- *Corresponding Author:
- P. K. Sarkar
Department of Rasashastra and Bhaishajya Kalpana including Drug Research,Jamnagar - 361 008, India
E-mail: prasantsarkar@indiatimes.com
| Date of Submission | 14 September 2006 |
| Date of Revision | 23 August 2007 |
| Date of Acceptance | 22 November 2007 |
| Indian J. Pharm. Sci., 2007, 69 (6): 791-795 |
Abstract
The present study was carried out to evaluate the haematinic effect of two ayurvedic preparations of iron on mercuric chloride-induced anemia in rats. Lauha bhasma and Mandura bhasma, two well-known ayurvedic iron preparations, are commonly used to treat anemia. In Charles Foster strain rats of either sex, anemia was induced by administering mercuric chloride (9 mg/kg). Lauha bhasma and Mandura bhasma (11 mg/kg) were evaluated for their haematinic activity. The observed results suggest that Lauha bhasma and Mandura bhasma possess significant (P<0.05) haematinic and cytoprotective activity.
Keywords
Lauha Bhasma, Mandura Bhasma, iron, mercuric chloride, anemia, haematinic
Mercury and its compounds have had a long history in medicine. While not as important in modern medicine today, certain mercury salts are still used widely in ayurvedic system of medicine. Metallic mercury is relatively non-toxic. The mercurous (Hg+) and mercuric (Hg++) cations are toxic [1]. Mercury vapor, however, is toxic. Mercury poisoning from inhaling mercury vapour is believed to have occurred in scientists working with mercury, in industrial situations and in people living near industrial plants emitting mercury vapour in the air [2,3]. The classic example used to illustrate mercury poisoning is that of the fishermen and their families living around Minamata Bay in Japan [4]. Mercury induced haematological effect among occupationally or accidentally exposed human beings are well established [5] and its effects on experimental animals are also well documented [6].
The empirical use of different preparations of iron in the treatment of anaemia dates from ancient times [7]. The incinerated iron preparations of ayurveda are known as Lauha bhasma, Mandura bhasma, Abhraka bhasma and so on. The preparation of these bhasmas are followed according to unique methods described by ancient scholars includes shodhana (purification), marana (incineration) and several grades of incineration under the term puta. Incineration of iron is done not only for making it finer but also for increasing its quality [8], so that it can be specifically effective for the eradication of different ailments.
Lauha bhasma and Mandura bhasma are the two most commonly used preparations of incinerated iron. These are indicated for the same as well as different diseases. So many studies have so far been carried out on individual preparation. Pandit et al, investigated Lauha bhasma for haematinic activity and haemoglobin regeneration efficacy on agar gel diet and phlebotomy induced iron deficiency anemia in rats and reported significant haematinic and haemoglobin regeneration efficiency in comparison to control and standard ferrous sulphate containing drug [9]. Kanase et al. studied curative effects of Mandura bhasma on liver and kidney of rats and noticed total recovery in two weeks [10]. But comparative scientific study of these two bhasma preparations at pharmacological level is very less. So the present programme was undertaken to evaluate the haematinic activity of these two bhasma preparations on mercuric chloride-induced anemia in Charles Foster rats. The present study confirms to the guidelines laid by the Institutional Animals Ethical Committee.
In this study, anemia was induced in rats by administering mercuric chloride solution. Accumulation of mercury in the blood of mice has already been proved using inorganic radio mercury [11]. Mercury induced anemia was also reported in mice exposed to 0.1 mg and 0.5 mg of HgCl2 for 100 d and 30 d respectively via drinking water [12].
Materials and Methods
Charles Foster rats of either sex weighing between 180-250 g were used for this study. They were obtained from the animal house attached to the pharmacology laboratory. They were housed in breeding cases at an ambient temperature with a natural day and night cycles. The animals had free access of Amrut brand rat pellet feed supplied by Pranav Agro Industries and tap water.
Lauha bhasma and Mandura bhasma were prepared in practical laboratory of Department of Rasashastra and Bhaishajya Kalpana, I.P.G.T. and R.A., Gujarat Ayurved University. The therapeutic dose of Lauha bhasma and Mandura bhasma is 65 mg to 250 mg/d8. In the present study, human dose of both the bhasma preparations has been decided to be 125 mg/d. The dose for experimental study of the drugs was calculated by extrapolating the human dose to animal dose based on the body surface area ratio. Drug suspension was prepared in 3% gum acacia solution (1 ml in 50 ml distilled water) and solution of HgCl2 was prepared by dissolving it in absolute alcohol (50 mg in 1 ml) and adding distilled water.
Experimental design
In the present study, mercuric chloride (HgCl2) was used to induce anemia in rats. Solution of HgCl2 was administered in 9 mg/kg dose through oral route by No.3 simple rubber catheter for 30 d. This protocol was arrived at after carrying out initial pilot studies. In the treated groups, suspension of test bhasmas (Lauha Mandura bhasma) was given in 11 mg/kg dose along with mercuric chloride solution (9 mg/kg). A control group was also kept to observe the significant occurrence of anemia.
Study protocol
A total of 24 Charles Foster rats of either sex weighing between 180-250 g were taken and divided randomly into 4 groups, each containing 6 animals, 3 male and 3 females each. The treatment schedule was as follows, group I comprised of vehicle (50 ml distilled water with 1 ml of absolute alcohol) treated control animals, group II animals received 9 mg/kg solution of HgCl2, group III animals were treated with 9 mg/kg solution of HgCl2 and 11 mg/kg suspension of Lauha Bhasma, and group IV animals were treated with 9 mg/kg solution of HgCl2 and 11 mg/kg suspension of Mandura Bhasma. Suspensions of test drugs were prepared in 2.2 mg/ml, concentration and solution of HgCl2 was prepared in 1.8 mg/ml, concentration.
The treatment schedule was continued for 30 d with daily doses of test drugs and vehicle. Gross behaviour was observed throughout the study period. On 31st day rats were sacrificed by stunning, blood was collected from jugular vein for haematological and biochemical tests, spleens were collected for the histopathological study.
Statistical analysis
All the values were expressed as mean±SEM (standard error of mean). The data were analysed by student’s ’t’ test and ANOVA (Dunnet’s ‘t’ test). A level of P<0.05 was considered as statistically significant. Level of significance was noted and interpreted accordingly.
Results and Discussion
The mean body weight of the albino rats in different treatment groups was recorded before and after drug treatment. The data pertaining to the effect of test drugs on body weight changes have been presented in Table 1. A significant decrease (P<0.05) in body weight was found in HgCl2-treated group. A slight but not significant decrease in body weight was obtained in Lauha bhasma with HgCl2 treated group. An apparent increase in body weight was observed in both Mandura bhasma with HgCl2-treated and control groups.
| Group | Dose (mg/kg) |
Body weight (g) | ||
|---|---|---|---|---|
| Initial | Final | Change in % | ||
| Control | - | 214.0±09.0 | 221.0±16.0 | 2.7±3.7 |
| HgCl2 | 09 | 215.3±07.7 | 201.0±11.5 | 6.9±2.5* |
| Lauhabhasma+ | 11+09 | 209.6±09.3 | 204.8±06.4 | 1.3±5.9 |
| HgCl2 | ||||
| Mandurabhasma+ | 11+09 | 203.6±10.0 | 209.2±10.4 | 3.2±5.4 |
| HgCl2 | ||||
Figures in this parenthesis indicate changes in body weight after 30 days of treatment. *P<0.05 by the student’s ‘t’ test
Table 1: Changes in body weight in control and treated rats
Loss of body weight is a common clinical feature of anemia. In Lauha bhasma with HgCl2-treated group the decrease in body weight was marginal and it was increased in Mandura bhasma with HgCl2-treated group. This reversal of body weight decrease by Mandura bhasma could be considered as a significant effect. It indicates reversal of the toxicant induced tissue degenerative changes. In this respect, Mandura bhasma is better in comparison to Lauha bhasma. Body weight change is the sum of the effects occurring in different parts of the body and reversal of the toxicant induced decrease is an index of good tissue or cytoprotective activity of the test drugs.
Post treated haematological parameters in vehicle control, HgCl2 control, Lauha bhasma and HgCl2 and Mandura bhasma and HgCl2-treated groups were estimated and have been presented in Table 2. A statistically significant (P<0.05) decrease in mean haemoglobin level, total RBC count and haematocrit value was obtained in HgCl2 control group in comparison to vehicle control group. A statistically significant (P<0.05) increase in mean haemoglobin level, total RBC count and haematocrit value was observed in Lauha bhasma with HgCl2- treated group in comparison to HgCl2 control group. A statistically significant increase (P<0.05) in mean haemoglobin level and haematocrit value and a statistically significant (P<0.05) increase in total RBC count was observed in Mandura bhasma with HgCl2- treated group in comparison to HgCl2 control group. Here Dunnet’s ‘t’ test to determine effect of test drugs against HgCl2 control group shows Mandura bhasma has more significant effect than Lauha bhasma on haemoglobin level, total RBC count and haematocrit value. Other haematological findings do not show significant adverse sign except marginal to moderate changes in platelet indices in HgCl2-treated group and most of the changes were found to be reversed by test drugs administration.
| Parameters | Groups (Dose mg/kg) | |||
|---|---|---|---|---|
| Control | HgCl2 (9) | Lauhabhasma + HgCl2 (11+9) | Mandurabhasma + HgCl2 (11+9) | |
| Hb g % | 15. 18±0.42 | 12.60±0.34* | 14.10±0.43*# | 14.54±0.37*# |
| RBC 1012/l | 8.58±0.32 | 7.19±0.20* | 8.20±0.26" | 8.44±0.39*# |
| Haematocrit % | 49.50±1.3 | 41.95±0.8* | 45.92±1.4" | 48.08±1.0*# |
| RDW % | 14.6±0.86 | 10.6±2.13 | 13.9±0.69 | 13.6±1.10 |
| MCV fl | 57. 85±0. 81 | 58.45±0.91 | 56.06±0.85 | 57.24±1.67 |
| MCH pg | 17.73±0.30 | 17.57±0.18 | 17.22±0.41 | 17.30±0.56 |
| MCHC g/dl | 30.67±0.31 | 30.07±0.52 | 30.72±0.49 | 30.22±0.28 |
| TLC 109/l | 7.1±1.4 | 7.0±1.7 | 8.2±0.8 | 5.2±0.7 |
| Polymorph % | 33.7±5.1 | 35. 5±6.6 | 30.2±6.0 | 23.8±1.4 |
| Lymphocyte % | 65.2±5.4 | 63.3±6.7 | 67.0±5.2 | 76.2±1.4 |
| Platelet 109/l | 1096.3±137.5 | 847.7±142.4 | 1168.0±196.1 | 1119.4±114.0 |
| Plateletcrit % | 11.1±0.95 | 08.5±1.27 | 09.2±2.07 | 07.3±0.89 |
| PDW % | 09.42±0.22 | 11.03±0.72 | 08.88±0.49* | 08.52±0.23* |
| MPV fl | 7.82±0.13 | 6.92±0.23* | 7.34±0.32 | 7.26±0.18 |
Table 2: Haematological observations after treatment in control and treated rats
HgCl2 is a severe corrosive and leads to irritability, stomatitis and colitis. It is toxic to liver and kidney leading to uremia [13]. Reduction in the serum erythropoietin due to liver and kidney damage and uremia cause hemolysis and bone marrow depression leads to decrease in haematocrit, RBC count and haemoglobin percentage [14]. The blood picture in the present study supports this feature. Here haemoglobin content of blood, haematocrit and total RBC content were decreased in HgCl2 treated group. And these were increased in the Lauha bhasma with HgCl2 and Mandura bhasma with HgCl2 administered groups, suggestive of haematinic effect of Lauha bhasma and Mandura bhasma. However the exact mechanism of alleviation of the HgCl2-induced anemia is not known from the available data. It would be interesting to ascertain whether the increase is due to increased availability of iron in the usable form or is due to cytoprotective effect of the preparations considering the fact that they are not simply iron preparations but a cocktail of many trace elements [15].
Observed biochemical parameters after treatment have been presented in Table 3. A statistically significant increase in blood sugar and blood urea and significant decrease in serum alkaline phosphatase and SGOT level was observed in HgCl2-treated group. Other parameters were not affected to significant extent. Both the drugs showed tendency towards reversal of these toxicant induced changes. The changes observed after HgCl2 administration can be mainly attributed to the toxicant induced liver damage. Reversal of most of these changes by test drugs administration indicates that they do have some element of cytoprotective activity.
| Parameters | Groups (Dose mg/kg) | |||
|---|---|---|---|---|
| Control | HgCl2 (9) | Lauhabhasma + HgCl2 (11+9) | Mandurabhasma + HgCl2 (11+9) | |
| Blood sugar (mg/dl) | 082.5±04.55 | 128.3±11.85* | 106. 3±06. 46 | 082.1±02.50* |
| Blood urea (mg/dl) | 36.82±1.78 | 44.42±3.03* | 29.64±1.72* | 31.46±0.98* |
| 5 Creatinine (mg/dl) | 1.02±0.04 | 0.96±0.02 | 0.90±0.03 | 0. 83±0 .03 14 |
| 5 Cholesterol (mg/d0 | 56.98±4.9 | 68.06±6.3 | 43.04±4.3" | 43.06±4.4* |
| S. Triglyceride (mg/dl) | 85.8±6.99 | 80.4±8.79 | 56.06±0.85 | 57.24±1.67 |
| S Total protein (g/dl) | 7.3±0.14 | 7.9±0.27k | 7.1±0.33 | 7. 3±0. 34 |
| SGOT (IWO | 482.5±19.2 | 294.2±52.2* | 366.8±57.0 | 368.4±32.6 |
| 5 Alkaline phos. (IUR) | 36.35±5.6 | 13.13±3.2* | 38.32±4.5* | 43.98±2.9* |
The pattern of changes in biochemical constituents after treatment for 30 days. Statistical significance at *P<0.05; by the student’s ‘t’ test. HgCl2 treated group is compared with control group; test drug treated groups are compared with HgCl2 treated group
Table 3: Biochemical observations after treatment in control and treated rats
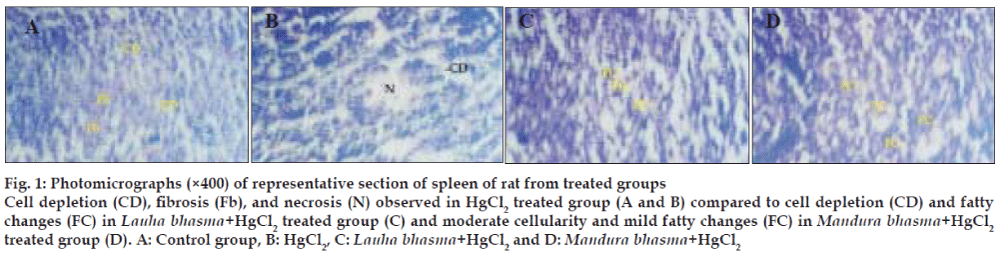
Microscopic examination of sections of spleen from different groups was observed under microscope at different magnifications. Normal cytoarchitecture of spleen was observed in control group, whereas cell depletion, fibrosis and necrosis were observed in the cytoarchitecture of spleen in HgCl2 treated group. In the Lauha Bhasma with HgCl2 treated group mild fatty changes and cell depletion was observed. In Mandura Bhasma with HgCl2 treated group only mild fatty changes with moderate cellularity in the cytoarchitecture of spleen was observed. Photographs from representative sections of spleen from different groups have been presented in fig. 1.
Fig. 1: Photomicrographs (×400) of representative section of spleen of rat from treated groups
Cell depletion (CD), fibrosis (Fb), and necrosis (N) observed in HgCl2 treated group (A and B) compared to cell depletion (CD) and fatty
changes (FC) in Lauha bhasma+HgCl2 treated group (C) and moderate cellularity and mild fatty changes (FC) in Mandura bhasma+HgCl2 treated group (D). A: Control group, B: HgCl2, C: Lauha bhasma+HgCl2 and D: Mandura bhasma+HgCl2
Spleen is the store house of dead RBC and breakdown of hemoglobin also occurs in the spleen. Hemolytic anaemia leads to accelerated breakdown of hemoglobin causing larger iron deposition in spleen [16]. This is likely to be the cause of fibrosis and necrosis observed in the spleen in HgCl2 treated group. This disturbance in the cytoarchitecture was significantly reversed by test drugs administration. In this respect Mandura bhasma was comparatively better because in addition to attenuating the fibrosis, it restored cellularity to moderate level thus inhibiting the toxicant induced cell depletion. Lauha bhasma also produced significant protective effect against necrosis and fibrosis but it did not reverse the cell depletion.
Analysis of the data of the haematinic study reveals both Lauha bhasma and Mandura bhasma have significant haematinic activity. Both are equiactive in this respect. However taking into consideration, changes in all the parameters it can be suggested that though both the drugs are effective as haematinic agents, Mandura bhasma has the better activity profile especially in the light of changes observed in ponderal and histopathological parameters. It seems to possess significant haematinic and cytoprotective activities.
References
- Grant N. Mercury in man. Environment 1971;13:1-15.
- McIntyre AR. The toxicities of mercury and its compounds. J ClinPharmacol New Drugs 1971;11:397-9.
- Goldwater LJ. Mercury in the environment. Sci Am 1971;224:15-21.
- Sharma DC. Man, mercury and minamata disease. Nagarjun 1980;9:19-21.
- Sauder PHF, Livardjani F, Jaeger A. Acute mercuric chloride intoxication, effects on hemodialysis and plasma exchange on mercury kinetics. ClinToxicol 1988;26:189-97.
- Rathore HS, Vaghese J. Effect of mercuric chloride on the survival, food-intake, body weight, histological and haematological changes in mice and their prevention with Liv-52. Ind J OccuptHlt 1994;37:42-54.
- Sharma RK, Dash B, editors. CarakaSamhita. Vol. III. 4th ed. Varanasi: Chowkhamba Sanskrit Series Office; 2000.
- Shastri KN, editor. Rasatarangini. 11 th ed. Varanasi: MotilalBanarasi Das; 2000.
- Pandit S, Biswas TK, Debnath PK, Saha AV, Chowdhury U, Shaw BP, et al. Chemical and pharmacological evaluation of different ayurvedic preparations of iron. J Ethnopharmacol 1999;65:149-56.
- Kanase A, Patil S, Thorat B. Curative effects of Mandurabhasma on liver and kidney of albino rats after induction of acute hepatitis by CCl 4. Indian J ExpBiol 1997;35:754-64.
- Mehra M, Kanwar KC. Absorption, distribution and excretion of Hg203 in mice. Bull Environ ContamToxicol 1979;21:733-8.
- Rathore HS, Siddiqui S. Prevention of mercuric chloride induced haematological effects in mice with a homeopathic drug. Indian Drugs 2000;37:383-5.
- Satoskar RS, Bhandarkar SD, Ainapure SS. Pharmacology and pharmacotherapeutics. Vol. 2. 15th ed. Mumbai: Popular Prakashan; 1997.
- Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. Vol. II. 15th ed. New Delhi: The McGraw Hill Companies; 1998.
- Sarkar PK, Prajapati PK, Choudhary AK, De S. Physico-chemical Evaluation of LauhaBhasma and ManduraBhasma . Indian Drugs 2007;44:21-6. Back to cited text no. 15
- Chatterjee CC. Human physiology. Vol. I. 10th ed. Kolkatta: Medical Allied Agency; 1994.