- *Corresponding Author:
- Siwei Yu
Department of Critical Care Medicine, Shanghai Electric Power Hospital, Changning, Shanghai 200031, China
E-mail: ysw1231237788@163.com
| This article was originally published in a special issue, “Advanced Targeted Therapies in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(1) Spl Issue “140-147” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In emergency and critical care medicine, lung ischemia-reperfusion injury is a prevalent disorder, which has significant morbidity and mortality. However, there is still a lack of effective means to block the lung ischemia-reperfusion injury. Established the lung ischemia-reperfusion injury model of mice; lung injury and histological analysis scoring were helpful to assess pathological injury in lung tissue; using an enzymelinked immunosorbent assay kit, expressed interleukin-6 and interleukin-1 in bronchoalveolar lavage fluids was determined. Seahorse analysis was used to examine the oxygen consumption rate and production of adenosine triphosphate. The expression of mitochondrial function-related genes was detected by realtime polymerase chain reaction; commercially available assay kits used for find the malondialdehyde production and catalase activity; apoptotic cells were detected using terminal deoxynucleotidyl transferase dUTP nick end labeling and protein expression was discovered using Western blotting. Ginsenoside alleviated the pathological changes caused by lung ischemia-reperfusion injury and reduced the lung injury score; ginsenoside Rd therapy reduced inflammatory cell infiltration significantly decreased lung bronchoalveolar lavage fluids interleukin-1 and interleukin-6 levels; ginsenoside Rd also increased the respiratory rate and the adenosine triphosphate production and regulated mitochondrial-related gene expression. Moreover, ginsenoside Rd ameliorated the lung ischemia-reperfusion injury-associated decrease in superoxide dismutase 2 expression, increase in malondialdehyde content and reduction in catalase activity. Additionally, ginsenoside Rd decreased lung ischemia-reperfusion injury-induced apoptosis. Ginsenoside Rd regulates the mitochondrial activity and thus protects against lung ischemiareperfusion injury.
Keywords
Inflammation, pulmonary embolism, apoptosis, reperfusion
The Acute Lung Injury (ALI) known as lifethreatening disease with high rates in morbidity and mortality. Reactive Oxygen Species (ROS) involved in pathogenesis of ALI. Lung Ischemia-Reperfusion Injury (LIRI) is a cause of the high mortality and it is widespread case of emergency and critical care medicine, with increased rates of pulmonary embolism, cardiopulmonary bypass and cardiopulmonary resuscitation in patients receiving lung transplants and pulmonary thrombosis[1-3]. Pathogenesis nature of LIRI is extremely difficult. Inflammation, oxidative stress, intracellular calcium excess, protease release and the depletion of protective mediators including lung surfactant and Nitric oxide (NO) have all been linked to it in studies[4-7]. However, there are currently no viable treatments for LIRI. Therefore, increasing attention has recently been given to investigating the pathophysiologic process of LIRI and identifying better treatments.
Mitochondrial dysfunction and damage are firmly and directly related to the pathogenesis of LIRI[8,9]. Since early 1960s, mitochondrial dysfunction was reported in cells of the lung in individuals and in experimental models of chronic and acute respiratory diseases. But it was only with the emergence of more useful tools and method that mechanism of this enigmatic organelle regulating cellular homeostasis in lung.
Mitochondria not only act as the “engines” of body cells but also produce ROS. It regulates the biosynthesis of intracellular calcium, iron and sulfur and participates in the transduction of intracellular signals of activities such as apoptosis[10]. ROS levels rise dramatically after reperfusion. Endogenous ROS can cause the permeability transition pore of mitochondria to be open, allowing Cytochrome c (Cyto c) and to enter the cytoplasm, where they work together to activate caspase and initiate apoptosis[9,11].
Ginsenoside Rd (GSRd) is a kind of ginsenoside that is mainly extracted from Panax ginseng (P. ginseng) or Panax notoginseng. It is an important bioactive substance in P. ginseng and is often used to prevent and treat ischemic and degenerative diseases of the central nervous system[12]. According to recent studies, numerous functions of GSRd include anti-inflammatory, anti-apoptosis, anti-cancer, anti-oxidative stress and induction of neurogenesis[13-16]. Notably, GSRd can ameliorate tissue damage and inhibit neuronal apoptosis caused by Ischemia/Reperfusion (I/R) through its antioxidation activity[17]. GSRd has been found in studies to successfully by lowering mitochondrial damage to neurons, it can prevent oxidative stressinduced nerve cell damage, assist energy metabolism system reconstitution and inhibit neuron death, making it a neuroprotective agent[18]. However, it remains unclear whether GSRd could ameliorate LIRI and whether this beneficial effect involves regulation of the mitochondrial damage. Therefore, this study’s objective was to investigate in a mouse model the preventive role on LIRI and the mechanism underlying it.
Materials and Methods
Drugs and reagents:
Tai-He Biopharmaceutical (Guangzhou, China) provided the GSRd, while cell signaling technology gave all of the antibodies (Danvers, MA, USA). The Terminal deoxynucleotidyl transferase dUTP Nick End Labeling (TUNEL) assay was acquired from Kangwei Biotechnology (Beijing, China); Lianke Biotechnology supplied the Interleukin (IL)-6 and IL-1 Enzyme-Linked Immunosorbent Assay (ELISA) kits; and Nanjing Jiancheng Bioengineering Institute (Nanjing, China) provided the Malondialdehyde (MDA) production and Catalase (CAT) activity assay kits (Hangzhou, China). Sigma–Aldrich provided the pentobarbital (MO, USA). Other reagents purchased from Invitrogen. Primers were conducted by Sangon Biotechnology (Shanghai, China).
Animals and study groups:
C57BL/6 mice (weighing 20-25 g) were donated by SLAC Laboratory Animal Co. Ltd (Shanghai, China). All the animal testing was done according with approved protocols that were approved from Ethics Committee. The six groups; sham, I/R, I/ R+saline, I/R+GSRd (10 mg/kg), I/R+GSRd (40 mg/kg) and I/R+GSRd (100 mg/kg) were chosen at random to receive the mice. The mice in the sham group also weren't clamped and only subjected to the left pulmonary. Mice in I/R+GSRd categories were injected intraperitoneally with varying amounts of GSRd 3 d prior to ischemia. Mice in the I/R+saline group received an intraperitoneal injection of the same quantity of saline 3 d prior to ischemia. The lung I/R model created accordance to Fei et al. methods[19]. The animals were anaesthetized by using pentobarbital (50 mg/kg) and after being clamped for an hour, the left pulmonary hilum underwent 6 h of reperfusion. The left lung was excised for additional research after modeling.
Histological analysis and lung injury scoring system:
Before being sliced in 5 m slices, the lung tissues preserved by using 4 % paraformaldehyde up to 24 h. Next, paraffin-embedded lung sample were stained by using Hematoxylin and Eosin (HE). Similar to how it was previously described, lung damage was graded[19]. Firstly, the degree of inflammatory cell aggregation or infiltration in vessel walls or air space: 1 suggests only a wall, 2 shows rare cells in the air space, 3 shows moderate and 4 represents severe (congested air space). Secondly, the severity of hyaline membrane formation and interstitial congestion in the lungs: 1=normal lung, 2=mild (>25 % of lung section), 3=intermediate (25 %-50 % of lung section) and 4=severe (>50 % of lung section). Hemorrhage was the 3rd criterion: 0 means no bleeding and 1 means it was present. Six fields were evaluated at 400 magnifications for each mouse. Two pathologists were blind to the animal category and evaluated the samples separately.
Ratio of lung Dry/Wet (D/W) weight:
Lung W/D weight ratio was used as an index of lung water accumulation after the instillation of the Adenosine Triphosphate (ATP). The animals were dissected under sevoflurane anesthesia and the lung weight was measured immediately after its excision (W weight). 6 h after reperfusion, the left lung's moist weight was determined. The lung specimens were then dried and incubated at 60° for 96 h.
Real-Time quantitative Polymerase Chain Reaction (RT-qPCR):
In accordance with the directions provided by the PrimeScript RT reagent kit, total Ribonucleic Acid (RNA) was extracted from lung tissues (TaKaRa, Japan) and the reaction system was configured for reverse transcription into complementary Deoxyribonucleic Acid (cDNA). Then reverse transcription-PCR was carried out as shown in Table 1.
| Gene | Forward | Reverse |
|---|---|---|
| Drp1 | ATGCCAGCAAGTCCACAGAA | TGTTCTCGGGCAGACAGTTT |
| Fis1 | CAAAGAGGAACAGCGGGACT | ACAGCCCTCGCACATACTTT |
| Mfn2 | TGCACCGCCATATAGAGGAAG | TCTGCAGTGAACTGGCAATG |
| OPA1 | ACCTTGCCAGTTTAGCTCCC | TTGGGACCTGCAGTGAAGAA |
| GAPDH | TTCCCGTTCAGCTCTGGG | CCCTGCATCCACTGGTGC |
Table 1: The Primers of Gene
ELISA:
The supernatant was collected for ELISA and the Bronchoalveolar Lavage Fluids (BALFs) centrifuged at 4° (400 g, 15 min). Following the manufacturer's instructions, the ELISA kits used to measure levels of IL-6 and IL-1.
Inflammation cells counts:
Under an optical microscope, neutrophils, total cells and BALFs from each group's lungs were counted using a cell counting plate.
Oxygen Consumption Rate (OCR) and ATP production:
Following the instructions of the manufacturer, 2 mm pieces of lung tissue were injected into the XF24 hippocampus plate and the Seahorse XF24 extracellular flux analyzer was used to assess the OCR (Seahorse Bioscience, North Billerica, MA, USA).
CAT activity and MDA content:
A commercially available MDA assay kit and a CAT assay kit were used to detect MDA generation and CAT activity in tissue, respectively, according to the manufacturer's methods.
TUNEL staining:
According to manufacturer's instructions, TUNEL tests utilized to compare the rates of apoptosis in various groups. The total cells were assessed by 4',6-diamidino-2-phenylindole labeling and TUNEL-positive cells were numbered under five non-continuous for each category. A laser scanning confocal microscope Zeiss LSM800 used to evaluate the slides (Zeiss, Wetzlar, Germany).
Western blotting:
Content of lung tissue proteins was quantified with the help of a bicinchoninic acid protein test kit. The proteins were separated using sodium dodecylsulfate- polyacrylamide gel electrophoresis before being transferred to a poly vinylidene fluoride membrane. After the secondary antibody had been incubated up to 1 h at room temperature, the primary antibody had been incubated on the membrane for an entire night at 4°. Using an increased detection enhanced chemiluminescence method, the protein bands were located.
Statistical analysis:
At least three repetitions are run through each experiment. The Statistical Package for the Social Sciences (SPSS) 22.0 statistical analysis programmed was used. The difference in variables among the experimental and control groups were ascertained using the student's t-test. A difference that is statistically significant is one with a p<0.05.
Results and Discussion
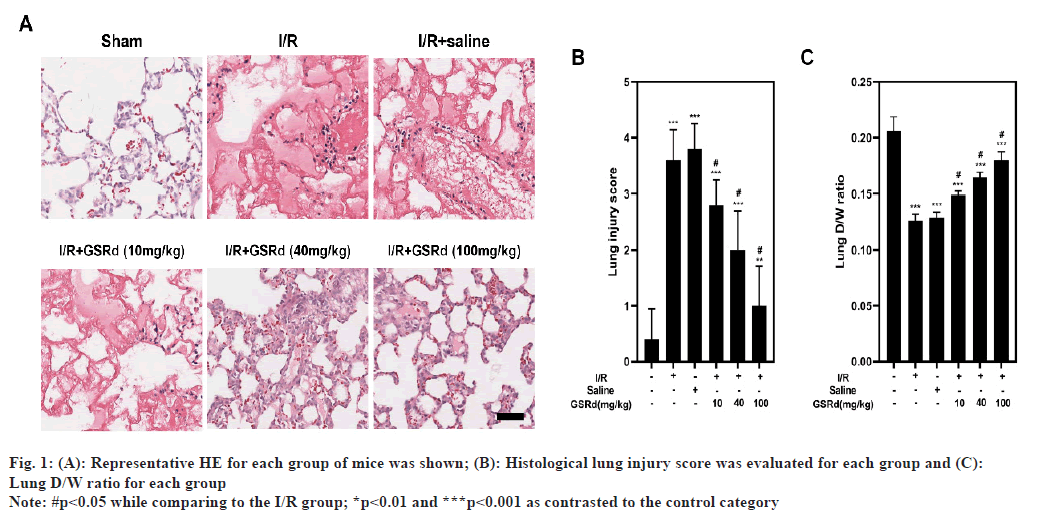
Mice with lung I/R acquire serious pathological abnormalities in their lungs. HE staining was used to assess and score morphological alterations in lung tissues (fig. 1A and fig. B). According to the findings of HE staining, the sham group's lung tissue had a typical organized architecture without any oedema, lesions or infiltration of inflammatory cells into the pulmonary stroma and alveolar cavity. Alveolar haemorrhage, interstitial oedema and inflammatory cell infiltration were all present in I/R group's lung tissue. A dosedependent level of pathological alterations alleviation was seen in the GSRd group. Additionally, the D/W ratio was decreased in I/R group when compared to the sham group and GSRd had dose-dependent effects to partially reverse this (fig. 1C). The D/W weight ratio is an indicator of lung oedema. These results confirmed that GSRd attenuated LIRI-induced pulmonary injury.
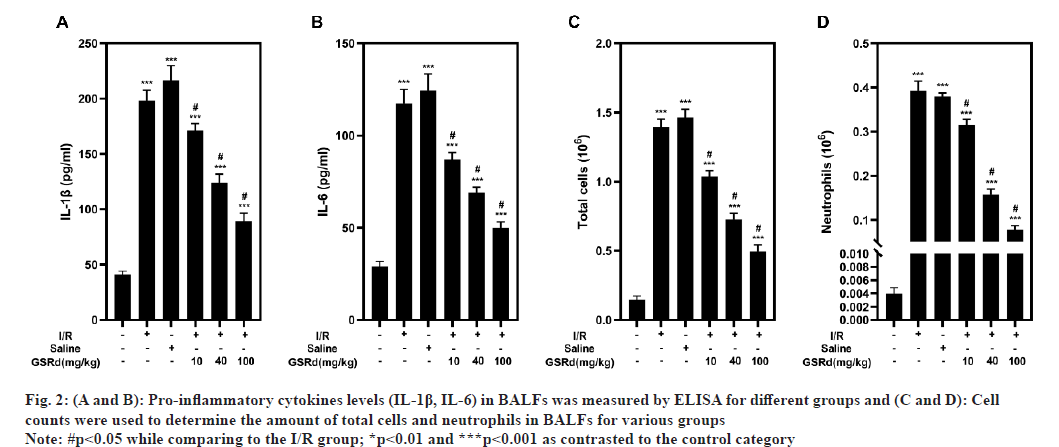
Consistent with the lung pathological injury caused by LIRI, an excessive inflammatory response was observed[20]. The inflammatory response was measured using the number of total cells and neutrophils in BALFs, as well as the IL-6 and IL-1 concentrations. By using ELISA to detect IL-6 and IL-1 protein levels, I/R group showed noticeably greater levels of both, as well as a higher number of the total cells and neutrophils evaluated by cell count in BALFs than the sham group, in a dose-dependent way, GSRd partially counteracted this impact (fig. 2). Findings demonstrated that GSRd can attenuate LIRI-induced inflammation response.
Fig. 2:(A and B): Pro-inflammatory cytokines levels (IL-1β, IL-6) in BALFs was measured by ELISA for different groups and (C and D): Cell
counts were used to determine the amount of total cells and neutrophils in BALFs for various groups
Note: #p<0.05 while comparing to the I/R group; *p<0.01 and ***p<0.001 as contrasted to the control category
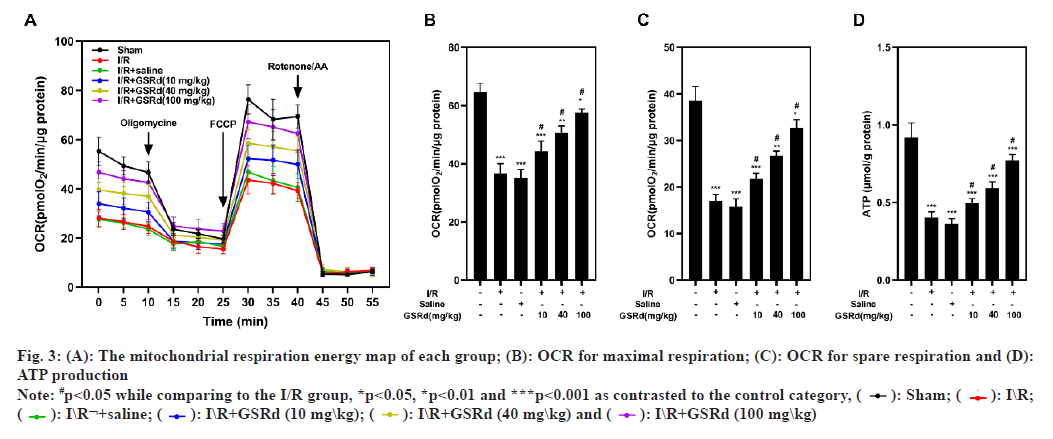
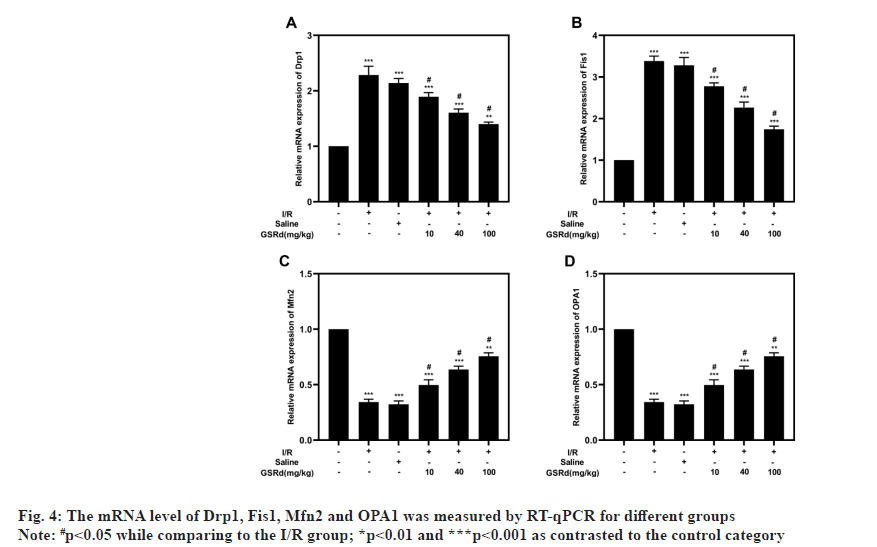
An analysis of the OCR by Seahorse assay performed to determine the GSRd affects the mitochondrial function of lung tissue. The results showed that the I/R group had considerably lower maximal respiration, spare respiration and ATP generation than the sham group, but that the I/R-induced declines were dosedependently mitigated by GSRd (fig. 3). Mitochondrial fission and fusion in a dynamic balance is also an important index of normal mitochondrial function. Our results showed that compared to the sham condition, mitochondrial fission proteins Dynamin-related protein 1 (Drp1) and Fission protein 1 (Fis1) both may express genetically more favorably under the influence of LIRI, but Mitofusins 2 (Mfn2) and Optic Atrophy 1 (OPA1) may not (mitochondrial fusion proteins). In a dosedependent way, GSRd partially restored this effect (fig. 4). These results indicated that GSRd protective effect on LIRI was linked to its modulation of mitochondrial activity.
Fig. 3: (A): The mitochondrial respiration energy map of each group; (B): OCR for maximal respiration; (C): OCR for spare respiration and (D):
ATP production
Note: #p<0.05 while comparing to the I/R group, *p<0.05, *p<0.01 and ***p<0.001 as contrasted to the control category, ( ): Sham; (
): Sham; ( ): I\R;
(
): I\R;
( ): I\R¬+saline; (
): I\R¬+saline; ( ): I\R+GSRd (10 mg\kg); (
): I\R+GSRd (10 mg\kg); ( ): I\R+GSRd (40 mg\kg) and (
): I\R+GSRd (40 mg\kg) and ( ): I\R+GSRd (100 mg\kg)
): I\R+GSRd (100 mg\kg)
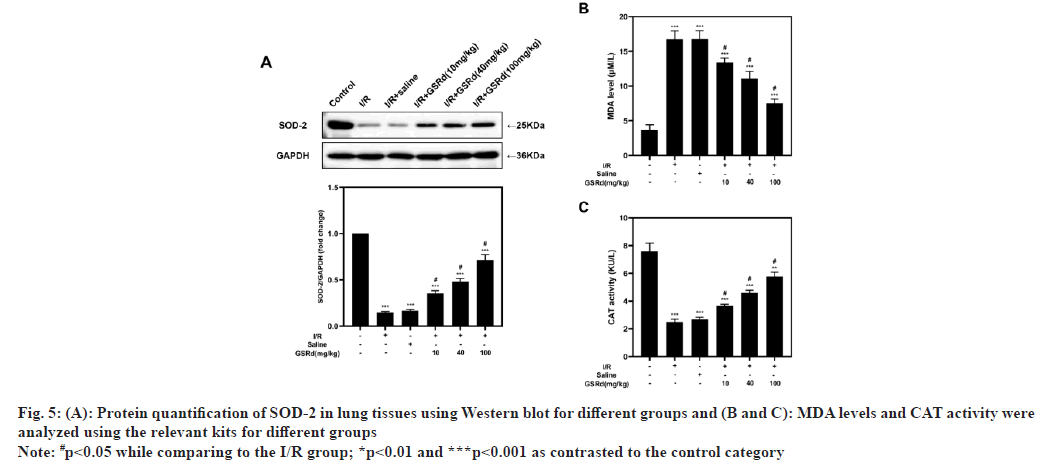
LIRI is linked to excessive oxidative stress[9] and it is still unclear whether GSRd could regulate LIRIinduced oxidative stress. Therefore, we investigated the expression of Superoxide Dismutase 2 (SOD- 2), the MDA content and the CAT activity in various populations. The results demonstrated that LIRI inhibited SOD-2 protein levels and CAT activity in comparison to the sham group, in a dose-dependent way, GSRd partially counteracted this impact. As anticipated, the LIRI increased MDA levels in comparison to the sham group and GSRd partially restored this effect in a dose-dependent way (fig. 5). According to these results, GSRd might shield mice from LIRI by halting the oxidative harm the virus causes.
Fig. 5: Protein quantification of SOD-2 in lung tissues using Western blot for different groups and (B and C): MDA levels and CAT activity were
analyzed using the relevant kits for different groups
Note: #p<0.05 while comparing to the I/R group; *p<0.01 and ***p<0.001 as contrasted to the control category
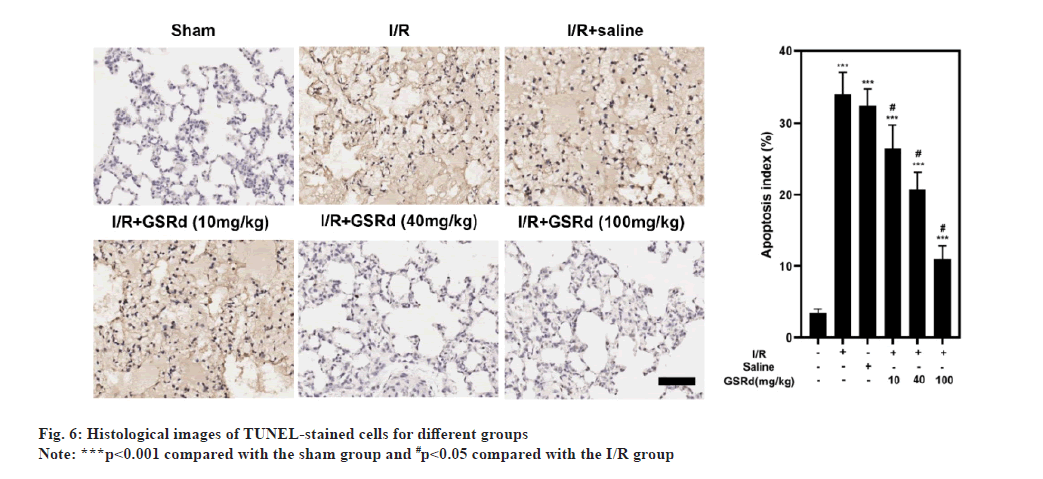
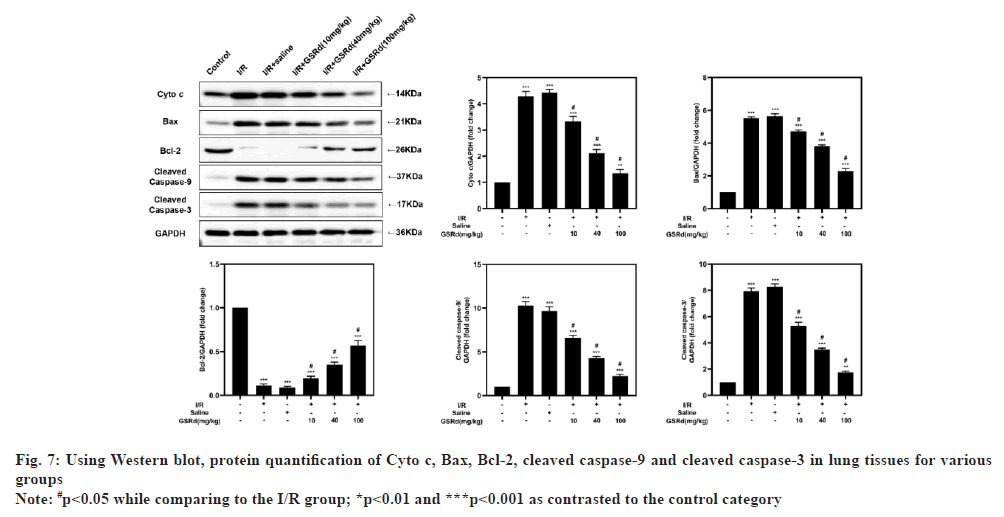
Because apoptosis appears to play a crucial role in LIRI[21], after GSRd treatment, the amount of apoptosis was measured. To begin, the number of LIRI-apoptotic cells determined using a TUNEL labeling test were used. As seen in fig. 6, there were more TUNELpositive pulmonary epithelial cells in I/R group than in the sham group. In I/R+GSRd groups, the proportion of the positive cells was significantly reduced. Then, using a Western blot, we looked at the apoptosis-related proteins. Fig. 7 demonstrates that compared to the sham group, I/R group had increased levels of Cyto c, Bcl-2-Associated X Protein (BAX), cleaved-caspase-9 and cleaved-caspase-3 expression. GSRd, in a dosedependent way, partially reversed this effect. These findings suggested that GSRd could alleviate LIRIinduced apoptosis.
In a number of neurological illnesses, GSRd, the active ingredient in ginseng, has been found to have neuroprotective properties. In rats with acute localized cerebral ischemia, GSRd reduces inflammatory injury and as a result, oxidative DNA and protein damage, lipid peroxidation and endogenous antioxidant system activity consumption[17]. Oedema and inflammatory cell infiltration driven by IL-1 and IL-6 characterize LIRI[2,8,21]. The pathogenesis of LIRI is based on the activation and inflammatory mediators are released in significant quantities, which causes an inflammatory response, resulting in impairment of endothelial barrier functions such as capillary endothelial cells and alveolar epithelial cells, resulting in pulmonary oedema, hypoxemia and other manifestations, eventually leading to a series of respiratory crises[21,22]. This research showed that GSRd has a protective effect against LIRI using a mice model. In our study, the pathological harm, which included interstitial oedema, alveolar haemorrhage, extensive inflammatory cell infiltration and an elevated D/W weight ratio was alleviated by GSRd. Generally, the D/W weight ratio is an indirect index for pulmonary oedema.
Mitochondrial damage is an important event in the progression of LIRI[22,23]. The reduction of ATP will directly affect the sodium pump function of the cell membrane, leading to mitochondria swelling and disintegration, endoplasmic reticulum swelling and vesicle formation, and finally lysosome rupture and autolytic enzyme release[23]. The OCR can be analyzed to study mitochondrial oxidative phosphorylation. We found that GSRd increased the respiratory rate and ATP generation in lung tissues following I/R. As a result, it is believed that prompt and effective intervention methods to correct the original mitochondrial fusion and division movement and produce a new ecological balance to adapt to the environment will slow the disease's progression. Compared with I/R induction alone, GSRd treatment reduced mRNA expression of mitochondrial division genes such as Drp1 and Fis1 and increases mitochondrial fusion gene mRNA expression, such as Mfn2 and OPA1. This research showed that mitochondrial biogenesis markers can be regulated by GSRd.
Mitochondria help to integrate and circulate the intracellular death signals such as oxidative stress and apoptosis[24]. Cells create a complex antioxidant defense system to protect the organism from oxidative damage, which includes SOD, CAT, GSH and other enzymes. SOD converts oxidative anions into hydrogen peroxide and CAT converts hydrogen peroxide into the water; thus, the toxic superoxide anions and hydrogen peroxide are converted into harmless water molecules[24]. When the function of mitochondria is damaged during LIRI, a huge amount of oxygen free radicals are produced in the lungs, resulting in increased production of the lipid peroxidation product MDA, damage to the free radical defense system, declines in the antioxidant activities of SOD and CAT, and further aggravation of tissue damage[9]. Following I/R, MDA levels increased, SOD-2 levels decreased and CAT activity decreased in the lungs of mice, showing that LIRI induced severe oxidative stress. This phenomenon was effectively suppressed via GSRd treatment. It indicates that GSRd can increase the antioxidant capacity of lung tissue, remove free radicals promptly and reduce lipid peroxidation to protect lung tissue from I/R injury. Furthermore, we found that GSRd inhibited LIRI-induced apoptosis BAX, cleaved-caspase-9 and cleaved-caspase-3 expression is down regulated while Bcl-2 expression is up regulated after taking Cyto c. The advantage of this conducted study was to show the role of GSRd in reducing LIRI in mice. But there are also limits, such as there are no clinical species, which still needed further experiment.
In conclusion, GSRd reduces LIRI in mice and the mechanisms underlying this protective effect may be linked to mitochondrial function modulation, according to the findings. Therefore, GSRd may have clinical prospects for LIRI.
Conflict of interest:
The authors declared no conflict of interests.
References
- Chen-Yoshikawa TF. Ischemia–reperfusion injury in lung transplantation. Cells 2021;10(6):1333.
[Crossref] [Google Scholar] [PubMed]
- Deng C, Zhai Z, Wu D, Lin Q, Yang Y, Yang M, et al. Inflammatory response and pneumocyte apoptosis during lung ischemia–reperfusion injury in an experimental pulmonary thromboembolism model. J Thromb Thrombolysis 2015;40(1):42-53.
[Crossref] [Google Scholar] [PubMed]
- Maltesen RG, Wimmer R, Rasmussen BS. A longitudinal serum NMR-based metabolomics dataset of ischemia-reperfusion injury in adult cardiac surgery. Sci Data 2020;7(1):198.
[Crossref] [Google Scholar] [PubMed]
- Saito M, Chen-Yoshikawa TF, Suetsugu K, Okabe R, Takahagi A, Masuda S, et al. Pirfenidone alleviates lung ischemia-reperfusion injury in a rat model. J Thorac Cardiovasc Surgery 2019;158(1):289-96.
[Crossref] [Google Scholar] [PubMed]
- Lv JL, Shi LN, Zhai CY, Wang GJ, Qu Y. Bag-1L protects against cell apoptosis in an in vitro model of lung ischemia-reperfusion injury through the C-terminal “Bag” domain. Biomed Res Int 2021;2021:8822807.
[Crossref] [Google Scholar] [PubMed]
- Laubach VE, Sharma AK. Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant 2016;21(3):246-52.
[Crossref] [Google Scholar] [PubMed]
- Pak O, Sydykov A, Kosanovic D, Schermuly RT, Dietrich A, Schröder K, et al. Lung ischaemia–reperfusion injury: The role of reactive oxygen species. Adv Exp Med Biol 2017:195-225.
[Crossref] [Google Scholar] [PubMed]
- Tian WF, Zeng S, Sheng Q, Chen JL, Weng P, Zhang XT, et al. Methylene blue protects the isolated rat lungs from ischemia–reperfusion injury by attenuating mitochondrial oxidative damage. Lung 2018;196(1):73-82.
[Crossref] [Google Scholar] [PubMed]
- Yang C, Yang W, He Z, Guo J, Yang X, Wang R, et al. Kaempferol alleviates oxidative stress and apoptosis through mitochondria-dependent pathway during lung ischemia-reperfusion injury. Front Pharmacol 2021;12:624402.
[Crossref] [Google Scholar] [PubMed]
- Simula L, Campanella M, Campello S. Targeting Drp1 and mitochondrial fission for therapeutic immune modulation. Pharmacol Res 2019;146:104317.
[Crossref] [Google Scholar] [PubMed]
- Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta 2010;1797(6-7):1171-7.
[Crossref] [Google Scholar] [PubMed]
- Chen YY, Liu QP, An P, Jia M, Luan X, Tang JY, et al. Ginsenoside Rd: A promising natural neuroprotective agent. Phytomedicine 2021;95:153883.
- Liu JF, Yan XD, Qi LS, Li L, Hu GY, Li P, et al. Ginsenoside Rd attenuates Aβ25–35-induced oxidative stress and apoptosis in primary cultured hippocampal neurons. Chem Biol Interact 2015;239:12-8.
[Crossref] [Google Scholar] [PubMed]
- WeiJia Z, Ying L, JieShu W, YaLi W. Ginsenoside Rd inhibits IL-1β-induced inflammation and degradation of intervertebral disc chondrocytes by increasing IL1RAP ubiquitination. Brazilian J Med Biol Res 2019;52(9):e8525.
[Crossref] [Google Scholar] [PubMed]
- Yang B, Wang R, Ji LL, Li XP, Li XH, Zhou HG, et al. Exploration of the function of ginsenoside RD attenuates lipopolysaccharide-induced lung injury: A study of network pharmacology and experimental validation. Shock 2022;57(2):212-20.
[Crossref] [Google Scholar] [PubMed]
- Zhou S, Ji Y, Yao H, Guo H, Zhang Z, Wang Z, et al. Application of ginsenoside RD in periodontitis with inhibitory effects on pathogenicity, inflammation and bone resorption. Front Cell Infect Microbiol 2022;12:813953.
[Crossref] [Google Scholar] [PubMed]
- Yang LX, Zhang X, Zhao G. Ginsenoside Rd attenuates DNA damage by increasing expression of DNA glycosylase endonuclease VIII-like proteins after focal cerebral ischemia. Chin Med J 2016;129(16):1955-62.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Li X, Wang X, Lau W, Wang Y, Xing Y, et al. Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via Akt/GSK-3β signaling and inhibition of the mitochondria-dependent apoptotic pathway. PLoS One 2013;8(8):e70956.
[Crossref] [Google Scholar] [PubMed]
- Fei L, Jifeng F, Tiantian W, Yi H, Linghui P. Glycyrrhizin ameliorate ischemia reperfusion lung injury through downregulate TLR2 signaling cascade in alveolar macrophages. Front Pharmacol 2017;8:389.
[Crossref] [Google Scholar] [PubMed]
- Zhao Q, Wu J, Lin Z, Hua Q, Zhang W, Ye L, et al. Resolvin D1 alleviates the lung ischemia reperfusion injury via complement, immunoglobulin, TLR4 and inflammatory factors in rats. Inflammation 2016;39(4):1319-33.
[Crossref] [Google Scholar] [PubMed]
- Ferrari RS, Andrade CF. Oxidative stress and lung ischemia-reperfusion injury. Oxid Med Cell Longev 2015;2015.
[Crossref] [Google Scholar] [PubMed]
- Merry HE, Phelan P, Doak MR, Zhao M, Hwang B, Mulligan MS. Role of toll-like receptor-4 in lung ischemia-reperfusion injury. Ann Thorac Surg 2015;99(4):1193-9.
[Crossref] [Google Scholar] [PubMed]
- Karabatsiakis A, Schönfeldt-Lecuona C. Depression, mitochondrial bioenergetics and electroconvulsive therapy: A new approach towards personalized medicine in psychiatric treatment-A short review and current perspective. Transl Psychiatry 2020;10(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 2018;217(6):1915-28.
[Crossref] [Google Scholar] [PubMed]