- *Corresponding Author:
- Lei Zhao Department of Pharmacology, Liaoning Academy of Traditional Chinese Medicine, The Second Hospital Affiliated to Liaoning University of Traditional Chinese Medicine, Shenyang, Liaoning 110034, China E-mail: zhaolei19@yeah.net
| Date of Received | 14 September 2021 |
| Date of Revision | 25 November 2022 |
| Date of Acceptance | 24 July 2023 |
| Indian J Pharm Sci 2023;85(4):1085-1091 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the influence of harpagide isolated from Scrophularia ningpoensis Hemsl. on human umbilical vascular endothelial cells damage induced by oxidized low-density lipoprotein and its possible mechanism. Human umbilical vascular endothelial cells were cultured in vitro. Different doses (20, 40, 80 μmol/l) of harpagide were applied to treat human umbilical vascular endothelial cells induced by oxidized low-density lipoprotein, human umbilical vascular endothelial cells overexpressing microRNA-140-5p were induced by oxidized low-density lipoprotein, and 80 μg/ml harpagide was applied to treat oxidized low-density lipoprotein induced human umbilical vascular endothelial cells with microRNA-140-5p downregulation. Enzyme-linked immunosorbent assay kits were used to detect malondialdehyde content as well as superoxide dismutase and glutathione peroxidase activities. Flow cytometry and Western blot were utilized to investigate cell apoptosis. Ribonucleic acid expression was analyzed by real-time quantitative reverse transcription polymerase chain reaction. Different doses of harpagide (20, 40, 80 μmol/l) reduced the malondialdehyde content and the rate of apoptosis in oxidized low-density lipoprotein-stimulated human umbilical vascular endothelial cells (p<0.05), while elevated superoxide dismutase as well as glutathione peroxidase activities (p<0.05). MicroRNA-140- 5p overexpression reduced the malondialdehyde content and apoptosis in oxidized low-density lipoproteinstimulated human umbilical vascular endothelial cells while elevated superoxide dismutase as well as glutathione peroxidase activities (p<0.05). Harpagide promoted microRNA-140-5p expression in human umbilical vascular endothelial cells after oxidized low-density lipoprotein stimulation (p<0.05). MicroRNA- 140-5p knockdown reversed the inhibitory effect of harpagide in human umbilical vascular endothelial cells (p<0.05). Harpagide up-regulates microRNA-140-5p to inhibit the oxidative stress and apoptosis of oxidized low-density lipoprotein-induced human umbilical vascular endothelial cells.
Keywords
Harpagide, human umbilical vascular endothelial cells, oxidative stress, apoptosis, microRNA- 140-5p
Atherosclerosis is able to reduce blood flow to tissues, seriously threatening human life and health and causing myocardial infarction as well as other complications[1,2]. Vascular endothelial cell dysfunction is initial process of atherosclerosis[3] and inhibition of vascular endothelial cell injury is helpful for atherosclerosis therapy. Oxidized Low- Density Lipoprotein (oxLDL) that can influence macrophages and microenvironment is one of the main factors inducing vascular endothelial cell injury, and the occurrence and development of atherosclerosis[4,5].
Traditional Chinese medicine or its main active ingredients have protective effects on vascular endothelial cells[6]. Harpagide is the main component of Scrophularia ningpoensis Hemsl. and belongs to iridoid compounds. Studies have shown that harpagide may inhibit Aβ25-35 induced apoptosis and oxidative stress of PC12 cells through activation of Phosphatidylinositol 3-Kinase (PI3K)/Protein Kinase B (Akt) signaling pathway, indicating its potential value in the treatment of Alzheimer's disease[7]. Harpagide exerts a neuroprotective effect via decreasing endoplasmic reticulum stress-related marker production, reduced the (Calcium ions (Ca2+)) and increased thapsigargin induced production of sarcoendoplasmic reticulum Ca2+-Adenosine Triphosphatase (ATPase)-linked proteins[8]. But the effect of harpagide on vascular endothelial cell dysfunction are still unknown.
As a microRNA (miRNA), miR-140-5p regulates apoptosis, oxidative stress, inflammation and other physiological or pathological processes, participating in the development of various diseases[9-11]. A previous study used oxLDL to induce macrophages to mimic an atherosclerosis model, and cell assays displayed overexpression of miR-140-5p could inhibit oxLDL induced macrophage injury via interaction with Toll- Like Receptor 4 (TLR4)[12]. Thus, the miRNA may be a molecular target for atherosclerosis therapy. Herein, we established a model of atherosclerosis like cell dysfunction by using Human Umbilical Vein Endothelial Cells (HUVECs) and oxLDL and then performed assays to analyze the influence of harpagide on oxLDL induced cell oxidative stress as well as apoptosis and its association with miR-140- 5p.
Materials and Methods
Cells and reagents:
HUVECs (Procell, Wuhan, China); harpagide (purity >98 %, Chengdu Ruifensi Biotechnology Co., Ltd); LipofectamineTM 2000 (Invitrogen, United states of America (USA)); miR-140-5p mimics, miR-NC, miR-140-5p inhibitor, anti-miR-NC, and Polymerase Chain Reaction (PCR) primers (Sangon, Shanghai, China); oxLDL, Ribonucleic acid (RNA) extraction kit, Dulbecco's Modified Eagle (DMEM) medium, Annexin V-Fluorescein Isothiocyante (FITC)/ Propidium Iodide (PI) Kit, Bicinchoninic acid (BCA) protein detection kit and Fetal Bovine Serum (FBS) (Soleibao, Beijing, China); Malondialdehyde (MDA), Superoxide Dismutase (SOD), Glutathione Peroxidase (GSH-Px) kit, Western blot detection kit and Western blot blocking solution (Nanjing Jiancheng Bioengineering Institute); reverse transcription kit as well as PCR kit (TaKaRa, Dalian, China), cleaved caspase-9 and cleaved caspase-3 antibodies (Santa Cruz, USA).
Cell culture and transfection:
HUVECs were resuscitated and cultured with Roswell Park Memorial Institute (RPMI) 1640 medium containing 10 % FBS in an incubator at 37° with 5 % Carbon dioxide (CO2) and 97 % humidity. 5.0×105 HUVECs were inoculated into each well of 6-well plates. Synthesized oligonucleotides were transfected by LipofectamineTM 2000 liposome method. The transfection time was 12 h, and then new medium was replaced. Reverse Transcriptionquantitative PCR (RT-qPCR) was used to verify transfection effect after 24 h of culture.
Cell grouping:
The untransfected HUVECs were treated with oxLDL and harpagide, and divided into the following groups including control, oxLDL, oxLD+low-dose harpagide, oxLD+medium-dose harpagide and oxLD+high-dose harpagide groups. Cells in the oxLDL group were cultured in medium containing 40 μg/ml oxLDL[13]. Cells in oxLDL+low-dose harpagide, oxLDL+medium-dose harpagide as well as oxLDL+high-dose harpagide groups were co-cultured with medium containing 20, 40 or 80 μmol/l harpagide and 40 μg/ml oxLDL for 24 h, respectively[7]. Cells in the control group were not subjected to any treatment.
HUVECs transfected with synthesized oligonucleotides were all inoculated in 6-well plates. After 4 h incubation, treated cells were subjected to administration according to the following methods. Cells were diluted and subjected to transfection with miR-140-5p mimics or miR-NC and cultured in medium with 40 μg/ml oxLDL, which were labeled as oxLDL+miR-140-5p group and oxLDL+miRNC group. HUVECs were diluted and subjected to transfection with anti-miR-140-5p and, anti-miR-NC and treatment with 80 μmol/l harpagide and 40 μg/ml oxLDL, recorded as oxLDL+harpagide+anti-miR- 140-5p group and oxLDL+harpagide+anti-miR-NC group. The incubation time was 24 h.
MDA level, SOD and GSH-Px activity analysis:
MDA, SOD and GSH-Px activity analysis was done by Enzyme-Linked Immunosorbent Assay (ELISA). Cells were collected and cleaned with phosphate buffer twice and cell lysis solution was added. The cell centrifugation (3500 r/min, 5 min) was performed and supernatant was harvested. MDA level, SOD and GSH-Px activity were detected, referring to the instructions of kits.
Apoptosis analysis:
Collected cells of each group were washed with phosphate buffer. HUVECs were added with 10 μl Annexin V-FITC and 5 μl PI successively and then subjected to incubation. Apoptosis was detected via flow cytometry.
Western blot:
Collected cells of each group were cleaned with phosphate buffer twice, and the total proteins in the cells were extracted with Radioimmunoprecipitation Assay (RIPA) reagent. After quantification by BCA method, 10 % Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) was conducted. Then membranes were exposed to 5 % skim milk powder. After that, the antibodies specific to cleaved caspase-9 (1:1000), cleaved caspase-3 (1:1000), Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) (1:1500), and secondary antibody (1:3000) solution. Chemiluminescent reagent was added and the gel imaging system was applied to expose and photograph the membranes.
RT-qPCR:
Collected cells of each group were cleaned with phosphate buffer twice, and total RNA was extracted with RNA extraction kit. After reverse transcription, complementary Deoxyribonucleic Acid (cDNA) was generated, and PCR amplification was performed. Amplification procedures are 95° 5 min, 95° 10 s, 60° 45 s and 72° 30 s, with a total of 35 cycles.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 22.0 software was performed to analyze the experimental data. Measurement data are expressed as mean±standard deviation (x̄ ±s). Independentsample t test or one-way analysis of variance was used for comparison. p<0.05 indicated statistically significant difference.
Results and Discussion
As shown in Table 1, after 40 μg/ml oxLDL intervention, MDA content in HUVECs was increased, while SOD as well as GSH-Px activities were inhibited (p<0.05). Harpagide at 20, 40 and 80 μmol/l decreased MDA level in oxLDL stimulated HUVECs and increased SOD and GSH-Px activities (p<0.05), suggesting that harpagide could inhibit oxLDL-triggered oxidative stress of HUVECs.
| Group | MDA (nmol/l) | SOD (U/ml) | GSH-Px (U/ml) |
|---|---|---|---|
| Control | 0.52±0.05 | 62.32±5.19 | 93.16±8.42 |
| oxLDL | 9.23±0.79* | 19.83±2.08* | 31.02±3.29* |
| oxLDL+low-dose harpagide | 6.64±0.56# | 29.49±2.69# | 49.53±4.49# |
| oxLDL+medium-dose harpagide | 4.03±0.34#& | 41.48±4.48#& | 67.51±5.22#& |
| oxLDL+high-dose harpagide | 1.51±0.14#&$ | 54.63±4.95#&$ | 80.81±7.56#&$ |
| F | 561.627 | 165.177 | 147.444 |
| p | 0.000 | 0.000 | 0.000 |
Table 1: Oxidative Stress Analysis in Huvecs (X̄±S, N=9)
Note: *p<0.05, #p<0.05, &p<0.05 and $p<0.05 indicated comparison with control, oxLDL, oxLDL+low-dose harpagide and oxLDL+medium-dose harpagide groups, respectively
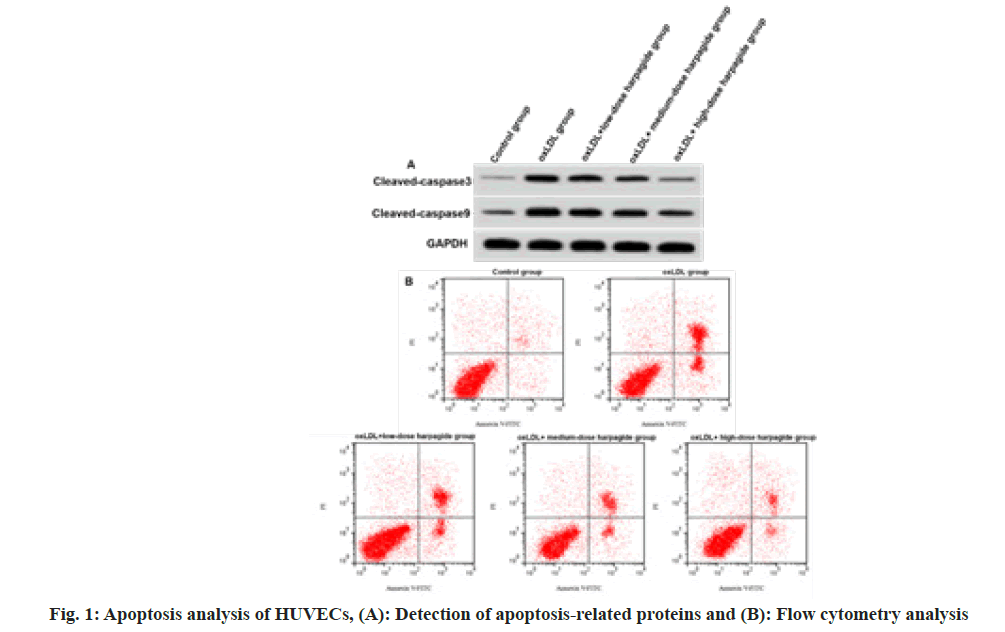
After performing a treatment with oxLDL, we observed HUVECs had increased apoptosis rate, cleaved caspase-3 and cleaved caspase-9 expressions (p<0.05) (fig. 1 and Table 2), indicating oxLDL induced HUVEC apoptosis. Harpagide at 20, 40, 80 μmol/l dose-dependently decreased oxLDL-induced HUVEC apoptosis rate, cleaved caspase-3 as well as cleaved caspase-9 protein expression (p<0.05) (fig. 1 and Table 2), suggesting that harpagide inhibited oxLDL induced HUVEC apoptosis.
| Group | Apoptotic rate (%) | Cleaved caspase-3 | Cleaved caspase-9 |
|---|---|---|---|
| Control | 5.91±0.53 | 0.12±0.02 | 0.26±0.02 |
| oxLDL | 33.28±2.25* | 0.56±0.04* | 0.75±0.05* |
| oxLDL+low-dose harpagide | 23.21±2.21# | 0.44±0.03# | 0.61±0.05# |
| oxLDL+medium-dose harpagide | 15.64±1.11#& | 0.31±0.03#& | 0.47±0.03#& |
| oxLDL+high-dose harpagide | 8.82±0.81#&$ | 0.19±0.02#&$ | 0.31±0.03#&$ |
| F | 459.316 | 345.321 | 261.25 |
| p | 0.000 | 0.000 | 0.000 |
Table 2: Harpagide-Mediated Effect on Oxldl-Induced Apoptosis (X̄±S, N=9)
Note: *p<0.05, #p<0.05, &p<0.05 and $p<0.05 indicated comparison with control, oxLDL, oxLDL+low-dose harpagide and oxLDL+medium-dose harpagide groups, respectively
As shown in Table 3, after 40 μg/ml oxLDL treatment, miR-140-5p content was decreased (p<0.05), indicating that ox-LDL inhibited miR-140- 5p. Harpagide at 20, 40, 80 μmol/l dose-dependently increased miR-140-5p after treatment with oxLDL in HUVECs (p<0.05), suggesting that harpagide promoted miR-140-5p level in oxLDL induced HUVECs.
| Group | miR-140-5p |
|---|---|
| Control | 1.00±0.00 |
| oxLDL | 0.33±0.03* |
| oxLDL+low-dose harpagide | 0.48±0.04# |
| oxLDL+medium-dose harpagide | 0.65±0.05#& |
| oxLDL+high-dose harpagide | 0.82±0.07#&$ |
| F | 321.013 |
| p | 0.000 |
Table 3: Influence of Harpagide on Mir-140-5p Expression after Treatment with Oxldl (X̄±S, N=9)
Note: *p<0.05, #p<0.05, &p<0.05 and $p<0.05 indicated comparison with control, oxLDL, oxLDL+low-dose harpagide and oxLDL+medium-dose harpagide groups, respectively
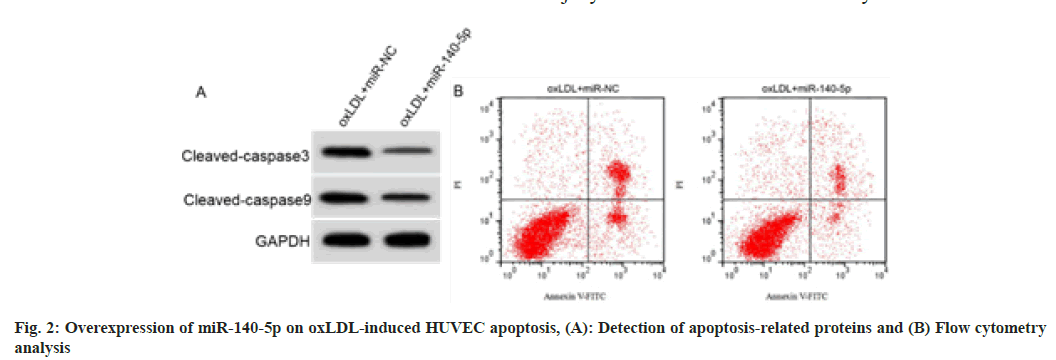
RT-qPCR analysis of cell samples indicated that HUVECs overexpressing miR-140-5p were successfully constructed (fig. 2 and Table 4). miR- 140-5p introduction decreased MDA level, cell apoptosis rate and cleaved caspase-3 and cleaved caspase-9 protein expression in oxLDL induced HUVECs, while promoted SOD and GSH-Px activities (p<0.05).
| Group | MDA (nmol/l) | SOD (U/ml) | GSH-Px (U/ml) | Apoptosis rate (%) | Cleaved caspase-3 | Cleaved caspase-9 |
|---|---|---|---|---|---|---|
| oxLDL+miR-NC | 9.46±0.66 | 17.94±1.57 | 29.04±3.22 | 34.07±3.31 | 0.58±0.05 | 0.78±0.06 |
| oxLDL+miR-140-5p | 2.44±0.23* | 44.01±4.27* | 71.23±6.84* | 12.01±1.08* | 0.24±0.02* | 0.38±0.03* |
| t | 30.132 | 17.191 | 16.742 | 19.008 | 18.941 | 17.889 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Table 4: Effect of Mir-140-5p on Oxldl-Induced Huvec Injury (X̄±S, N=9)
Note: *p<0.05 indicated comparison with oxLDL+miR-NC group
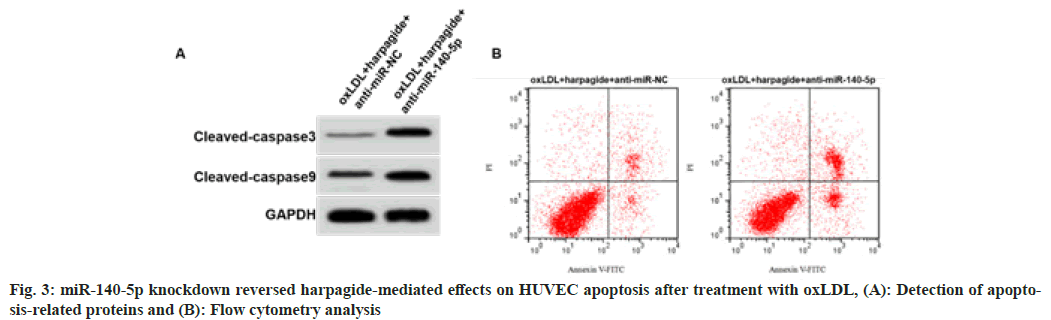
As shown in fig. 3 and Table 5, HUVECs with miR-140-5p downregulation were successfully constructed. As shown in fig. 3 and Table 5, the content of MDA, cell apoptosis rate and cleaved caspase-3 and cleaved caspase-9 protein expression in oxLDL induced HUVECs treated with 80 μmol/l harpagide was increased, SOD and GSH-Px activities were decreased after miR-140-5p knockdown (p<0.05).
| Group | MDA (nmol/l) | SOD (U/ml) | GSH-Px (U/ml) | Apoptosis rate (%) | Cleaved caspase-3 | Cleaved caspase-9 |
|---|---|---|---|---|---|---|
| oxLDL+harpagide +anti-miR-NC | 1.48±0.11 | 55.73±5.13 | 84.11±7.01 | 8.53±0.59 | 0.18±0.02 | 0.30±0.03 |
| oxLDL+harpagide +anti-miR-140-5p | 8.22±0.68* | 28.89±2.67* | 43.11±3.96* | 26.35±2.22* | 0.48±0.04* | 0.66±0.05* |
| t | 29.354 | 13.923 | 15.277 | 23.273 | 20.125 | 18.522 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Table 5: Mir-140-5p Knockdown Reversed Harpagide-Induced Effects (X̄±S, N=9)
Note: *p<0.05 indicated comparison with oxLD+harpagide+anti-miR-NC group
Cardiovascular and cerebrovascular diseases have very high rates of disability and fatality, which seriously threaten human life and health. Atherosclerosis has attracted increasing attention owing to its association with cardiovascular and cerebrovascular risks. The dysfunction of vascular endothelial cells caused by oxLDL is the key to the occurrence of atherosclerosis[14]. Thus, in-depth study on the inhibition of vascular endothelial cell dysfunction triggered via oxLDL is particularly important for atherosclerosis therapy. In this study, after HUVECs were induced by 40 μg/ml oxLDL, MDA content and apoptosis of the cells were significantly elevated, but SOD and GSH-Px activities were inhibited, which was consistent with relevant reports[15], indicating that the atherosclerosis-like injury cell model was successfully established.
In a normal body, oxidation and antioxidation are in dynamic balance. But in a pathological state, the balance is broken, and intracellular Reactive Oxygen Species (ROS) production is increased, inducing oxidative stress in cells. Oxidative stress is a way of oxLDL caused vascular endothelial cell injury, and reducing vascular endothelial cell oxidative stress can reduce vascular endothelial cell oxidative injury[16]. MDA, a product of lipid peroxidation is a marker of oxidative stress[17]. As an important antioxidant enzyme in the body, SOD can remove oxygen free radicals and reduce the damage of free radicals to cells and tissues[18]. GSH-Px is also an antioxidant enzyme, which can cooperate with SOD to play an antioxidant role. Harpagide inhibited oxidative stress and apoptosis of rat cardiomyocytes induced by hydrogen peroxide and has potential value in cardiovascular disease therapy[19]. Our data indicated harpagide decreased MDA production in oxLDL induced HUVECs and promoted SOD and GSH-Px activities, indicating that harpagide could inhibit oxLDL induced oxidative stress.
Increasing evidence demonstrates that excessive oxidative stress can induce cell apoptosis and excessive apoptosis of vascular endothelial cells can destroy cardiovascular homeostasis, finally causing endothelial dysfunction and promoting the development of atherosclerosis[20]. Our data indicated harpagide reduced oxLDL induced HUVEC apoptosis, suggesting that harpagide could effectively reduce oxLDL induced HUVEC apoptosis. Apoptosis is influenced via various gene molecules and signaling pathways, among which caspase cascade regulates cell apoptosis. Caspase-9 and caspase-3 are key molecules in the caspase cascade, among which caspase 9, as the initial molecule of the caspase cascade, is activated to cleaved caspase 9 when receiving apoptotic signals, which transmits the apoptotic signals down. Caspase 3 is at the core of the caspase cascade, cleaved caspase 3 is generated when stimulated by upstream signals[21]. In this study, harpagide effectively reduced cleaved caspase-9 and cleaved caspase-3 expression after oxLDL treatment, suggesting harpagide may reduce oxLDL induced HUVEC apoptosis by inhibiting the caspase cascade.
To further determine the molecular mechanism of harpagide-mediated inhibition in HUVEC injury, this work continued to examine the influence of harpagide on miR-140-5p expression after oxLDL treatment. As expected, oxLDL inhibited miR-140- 5p content in HUVECs, and harpagide promoted its expression after oxLDL treatment, suggesting that harpagide may affect oxLDL induced HUVEC injury through interaction with miR-140-5p. Considerable studies have shown that miR-140- 5p is downregulated in a mouse cell model of acute lung injury and the overexpression of miR- 140-5p can repress TLR4/Myeloid Differentiation Primary Response Protein 88 (MyD88)/Nuclear Factor Kappa B (NF-κB) signaling pathway as well as inflammation induced by acute lung injury[22]. miR-140-5p can protect cisplatin-induced oxidative stress in a mouse cell model of acute kidney injury via activating Nuclear Factor Erythroid 2–Related Factor 2 (Nrf2)-dependent antioxidant pathways[23]. In this study, overexpression of the miRNA decreased oxLDL induced oxidative stress as well as reduced HUVEC apoptotic rate. Additionally, knockdown of miR-140-5p reversed harpagide induced inhibition of oxLDL induced HUVEC injury, suggesting that harpagide inhibited oxLDL induced HUVEC injury via upregulating miR-140-5p.
In conclusion, harpagide inhibited oxLDL induced oxidative stress and apoptosis of HUVECs and its mechanism may be associated with increased level of miR-140-5p in cells, which may be used as a drug for atherosclerosis. However, the work only used in vitro cell experiments for preliminary exploration, and animal experiments are needed to verify the influence of harpagide on atherosclerosis.
Funding:
This work was supported by Natural Science Foundation of Liaoning (NO.20180540141).
Author’s contributions:
He Huang and Lei Zhao have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests
References
- Li M, Wang ZW, Fang LJ, Cheng SQ, Wang X, Liu NF. Programmed cell death in atherosclerosis and vascular calcification. Cell Death Dis 2022;13(5):467.
[Crossref] [Google Scholar] [PubMed]
- Keeter WC, Ma S, Stahr N, Moriarty AK, Galkina EV. Atherosclerosis and multi-organ-associated pathologies. Semin Immunopathol 2022;44(3):363-74.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Zhang C, Liu Q H. Allicin alleviates the apoptosis of vascular endothelial cells induced by oxidative low-density lipoprotein by inhibiting lectin-like oxidation of low-density lipoprotein receptor-1. Acta Nutrimenta Sin 2019;41(4):73-9.
- Guo JT, Wang L, Yu HB. Knockdown of NEAT1 mitigates ox-LDL-induced injury in human umbilical vein endothelial cells via miR-30c-5p/TCF7 axis. Eur Rev Med Pharmacol Sci 2020;24(18):9633-44.
[Crossref] [Google Scholar] [PubMed]
- Jiang H, Zhou Y, Nabavi SM, Sahebkar A, Little PJ, Xu S, et al. Mechanisms of oxidized LDL-mediated endothelial dysfunction and its consequences for the development of atherosclerosis. Front Cardiovasc Med 2022;9:925923.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Lin F, Yan Z, Chen Z, Chen Y, Zhao Y, et al. Salidroside downregulates microRNA-133a and inhibits endothelial cell apoptosis induced by oxidized low-density lipoprotein. Int J Mol Med 2020;46(4):1433-42.
[Crossref] [Google Scholar] [PubMed]
- Wang F. Protection of harpagide on cytotoxicity in PC12 cells induced by Aβ25-35 peptides. Chin Tradit Herbal Drugs 2015;46(23):3539-43.
- Wang K, Wang T, Xu H, Zhong X, Huang Z. Harpagide exerts a neuroprotective effect by inhibiting endoplasmic reticulum stress via SERCA following oxygen-glucose deprivation/reoxygenation injury. Neurosci Lett 2021;753:135874.
[Crossref] [Google Scholar] [PubMed]
- Sun MY, Li LP. miR-140-5p targets BCL2L1 to promote cardiomyocyte apoptosis. Eur Rev Med Pharmacol Sci 2020;24(11):6311-22.
[Crossref] [Google Scholar] [PubMed]
- Huang X, Qiao F, Xue P. The protective role of microRNA-140-5p in synovial injury of rats with knee osteoarthritis via inactivating the TLR4/Myd88/NF-kappa B signaling pathway. Cell Cycle 2019;18(18):2344-58.
[Crossref] [Google Scholar] [PubMed]
- Wang S, Cui Y, Xu J, Gao H. miR-140-5p attenuates neuroinflammation and brain injury in rats following intracerebral hemorrhage by targeting TLR4. Inflammation 2019;42(5):1869-77.
[Crossref] [Google Scholar] [PubMed]
- Liu H, Mao Z, Zhu J, Shen M, Chen F. miR-140-5p inhibits oxidized low-density lipoprotein-induced oxidative stress and cell apoptosis via targeting toll-like receptor 4. Gene Ther 2021;28(7-8):413-21.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z J, Hao Y, Wan TT. Effects of saggliptin on vascular endothelial cell injury induced by ox-LDL and expression of miR-590/TLR4/NF-κB. Chin J Arterioscler 2019;27(11):23-8.
- Zhang B, Zhang YF, Li R, Zhao L, Qin SG, Pan LF, et al. miR-217 inhibits apoptosis of atherosclerotic endothelial cells via the TLR4/PI3K/Akt/NF-κB pathway. Eur Rev Med Pharmacol Sci 2020;24(24):12867-77.
[Crossref] [Google Scholar] [PubMed]
- Zhu L, Gong X, Gong J, Xuan Y, Fu T, Ni S, et al. Notoginsenoside R1 upregulates miR-221-3p expression to alleviate ox-LDL-induced apoptosis, inflammation and oxidative stress by inhibiting the TLR4/NF-κB pathway in HUVECs. Brazil J Med Biol Res 2020;53(6):e9346.
[Crossref] [Google Scholar] [PubMed]
- Zhang ZL, Ren QZ, Meng XZ. Protective effect of acteoside on oxLDL-induced human umbilical vein endothelial cells injury by regulating CREG expression. Chin J Pharm 2020;23(9):1725-30.
- Yao L, Yan H. miR-182 inhibits oxidative stress and epithelial cell apoptosis in lens of cataract rats through PI3K/Akt signaling pathway. Eur Rev Med Pharmacol Sci 2020;24(23):12001-8.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Zhang S, Liu X, Dai J, Yan Z, Wu C. Effects of dendrobium polysaccharides on human brain microvascular endothelial cell injury induced by ox-LDL via regulating the miR-378 expression. Cell Mol Biol 2020;66(7):66-71.
[Google Scholar] [PubMed]
- Xu B, Guo W, Liu DP. Protective effect of harpagide on H2O2-induced oxidative stress injury of rat cardiomyocytes H9C2. Chin J Integ Med Cardio Cerebrovasc Dis 2019;17(15):2276-81.
- Xu Y, Miao C, Cui J, Bian X. miR-92a-3p promotes ox-LDL induced-apoptosis in HUVECs via targeting SIRT6 and activating MAPK signaling pathway. Braz J Med Biol Res 2021;54(3):e9386.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Duan JZ, He Q, Wang CQ. miR-155 modulates high glucose-induced cardiac fibrosis via the Nrf2/HO-1 signaling pathway. Mol Med Rep 2020;22(5):4003-16.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Liu D, Xi Y, Li J, Liu B, Li J. Upregulation of miRNA-140-5p inhibits inflammatory cytokines in acute lung injury through the MyD88/NF-κB signaling pathway by targeting TLR4. Exp Ther Med 2018;16(5):3913-20.
[Crossref] [Google Scholar] [PubMed]
- Liao W, Fu Z, Zou Y, Wen D, Ma H, Zhou F, et al. microRNA-140-5p attenuated oxidative stress in cisplatin induced acute kidney injury by activating Nrf2/ARE pathway through a Keap1-independent mechanism. Exp Cell Res 2017;360(2):292-302.
[Crossref] [Google Scholar] [PubMed]