blocked in a solution of 5 % nonfat milk for 2 h at ambient temperature (1:800 dilution), N-cadherin (1:500 dilution), GAPDH antibody (1:1000 dilution) for 12 h at 4°; then, the membrane was incubated with goat-antimouse IgG secondary antibody (1:3000 dilution) for 1 h at 25°. After the bands were developed, the net gray value of the protein of interest was determined with Quantity One software.
RT-qPCR assay for miR-1269 expression: Total RNA was extracted by TRIzol reagent from TU177 of each group. After reverse transcription, RTqPCR was performed using miRNA fluorescence quantitative PCR kit with complementary Deoxyribonucleic Acid (cDNA) as template. The reaction system consisted of 2× miRNA qPCR master mix 10 μl, ROX reference dye 1 μl and template 2 μl. The upstream primer (10 μM) 0.5 μl and the reverse primer (10 μM) 0.5 μl. Doubledistilled water (ddH2O) was supplemented to a total volume of 20 μl. miR-126, upstream primer 5′-ATCCAGTGCGTGTCGTG-3′, reverse primer 5′-TGCTGGTCATCGTGCCGAG-3′; U6: upstream primer 5 ′ - G C T T C G G C A G C A C ATATA C TA A A AT- 3 ′ and reverse primer 5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Statistical methods:
Each experiment was independently repeated 3 times with 3 parallel per setup, and data are presented as x±s. Statistical significance was assumed at the p<0.05 level. Statistical testing was performed using Statistical Package for the Social Sciences (SPSS) 22.0 with the independent samples t-test being used to compare pairs of groups, and the one-way Analysis of Variance (ANOVA) and Least Significant Difference (LSD)-t test being used to compare sets of groups.
Results and Discussion
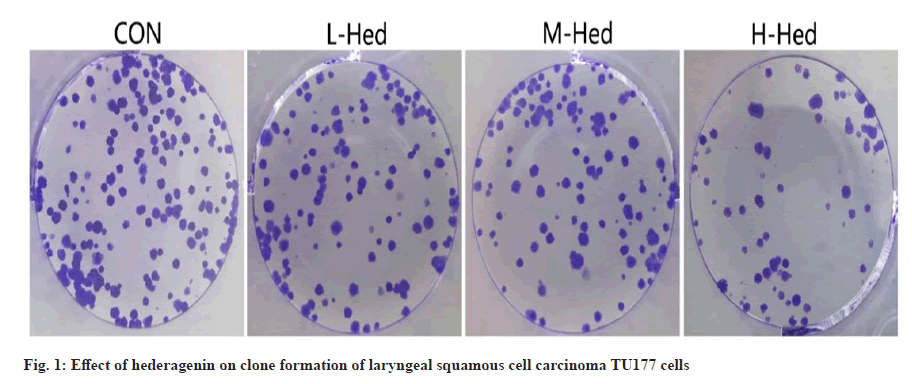
Hederagenin's effect on the growth and clonal expansion of TU177 laryngeal squamous carcinoma cells. Sequentially treating TU177 cells with modest doses of doxorubicin resulted in a higher inhibition rate compared to the control group, middle and high dosage of hederagenin, and the number of clone formation cells was decreased sequentially (p<0.05) as shown in fig. 1 and Table 1.
| Group | Inhibition rate (%) | Cell number of clone formation (s) |
|---|---|---|
| CON | 0.00±0.00 | 97.74±6.34 |
| L-Hed | 14.81±1.26* | 78.04±5.91* |
| M-Hed | 32.94±3.22*# | 62.71±4.75*# |
| H-Hed | 57.74±4.29*#& | 48.23±4.48*#& |
| F | 733.694 | 137.565 |
| p | 0.000 | 0.000 |
Note: *p<0.05, vs. the M-Hed group; #p<0.05, vs. the L-Hed group and &p<0.05, vs. the control group category
Table 1: Effect of Hederagenin on Proliferation and Colony Formation of Laryngeal Squamous Cell Carcinoma Tu177 Cells (X±S, N=9)
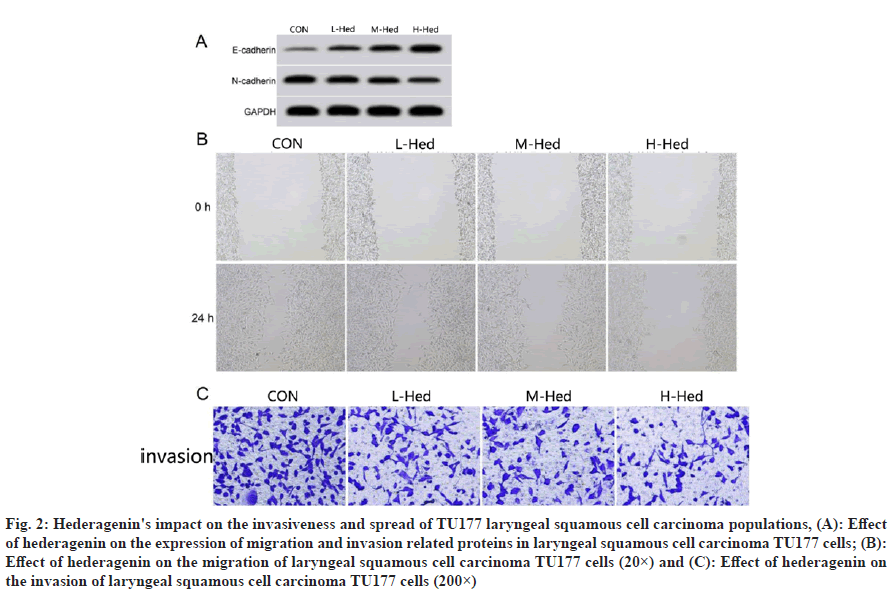
Hederagenin's impact on the invasiveness and spread of TU177 laryngeal squamous cell carcinoma cells compared with the control group, the number of invaded cells, scratch healing rate, protein N-cadherin expression were decreased in turn and E-cadherin expression was increased sequentially after low, medium and high dosage of hederagenin treatment in TU177 cells (p<0.05) as shown in fig. 2 and Table 2.
Fig. 2: Hederagenin's impact on the invasiveness and spread of TU177 laryngeal squamous cell carcinoma populations, (A): Effect of hederagenin on the expression of migration and invasion related proteins in laryngeal squamous cell carcinoma TU177 cells; (B): Effect of hederagenin on the migration of laryngeal squamous cell carcinoma TU177 cells (20×) and (C): Effect of hederagenin on the invasion of laryngeal squamous cell carcinoma TU177 cells (200×)
| Group | Scratch healing rate (%) | Number of invasion cell (s) | E-cadherin protein | N-cadherin protein |
|---|---|---|---|---|
| CON | 72.16±5.66 | 119.34±12.89 | 0.23±0.02 | 0.87±0.06 |
| L-Hed | 56.91±4.85* | 85.88±7.36* | 0.37±0.03* | 0.71±0.06* |
| M-Hed | 38.93±3.31*# | 70.67±5.37*# | 0.49±0.04*# | 0.54±0.04*# |
| H-Hed | 24.22±2.19*#& | 52.23±5.05*#& | 0.64±0.05*#& | 0.36±0.03*#& |
| F | 220.586 | 105.903 | 202.833 | 178.887 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: When comparing each group to the control group (*p<0.05), the L-Hed group (#p<0.05), and the M-Hed group (&p<0.05), statistical significance was found. *p<0.05, vs. the control group; #p<0.05, vs. the low hederagenin group and &p<0.05, vs. the medium ivy sapogenin group
Table 2: Hederagenin's Impact on the Invasiveness and Spread of Tu177 Laryngeal Squamous Carcinoma Cells (X±S, N=9)
Modulating miR-1269 expression in TU177 laryngeal squamous cell carcinoma cells with hederagenin. Following administration with low, medium, and high doses of hederagenin, the miR-1269 transcript level in larynx squamous cell adenocarcinoma TU177 cells dropped progressively (p<0.05) compared with the control group, as shown in Table 3.
| Group | miR-1269 |
|---|---|
| CON | 1.00±0.00 |
| L-Hed | 0.77±0.06* |
| M-Hed | 0.58±0.05*# |
| H-Hed | 0.38±0.04*#& |
| F | 328.013 |
| p | 0.000 |
Note: When comparing each group to the control group (*p<0.05), the L-Hed group (#p<0.05), and the M-Hed group (&p<0.05), statistical significance was found
Table 3: Effect of Hederagenin on Mir-1269 Expression in Laryngeal Squamous Cell Carcinoma Tu177 Cells (X±S, N=9)
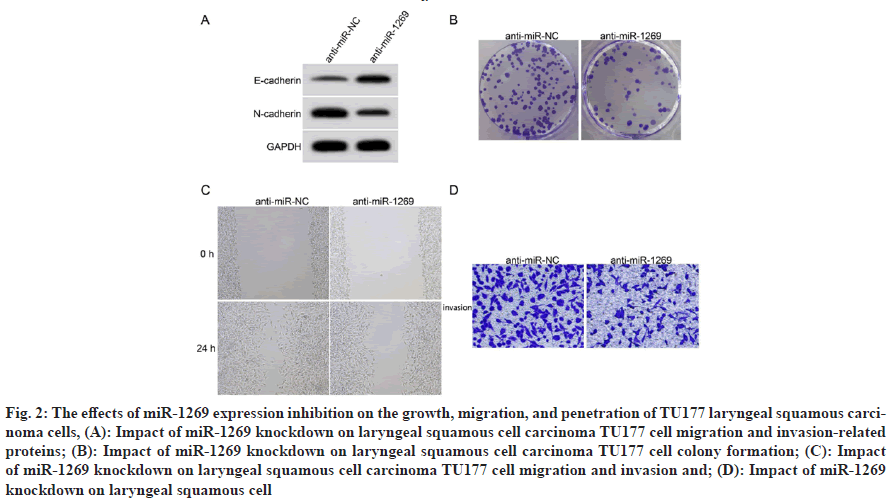
Increasing numbers of migratory and invading TU177 cells transfection with anti-miR-1269 was effective, as miR-1269 expression levels were considerably reduced in the anti-miR-1269 group compared to the anti-miR NC group (p<0.05). When comparing the anti-miR-1269 group with the anti-miR-NC group, we found that the proliferation inhibition rate and E-cadherin protein conveying were substantially increased (p<0.05), while the number of clones formed, N-cadherin protein communication, assault, and the ground up healing rate of TU177 cells were substantially reduced (p<0.05) as shown in fig. 3 and Table 4.
Fig. 3: The effects of miR-1269 expression inhibition on the growth, migration, and penetration of TU177 laryngeal squamous carcinoma cells, (A): Impact of miR-1269 knockdown on laryngeal squamous cell carcinoma TU177 cell migration and invasion-related proteins; (B): Impact of miR-1269 knockdown on laryngeal squamous cell carcinoma TU177 cell colony formation; (C): Impact of miR-1269 knockdown on laryngeal squamous cell carcinoma TU177 cell migration and invasion and; (D): Impact of miR-1269 knockdown on laryngeal squamous cell
| Group | miR-1269 | Inhibition rate (%) | Cell number of clone formation (s) | Scratch healing rate (%) | Number of invasion cell (s) | E-cadherin protein | N-cadherin protein |
|---|---|---|---|---|---|---|---|
| Anti-miR-NC | 1.00±0.00 | 5.89±0.48 | 99.69±8.44 | 73.11±6.39 | 118.02±11.84 | 0.22±0.02 | 0.88±0.06 |
| Anti-miR-1269 | 0.37±0.04 | 48.98±4.62 | 55.56±5.01 | 33.28±3.02 | 61.36±5.13 | 0.56±0.04 | 0.44±0.03 |
| t | 47.250 | 27.831 | 13.489 | 16.906 | 13.176 | 22.808 | 19.677 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Table 4: Detection of Proliferation, Migration and Invasion of Laryngeal Squamous Cell Carcinoma Tu177 Cells after Interfering with Mir-1269 Expression (X±S, N=9)
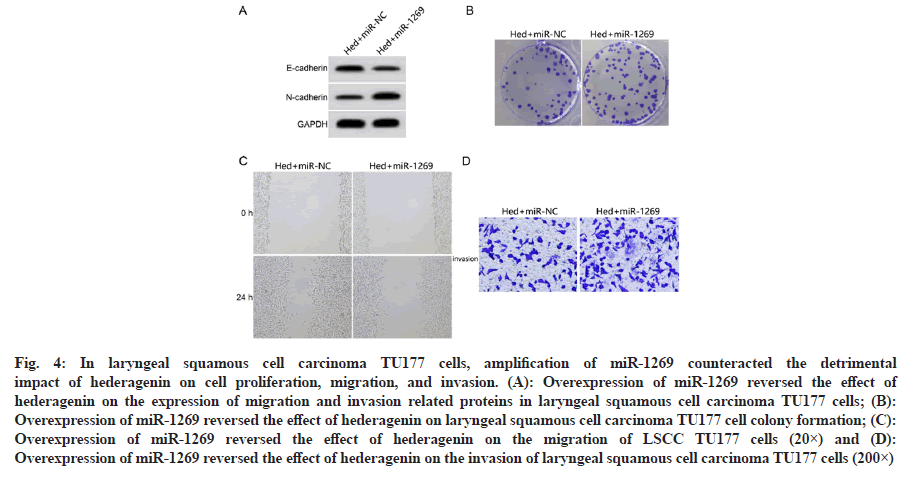
Over-expression of miR-1269 reversed the effects of hederagenin (20 μM) on proliferation, migration and invasion of laryngeal squamous cell carcinoma TU177 cells compared with the Hed+miR-NC group, the number of TU177 cell clone formation, miR-1269 expression level, invasion number, protein N-cadherin expression, scratch healing rate were significantly higher and the inhibition rate, protein E-cadherin expression were significantly decreased in the Hed+miR-1269 group (p<0.05) as shown in fig. 4 and Table 5.
Fig. 4: In laryngeal squamous cell carcinoma TU177 cells, amplification of miR-1269 counteracted the detrimental impact of hederagenin on cell proliferation, migration, and invasion. (A): Overexpression of miR-1269 reversed the effect of hederagenin on the expression of migration and invasion related proteins in laryngeal squamous cell carcinoma TU177 cells; (B): Overexpression of miR-1269 reversed the effect of hederagenin on laryngeal squamous cell carcinoma TU177 cell colony formation; (C): Overexpression of miR-1269 reversed the effect of hederagenin on the migration of LSCC TU177 cells (20×) and (D): Overexpression of miR-1269 reversed the effect of hederagenin on the invasion of laryngeal squamous cell carcinoma TU177 cells (200×)
| Group | miR-1269 | Inhibition rate (%) | Cell number of clone formation (s) | Scratch healing rate (%) | Number of invasion cell (s) | E-cadherin protein | N-cadherin protein |
|---|---|---|---|---|---|---|---|
| Hed+miR-NC | 1.00±0.00 | 58.35±4.72 | 44.73±4.14 | 23.07±3.02 | 50.74±5.01 | 0.67±0.05 | 0.32±0.03 |
| Hed+miR-1269 | 3.12±0.25 | 19.62±1.16 | 83.24±6.71 | 58.02±4.36 | 91.94±7.83 | 0.35±0.03 | 0.75±0.05 |
| t | 25.440 | 23.905 | 14.653 | 19.769 | 13.297 | 16.464 | 22.123 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Table 5: Overexpression of Mir-1269 Reversed Hederagenin's Inhibitory Effects on Cellular Proliferation, Migration and Penetration in Tu177 Laryngeal Squamous Cell Carcinoma (X±S, N=9)
One potential new approach to treating laryngeal squamous cell carcinoma would be to identify drugs which successfully inhibit the cancerous development of the throat squamous cell carcinoma cells, which is an important reason of therapeutic failure in individuals. Hederagenin has shown promise as an anti-tumor agent, with research showing that it boosts the toxicity of cisplatin and paclitaxel against lung tumor cells by inhibiting respiration and so inducing the buildup of reactive oxygen species[8]. In cisplatin-resistant carcinoma of the head and neck tissues, hederagenin may also block the antioxidant system and cause apoptosis[9]. Hederagenin also suppresses prostate cancer cell’s ability to spread and multiply malignantly[10]. Hederagenin was shown to have an inhibitory impact on TU177 cell proliferation and clone creation abilities in the current investigation, with the degree of inhibition increased as one increases the medication dose. Since metastasis was a hallmark of tumor cells, the fact that hederagenin greatly reduced the wound mending rate and invasion quantity of TU177 cells after treatment suggested that it may block the migratory and invasion capacities of TU177 cells. Throughout the process of Epithelial Mesenchymal Transition (EMT), epithelial type markers like E-cadherin are downregulated while mesenchymal phenotypic markers like N-cadherin are upregulated[11]. The aggressiveness and poor prognosis of laryngeal squamous cell carcinoma have both been linked to EMT[12]. Hederagenin inhibited laryngeal squamous cell carcinoma cell TU177 migration and invasion by blocking the EMT process, as shown by a dosedependent reduction in N-cadherin expression and an increase in E-cadherin expression. This finding is consistent with that of Chen et al.[13], who reported hederagenin's anti-EMT consequence in colorectal cancer.
Multiple studies have shown that miRNAs have a crucial role in controlling many different aspects of laryngeal squamous cell carcinoma cells, including their proliferation, metabolism, apoptosis and metastasis[14,15]. Due to their significance, miRNAs have been studied as potential biomarkers and therapeutic targets for laryngeal squamous cell carcinoma[16]. The function of miR-1269 in many types of malignancies has been established. Interruption of miR-1269 expression causes lung cancer cells to undergo apoptosis and is related with advanced tumor stages and lymphatic metastasis[17]. Liver cancer tissues had significantly higher miR- 1269 expression than adjacent non-cancerous liver tissues; miR-1269 conveying had a significant association with TNM stage, vascular invasion, and metastasis; and miR-1269 communication inhibition could decrease the development capacity utilization and the development of tumors of liver cancer cells, impede cell cycle transition, and inhibit liver cancer progression[18,19]. The results of this research show that hederagenin significantly reduces miR-1269 expression in TU177. AntimiR- 1269 transfection reduced clone formation, scratch healing rate, and incurable cells in TU177 cells, raised the cell inhibition rate, downregulated N-cadherin communication, and upregulated E-cadherin expression, all of which correlated with hederagenin is ability to inhibit cell proliferation, migration and invasion in laryngeal squamous cell carcinoma. This research uncovered evidence that hederagenin’s anti-cancer effects in laryngeal squamous cell carcinoma are mediated by miR- 1269’s low expression. Reversion assay results showed that hederagenin’s effects on TU177 cell proliferation, clone formation, migration and invasion, as well as the expression of N-cadherin and E-cadherin, were partially decreased by transferring miR-1269 mimics. This result further suggested that miR-1269 is the downstream target of hederagenin and suggested that hederagenin exerts effects against cancer in laryngeal squamous cell carcinoma.
In conclusion, hederagenin may reduce the growth, migration and invasion of TU177 laryngeal squamous carcinoma cells, and its anti-cancer mechanism is linked to miR-1269 down-regulation. Hederagenin shows promise as a therapeutic agent against laryngeal squamous cell carcinoma, suggesting it might be used for both treating and preventing the disease in people.
Author’s contributions:
Hui Peng and Shanshan Liu have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394-424.
[Crossref] [Google Scholar] [PubMed]
- Fang L, Liu M, Cai L. Hederagenin inhibits proliferation and promotes apoptosis of cervical cancer CaSki cells by blocking STAT3 pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2019;35(2):140-5.
[Google Scholar] [PubMed]
- Liu BX, Zhou JY, Li Y, Zou X, Wu J, Gu JF, et al. Hederagenin from the leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo colon cells through the mitochondrial pathway. BMC Complement Altern Med 2014;14(1):412.
[Crossref] [Google Scholar] [PubMed]
- Zi LB, Ping WR, Xi Z, Yong ZJ. The effect of hederagenin on the proliferation, adhesion, invasion and migration of human colon cancer cells LoVo. J Nanjing Univ Tradit Chin Med 2013;29(1):44-7.
- Liu WL, Wang HX, Shi CX, Shi FY, Zhao LY, Zhao W, et al. microRNA-1269 promotes cell proliferation via the AKT signaling pathway by targeting RASSF9 in human gastric cancer. Cancer Cell Int 2019;19(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Bu P, Wang L, Chen KY, Rakhilin N, Sun J, Closa A, et al. miR-1269 promotes metastasis and forms a positive feedback loop with TGF-?. Nat Commun 2015;6(1):6879.
[Crossref] [Google Scholar] [PubMed]
- Zuo F, Chen C, Gu C. Anti-breast cancer effect of hederagenin and its mechanism in vitro. Chin J Industry Med 2023;3:21.
- Wang K, Liu X, Liu Q, Ho IH, Wei X, Yin T, et al. Hederagenin potentiated cisplatin-and paclitaxel-mediated cytotoxicity by impairing autophagy in lung cancer cells. Cell Death Dis 2020;11(8):611.
[Crossref] [Google Scholar] [PubMed]
- Kim EH, Baek S, Shin D, Lee J, Roh JL. Hederagenin induces apoptosis in cisplatin-resistant head and neck cancer cells by inhibiting the Nrf2-ARE antioxidant pathway. Oxid Med Cell Longev 2017;2017:5498908.
[Crossref] [Google Scholar] [PubMed]
- Zhenxia Z, Zhenmin Z, Chengguang Z. Effects of hederagenin on proliferation, migration and invasion of prostate cancer DU145 cell. Pharmacol Clin Chin Mater Med 2017;33(1):37-41.
- Zhang L, Yang Y, Su F. Study on the correlation between the levels of CX26, CX32, CX43 and epithelial mesenchymal transformation-associated protein, and colorectal primary carcinoma and liver metastasis. J Bengbu Med Coll 2019;44(12):1621.
- Wu K, Shen B, Jiang F, Xia L, Fan T, Qin M, et al. TRPP2 enhances metastasis by regulating epithelial-mesenchymal transition in laryngeal squamous cell carcinoma. Cell Physiol Biochem 2016;39(6):2203-15.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Xi S, Teng YH. Effects of hederagenin in suppressing epithelial-mesenchymal transition and invasion of colon cancer cells SW480. Chin J Exp Tradit Med Formula 2016;22(12):133.
- Wang J, Yang S, Ge W, Wang Y, Han C, Li M. miR-613 suppressed the laryngeal squamous cell carcinoma progression through regulating PDK1. J Cell Biochem 2018;119(7):5118-25.
[Crossref] [Google Scholar] [PubMed]
- Hui L, Zhang J, Guo X. miR-125b-5p suppressed the glycolysis of laryngeal squamous cell carcinoma by down-regulating hexokinase-2. Biomed Pharmacother 2018;103(1):1194-201.
[Crossref] [Google Scholar] [PubMed]
- Guo Y, Chen Y, Liu H, Yan W. Alpinetin inhibits oral squamous cell carcinoma proliferation via miR-211-5p upregulation and notch pathway deactivation. Nutr Cancer 2020;72(5):757-67.
[Crossref] [Google Scholar] [PubMed]
- Bao M, Song Y, Xia J, Li P, Liu Q, Wan Z. miR-1269 promotes cell survival and proliferation by targeting tp53 and caspase-9 in lung cancer. Onco Targets Ther 2018;11(1):1721-32.
[Crossref] [Google Scholar] [PubMed]
- Gan TQ, Tang RX, He RQ, Dang YW, Xie Y, Chen G. Upregulated miR-1269 in hepatocellular carcinoma and its clinical significance. Int J Clin Exp Med 2015;8(1):714-21.
[Google Scholar] [PubMed]
- Yang XW, Shen GZ, Cao LQ, Jiang XF, Peng HP, Shen G, et al. microRNA-1269 promotes proliferation in human hepatocellular carcinoma via downregulation of FOXO1. BMC Cancer 2014;14(1):909.
[Crossref] [Google Scholar] [PubMed]