- *Corresponding Author:
- P. Thakkar
Pharmacy Department, Faculty of Technology and Engineering, The Maharaja Sayajirao University of Baroda, Vadodara-390 001, India
E-mail: hetal_thakkar11@yahoo.com
| Date of Submission | 26 March 2013 |
| Date of Revision | 18 October 2013 |
| Date of Acceptance | 22 October 2013 |
| Indian J Pharm Sci 2013; 75(6): 707-715 |
Abstract
The objective of the present work was to formulate gemcitabine hydrochloride loaded functionalised carbon nanotubes to achieve tumour targeted drug release and thereby reducing gemcitabine hydrochloride toxicity. Multiwalled carbon nanotubes were functionalised using 1,2-distearoylphosphatidyl ethanolamine-methyl polyethylene glycol conjugate 2000. Optimised ratio 1:2 of carbon nanotubes:1,2-distearoylphosphatidyl ethanolamine-methyl polyethylene glycol conjugate 2000 was taken for loading of gemcitabine hydrochloride. The formulation was evaluated for different parameters. The results showed that maximum drug loading efficiency achieved was 41.59% with an average particle size of 188.7 nm and zeta potential of −10.1 mV. Scanning electron microscopy and transmission electron microscopy images confirmed the tubular structure of the formulation. The carbon nanotubes were able to release gemcitabine hydrochloride faster in acidic pH than at neutral pH indicating its potential for tumour targeting. Gemcitabine hydrochloride release from carbon nanotubes was found to follow Korsmeyer-Peppas kinetic model with non-Fickian diffusion pattern. Cytotoxic activity of formulation on A549 cells was found to be higher in comparison to free gemcitabine hydrochloride. Stability studies indicated that lyophilised samples of the formulation were more stable for 3 months under refrigerated condition than at room temperature. Thus carbon nanotubes can be promising carrier for the anticancer drug gemcitabine hydrochloride.
Keywords
Noncovalent functionalisation, DSPE-mPEG2000, passive targeting, anticancer agent, cell cytotoxicity
Cancer has become one of the leading causes of death for the past 50 years. Every year, more than 10 million people in the world are diagnosed with cancer and more than 6 million die due to cancer [1]. Despite several modes of treatment options available such as chemotherapy, radiotherapy and immunotherapy, cure of cancer has still remained a challenge because of unavoidable cytotoxicity to normal cells. Radiotherapy may cause infertility and internal organ damage [1,2]. Cells in the lining of tongue, stomach and bowel may be degraded and bone marrow gets affected by use of chemotherapeutics [2]. In immunotherapy, variability in the patients’ antigens as well as mutations in the tumour cells and antigens makes this therapy mode ineffective [3]. Hence, because of all these limitations, there is a vital need to target a drug molecule exclusively to the tumour cells and thus minimise the toxicity of chemotherapeutic agents.
To increase therapeutic efficacy and reduce undesired effects produced by various chemotherapeutic agents, different novel formulations have been developed and studied in vitro and in vivo [4]. Various drug delivery systems, with or without targeting moiety, have been investigated such as synthetic liposomes [5], nanogels [6], micro- or nanospheres [7], micelles [8] and polymer-drug conjugates [9] for safe and effective treatment of cancer. Targeting of these formulations can be achieved by two means, passive targeting which is governed by size and surface properties of formulation and active targeting, which uses specific ligand to locate the site of interest [10]. Nanosized delivery systems are able to passively accumulate drugs in solid tumours by enhanced permeation and retention phenomenon [4]. However, these carriers also have drawbacks, like low drug loading capacity, which ultimately restricts their use from laboratory to clinics. Liposome shows physical instability in solution due to its amphiphilic nature and superficial toxicity due to prolonged circulation [11]. Dendrimers, themselves, are cytotoxic in nature and provide slow release rate [12].
Carbon nanotubes (CNTs) have recently received considerable attention due to their excellent cell penetration capacity and high loading for cargo molecules, which makes it beneficial in chemotherapy. CNTs have the potential to deliver the drug molecule to target cells for selective destruction, which reduce the distribution of drug to normal cells and hence avoidance of toxicity to noncancerous cells [13]. Functionalised carbon nanotubes (f-CNTs) can cross the cell membrane and body do not recognise them as harmful intruders [14]. CNTs are of two types, either singlewalled carbon nanotubes (SWCNTs) or multiwalled carbon nanotubes (MWCNTs). MWCNTs offer high drug loading because of the higher surface area [15]. CNTs tend to agglomerate resulting in poor solubility in aqueous and organic solvents and hence are difficult to disperse. This difficulty can be overcome by functionalisation of CNTs, which involves adsorption of surfactants on the surface of the sidewalls of CNTs that are hydrophobic in nature. This ultimately reduces agglomeration and hence increases the aqueous solubility of CNTs [12,16]. Moreover upon polyethylene glycolylation (PEGylation) CNTs avoids reticulo-endothelial system uptake and hence remains in circulation for extended periods of time maintaining sustained drug release [17]. Moreover, by virtue of their small particle size, CNTs can be selectively taken up by tumour tissues by enhanced permeation and retention phenomenon. Availability of free functional groups at the surface of CNTs can be utilised for conjugation of targeting moieties like ligands including antibodies, which renders site-specific delivery of drug. CNTs also bear high surface area and polyaromatic structure, which favour supramolecular chemistry with a wide range of drug molecules [4]. Functionalisation of CNTs can be achieved covalently or noncovalently. A covalent functionalisation method involves organic reaction, whereas noncovalent fuctionalisation method uses various surfactants or polymers to solubilise CNTs. Thereafter drug loading is performed on f-CNTs [16].
Gemcitabine hydrochloride (GEM HCl) is an antimetabolite nucleoside analogue, preventing cells from producing DNA and RNA that in turn inhibits cell growth and ultimately causes cell death. It is approved for use in treatment of various cancers like breast cancer, pancreatic cancer, bladder cancer and non-small cell lung cancer. Moreover, it is used experimentally in various types of tumours including oesophageal cancer [18]. Unfortunately, its high volume of distribution (370 l/m2) and rapid metabolism leads to its rapid elimination (plasma half-life 34–96 min) and hence its cytotoxic effect is only exposure-time dependent. Hence to get prolonged drug effects, repeated application of relatively sufficient high doses is required. This ultimately results in a dose-limiting systemic toxicity [19]. GEM HCl exhibits pH dependent solubility whereby it is more soluble at pH 4–5 (pH of the tumour interstitium) than at pH 7.4 (pH of blood). GEM HCl loaded f-CNTs are expected to remain in circulation for prolonged periods due to steric stabilisation owing to the presence of surfactants. While in circulation, minimum drug release is expected due to low solubility. Moreover, due to enhanced permeation and retention effect, the f-CNTs are expected to be taken up by the tumour interstitium and release the drug. Extracellular tissues and intracellular lysosomes and endosomes are acidic hence facilitates the GEM HCl release [18,19]. Thus, pH dependent delivery system of GEM HCl could be developed through use of f-CNTs. The objective of the present work was to develop GEM HCl-loaded f-CNTs as potential tumour targeted drug delivery system.
Materials and Methods
GEM HCl was gifted by Sun Pharma Advance Research Center, Vadodara, India. MWCNTs were purchased from Sigma Aldrich, Vadodara, India. DSPE-mPEG2000 (1,2-distearoylphosphatidyl ethanolamine-methyl polyethylene glycol conjugate 2000) was gifted by Cipla Ltd., Mumbai, India. Potassium dihydrogen phosphate and disodium hydrogen phosphate were purchased from Suvidhinath Lab., Vadodara, India. Sodium chloride was purchased from Loba Chemie Pvt. Ltd., Vadodara, India. All other chemicals and solvents were of analytical or HPLC grade.
Functionalisation of CNTs
For functionalisation of CNTs, covalent functionalisation was tried using mixture of 1:3 ratio of sulphuric acid:nitric acid [20]. However, the CNTs sedimented within 24 h and hence, the method did not yield stable suspension. Therefore, noncovalent functionalisation method was used [11]. In this method, MWCNTs (10 mg) and DSPE-mPEG2000 were mixed in 10 ml of phosphate buffer saline (PBS) pH 7.4. Mixture was immediately sonicated using bath sonicator (5 min×18 cycles) (Modern industrial corporation, Mumbai, India). After sonication, the mixture was centrifuged (Remi C-24, Mumbai) at 25 000 rpm for 30 min to remove unbound surfactant. Different batches were prepared by keeping the amount of MWCNTs constant (1 mg/ml) and varying concentration of DSPE-mPEG2000 to obtain optimal ratio of CNTs:DSPE-mPEG2000 as shown in Table 1. Average particle size of all batches was measured and the one showing minimum particle size was selected for drug loading [21,22]. Photographic images of MWCNTs before and after functionalisation was taken.
| Batch no. | Ratio of CNT:DSPE-mPEG2000 |
Particle size (nm)±SD (n=3) |
|---|---|---|
| 1 | 1:1 | 265±2.11 |
| 2 | 1:1.5 | 251.8±3.21 |
| 3 | 1:2 | 178.23±8.43 |
| 4 | 1:2.5 | 221.70±3.76 |
| 5 | 1:3 | 235.67±5.63 |
| 6 | 1:3.5 | 268.97±11.19 |
| 7 | 1:4 | 340.20±6.49 |
| 8 | 1:4.5 | Aggregation |
| 9 | 1:5 | Aggregation |
| 10 | 1:5.5 | Aggregation |
CNT is Carbon nanotubes, DSPE-mPEG2000 is 1,2-distearoylphosphatidyl ethanolamine-methyl polyethylene glycol conjugate-2000, SD is standard deviation for n=3 observations.
Table 1: Optimisation of ratio of cnt:dspe-mPEG2000
Fourier transform IR spectroscopy
To study the functionalisation of MWCNT, chemical characterisation was done by Fourier transform infrared (FT-IR) spectroscopy study. The study was performed for MWCNT, f-CNTs and DSPE-mPEG2000. The IR spectrum of the pellet, obtained by pressing the sample and KBr powder mixture by a press, was recorded (Perkin-Elmer FT-IR spectroscope, Spectra One, USA) [23].
Drug loading on f-CNTs
For drug loading in f-CNTs, various batches having constant amount of f-CNTs (1 mg/ml) and varying concentrations of GEM HCl were taken as shown in Table 2. GEM HCl solution in water was mixed with 1 ml of f-CNTs solution. The mixture was bath sonicated for 5 min to ensure proper mixing of f-CNTs and GEM HCl and was further allowed to stand overnight. Then the precipitates formed were redispersed by bath sonication for 1–2 min. Free drug separation was done by centrifugation at 25 000 rpm for 30 min (Sigma centrifuge, 3K30, Sigma Aldrich, Germany). The supernatant was collected and particle size of GEM HCl-loaded f-CNTs was measured. Absorbance of the sediment containing free drug, after proper dilution with PBS (pH 7.4) was measured at 268 nm against blank (PBS pH 7.4) by UV/Vis spectrophotometer (Schimadzu UV-1700, Pharma Spec, Tokyo, Japan). The amount of free drug was quantified relative to the calibration curve recorded under same conditions allowing the determination of drug loading efficiency [22,24]. Percent drug loaded (PDL) was calculated by subtracting amount of entrapped drug from total amount of drug added and dividing by total amount of drug added. The batch showing higher drug entrapment along with desired particle size range of less than 200 nm was selected as final batch and was characterised for different parameters.
| Batch no. |
Concentration of GEM HCl (mg/ml) |
PDL (%)±SE (n=3) |
Particle size (nm)±S.E (n=3) |
|---|---|---|---|
| 11 | 1 | 17.89±9.09 | 212.2±5.3 |
| 12 | 2 | 25.05±2.26 | 182.1±8.2 |
| 13 | 3 | 41.57±5.70 | 188.7±4.6 |
| 14 | 4 | 33.53±0.43 | 218.7±3.9 |
| 15 | 5 | 31.77±9.49 | 216.0±4.1 |
| 16 | 6 | 30.76±4.27 | 233.7±1.9 |
| 17 | 7 | 30.29±2.73 | 214.5±2.3 |
| 18 | 8 | - | Aggregation |
| 19 | 9 | - | Aggregation |
| 20 | 10 | - | Aggregation |
GEM HCl is gemcitabine hydrochloride, PDL is % drug loaded, SE is standard error
Table 2: Optimisation of concentration of gem hcl in the formulation
The final batch of GEM HCl-loaded f-CNTs was freeze dried using sucrose as the cryoprotectant in the ratio of 3:1 (sucrose:total solid content of formulation). The formulation was deep freezed at −70° for 24 h in deep freezer (Amancio Lab, Mumbai, India). The formed ice cake containing vials were transferred to the Heto Freeze-dry system (Heto dry, Denmark) and lyophilised under vacuum at 50 mbar for 24 h.
Particle size and zeta potential analysis
The particle size and zeta potential of f-CNTs and GEM HCl-loaded f-CNTs were measured using Malvern Zetasizer, Zeta-nano particle electrophoresis analyzer setup (Nano ZS, Zen 3600, Malvern Instruments Ltd., UK) equipped with 5 mV He-Ne laser (633 nm) and an in-built software that uses Helmholtz-smoluchowski equation [4]. The measurements were done in triplicate and average particle size and average zeta potential were recorded.
Surface morphology
The surface morphology of GEM HCl loaded f-CNTs was studied by Scanning Electron Microscopy (Jeol JSM-5610LV, Japan) and Transmission Electron Microscopy (Philips Technai 20, Holland) [4,22].
In vitro release study
The study was carried out for plain drug and unlyophilised sample of drug loaded f-CNTs. Apparatus for study consisted of donor and receptor compartments, differentiated by diffusion membrane (MWCO-12 000, Sigma-Aldrich) [25]. The activated dialysis membrane (activation procedure as given by manufacturer) was washed with PBS (pH 7.4). The CNTs suspension and plain GEM HCl solution equivalent to 1.5 mg of GEM HCl were accurately transferred into two different sacs, which thus became the donor compartments. Open end of sacs were tied up using thread and then were suspended in two different glass beakers containing 25 ml of PBS (pH 7.4) each, which acted as a receptor compartments. The same procedure was followed when phosphate buffer (pH 5.5) was used as a receptor compartment. The contents of the beakers were stirred at 100 rpm using teflon coated bar magnet and were covered with the aluminium foil to prevent any evaporative losses during the experiment run. The temperature of bulk of the solution was maintained at 37±0.5°. Five millilitres of samples were collected at predetermined time points for 36 h, from the receptor compartment and were subjected to analysis. Fresh buffer was used to replenish the receptor compartment. As GEM HCl shows pH dependent solubility, release from CNTs was examined as a function of pH. After performing drug release study, data were fixed in various models and best fitting model was analysed by correlation coefficient (R2) value. R2 value nearer to 1 indicates best suitable release model. The different models, which were applied for drug release from CNTs, were Zero order release, First order release, Higuchi’s model, Hixon–Crowell model and Korsmeyer–Peppas model.
In vitro cytotoxicity study
A549 lung cancer cells (104 cells/well) in its exponential growth phase was plated in 96-well flat bottom tissue culture plates and incubated at 37°, with 5% CO2 in incubator for 24 h, during which cells were allowed to adhere and to grow as monolayer. Samples studied were GEM HCl solution and GEM HCl-loaded f-CNTs, which were diluted with culture media to make various concentrations and were added in triplicate (200 μl each). Control wells were treated with equivalent volumes of GEM HCl free media. After 36 h of incubation period, supernatant was removed and washed with 100 μl PBS. Next, MTT (3-(4,-dimethylthiazol-2yl)-2,5- diphenyl-tetrazolium, 1 mg/ml) in culture medium was added to each well and again incubated for 4 h. The unreduced MTT and medium were then discarded and 200 μl of DMSO was added to dissolve the MTT formazan crystals [26,27]. Plates were shaken and absorbance was measured at 595 nm using the microplate reader (ELISA Reader, Bio-Rad, USA). Cell viability was determined in percentage on dividing mean absorbance of sample by mean absorbance of control.
The IC50 values (i.e. concentration resulting in 50% growth inhibition) of GEM HCl were graphically calculated from concentration-effect curves, considering the optical density of the control well as 100%.
Stability study
Lyophilised formulation was subjected to stability studies in triplicate at conditions according to International Conference on Harmonisation (ICH) guidelines. The formulations were stored at 5±3° and 30±2° with 65±5% RH for 3 months [28]. At the interval of 15 days, samples were withdrawn from the vials and rehydrated with distilled water and evaluated for particle size and percent drug retained.
Statistical analysis
Quantitative data were expressed as mean±standard deviation. Means were compared using Student t-test. P<0.05 were considered statistically significant.
Results and Discussion
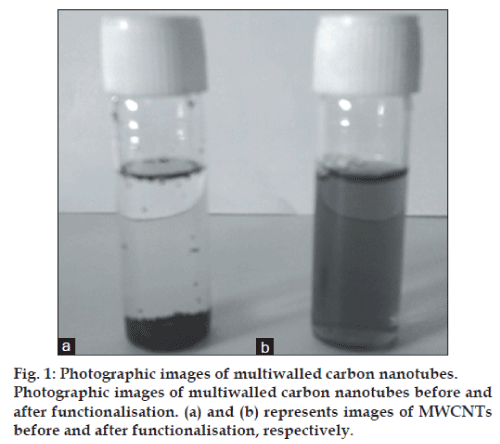
The sidewalls of CNTs are highly hydrophobic because of which it is difficult to disperse them both in aqueous and organic media. For the use of CNTs as carriers for drug delivery, it is often necessary to disperse them uniformly in aqueous medium. Hence, functionalisation of CNTs was carried out to impart dispersibility to CNTs in different media. Moreover, for functionalisation PEG was used, which also provides reduced reticuloendothelial system (RES) uptake resulting in prolonged circulation in blood. This property was attributed to the irreversible surface adsorption of PEG molecules on MWCNTs [29]. This surface adsorption was possible by using DSPE, which attaches noncovalently to both CNTs surface and PEG molecules. Hence, DSPE-mPEG2000 was used for functionalisation of MWCNTs in ratio of 1:2 w/w of DSPE:mPEG2000. Different batches prepared with varying ratios of CNTs:DSPE-mPEG2000 revealed that initially particle size of formulation decreased as the amount of DSPE-mPEG2000 increased up to the ratio of 1:2 of CNTs:DSPE-mPEG2000. Further when the amount of DSPE-mPEG2000 was increased, the particle size also increased and reaggregation was observed after 1:4.5 ratio due to presence of excess of DSPE-mPEG2000. Desirable particle size of 178.23 nm (Table 1) was found in ratio of 1:2, hence this CNTs: DSPE-mPEG2000 was taken as optimised ratio and was used for loading of GEM HCl and further characterisation. The f-CNTs had better dispersibility than CNTs because of PEGylation. The suspension remained stable as f-CNTs remained dispersed even after 24 h. This confirmed that the functionalisation of CNTs using PEGylated phospholipids gives stable formulations. Photographic image of CNTs before and after functionalisation is shown in fig. 1. As seen in the figure, MWCNTs remained settled as such in distilled water before functionalisation, while after functionalisation, f-CNTs could been seen suspended since clear solution was obtained. This further confirms that functionalisation has taken place.
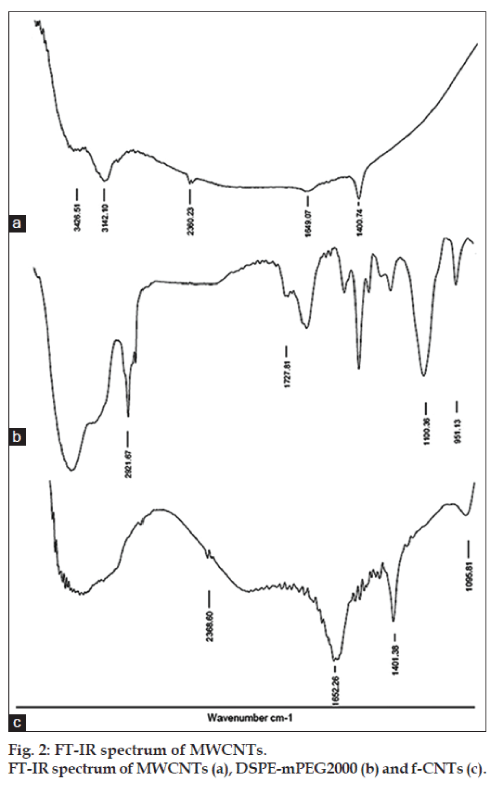
Moreover, FT-IR spectrum was recorded and results are shown in fig. 2. The prominent peak in spectra of MWCNTs (fig. 1a) seen at 1400 and 1649 cm−1 may be due to stretching vibrations carbon nanotube backbone. The broad peak at 3426 cm−1 may be due to O–H stretching of the hydroxyl group, which may be due to oscillation of carboxyl groups. These carboxyl groups may be due to partial oxidation of the surfaces of MWCNTs during purification by the manufacturer [30]. The peak at 1721 cm−1 in DSPEmPEG 2000 (fig. 1b) represents the carbonyl bond vibrations, peak at 1100 cm−1 relates to C–O secondary alcohol while at 951 cm−1 represents P–O–C aliphatic stretching vibrations. These peaks seemed to be merged with the CNTs peaks as seen in the f-CNTs spectra (fig. 1c). There may be noncovalent interaction between MWCNTs and DSPE-mPEG2000. Hence, the skeletal vibrations, which were seen in MWCNTs spectra, were also seen in f-CNTs spectra [31].
Further, upon drug loading in f-CNTs, with an increase in the concentration of GEM HCl, the loading also increased possibly due to availability of higher amount of drug. Maximum drug loading of 41.57% was found when ratio of f-CNTs:GEM HCl was 1:3 (Table 2) while particle size decreased. The average particle size increased more than desirable size (<200 nm) upon further increase in GEM HCl concentration and aggregation of particles was also observed when ratio was 1:8. Hence, it was observed that 1 mg of f-CNTs could hold a maximum of 3 mg of GEM HCl. Hence, 1:3 of f-CNTs:GEM HCl was taken as optimised ratio for later studies since further increase in GEM HCl amount led to an increase in particle size and further aggregation. GEM HCl has pH dependent solubility with higher solubility at low acidic pH. Thus, during drug loading, pH of medium is higher leading to adsorption of GEM HCl on CNTs via π-stacking and hydrophobic interaction between GEM HCl molecule and MWCNTs walls [30].
The mean particle size of f-CNTs before drug loading was found to be 178 nm with polydispersibility index (PDI) of 0.339. Moreover, mean particle size of GEM HCl-loaded f-CNTs was found to be 188.7 nm with PDI of 0.324. This increase in particle size was observed after drug loading demonstrated that GEM HCl was adsorbed on the MWCNTs. However, the size of GEM HCl-loaded f-CNTs (188.7 nm) was in the desirable nanometric size range (below 200 nm). The surface charge of particles as well as the electrostatic stabilisation of the formulation was measured through zeta potential analysis. There was an increase in average zeta potential from −48.1 to −10.1 mV after GEM HCl loading that could be attributed to the cationic nature (amino groups) of GEM HCl molecules that were present on the surface of MWCNTs. Zeta potential plays an important role in stabilisation of the nanoparticles in dispersion form. However, the formulation was lyophilised and hence the value of −10.1 mV also gave stable formulation.
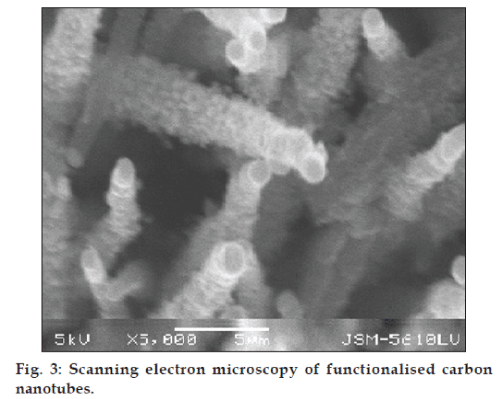
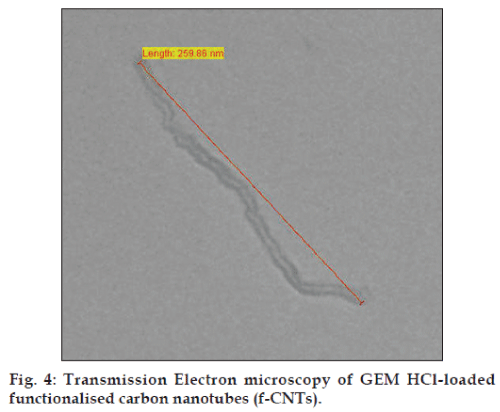
The scanning electron microscopic image (SEM) of f-CNTs was recorded as shown in fig. 3. Also, the transmission electron microscopic image (TEM) of GEM HCl-loaded f-CNTs was recorded (fig. 4). The length of f-CNTs was observed to be of 259.86 nm calculated on linear basis. Both the images confirmed the tubular structure of the formulation.
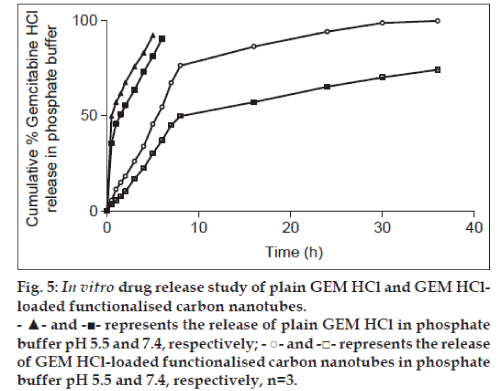
Drug loading on f-CNTs and its release can be controlled by varying pH. So, comparative diffusion studies were carried out for plain GEM HCl and f-CNTs-drug formulations using diffusion cell for a period of 36 h in PBS pH 5.5 and PBS (pH 7.4). Cumulative percent GEM HCl release was plotted against time (t). The in vitro release in PBS pH 7.4 at the end of 6 h was found to be 90.36% from plain GEM HCl while it was only 37.12% from GEM HCl loaded f-CNTs and took 36 h to release 74.2% of GEM HCl. Moreover, in phosphate buffer pH 5.5, at the end of 5 h, 92.23% release was obtained for plain GEM HCl and 45.53% from GEM HCl-loaded f-CNTs and took 36 h to release 99.79% from it (fig. 5). The plain drug diffused rapidly from the dialysis membrane in both media indicating that this membrane does not control the drug release from formulation. Also, this drug release was found to be higher (P<0.05) in phosphate buffer pH 5.5 than in phosphate buffer pH 7.4 but significant difference was not obtained. Moreover, as compared with free drug, f-CNTs provided sustained drug delivery. In acidic media (pH 5.5), more and rapid GEM HCl release was obtained as compared with physiological pH (pH 7.4) possibly due to the pH dependent π stacking interaction between GEM HCl molecule and MWCNTs [31]. This higher release rate obtained in acidic media will be beneficial in vivo since at tumour sites, the pH of interstitial fluid (which is around pH 5–5.8) and of endosomes (which is around pH 5.0) are acidic as compared with pH at noncancerous cells. Hence, GEM HCl release from f-CNTs will be more at tumour sites than to the noncancerous cells. This would ultimately reduce the toxicity of drug to the normal cells and successful pH dependent drug delivery systems out of f-CNTs to targeted tumours could be obtained. Further, these results were fitted in different kinetic models to determine GEM HCl release mechanism from f-CNTs. R2 values were obtained as shown in Table 3. From the table, R2 values of Higuchi’s model were closer to 1.0 as compared with other models. Hence, the drug release from CNTs was observed to follow this model indicating diffusion controlled release [32].
Fig. 5: In vitro drug release study of plain GEM HCl and GEM HClloaded functionalised carbon nanotubes.
- ▲- and -■- represents the release of plain GEM HCl in phosphate
buffer pH 5.5 and 7.4, respectively; - ○- and -□- represents the release
of GEM HCl-loaded functionalised carbon nanotubes in phosphate
buffer pH 5.5 and 7.4, respectively, n=3.
| pH | Linear correlation coefficient (R2) | ||||
|---|---|---|---|---|---|
| Zero order |
First order |
Higuchi’s model |
Hixoncrowell model |
Korsmeyer–Peppas model |
|
| 7.4 | 0.7517 | 0.5184 | 0.9236 | 0.9165 | 0.9171 |
| 5.5 | 0.5290 | 0.7866 | 0.9050 | 0.8550 | 0.7570 |
Table 3: Linear correlation coefficient (r2) values in different models
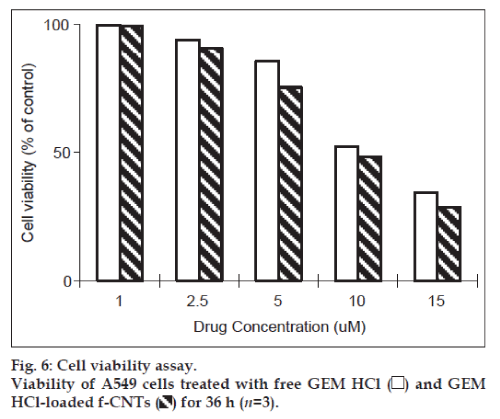
The cytotoxicity of GEM HCl-loaded in f-CNTs against A549 lung cancer cells were determined by using MTT dye reduction assay and was compared with that of plain GEM HCl. The results obtained are shown in fig. 6. There was no cytotoxic effect found in A549 cells treated with blank f-CNTs. With increasing concentrations, the percent cell viability was found to be decreasing. IC50 value of GEM HClloaded was found to be 10.4 μM and of GEM HCl loaded f-CNTs was found to be 9.1 μM. Although, GEM HCl-loaded f-CNTs exhibited a little more cytotoxicity as compared to free GEM HCl, there were no significant difference (P>0.05) between both at equal GEM HCl concentrations. Hence, the activity of GEM HCl was not adversely influenced during formulation step and cytotoxicity of main active component from GEM HCl loaded f-CNTs were GEM HCl. Moreover, no cytotoxicity found by f-CNTs at these concentrations suggests their safe biological applications at these concentrations [33].
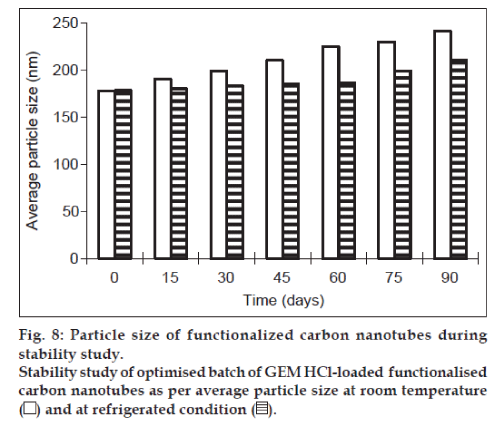
Lyophilisation of formulation helps in keeping the product stable during long-term storage. This can be achieved by using cryoprotectant such as trehalose, sucrose, mannitol and dextrose. Therefore for stability studies, the formulation was lyophilised using sucrose as cryoprotectant, which is widely used in industry because of its easy availability and low cost. The results in terms of percent drug retained and particle size are shown graphically in figs. 7 and 8, respectively. There were no changes in physical properties of f-CNTs. At the end of 3 months, the particle size and percent drug retained of lyophilised formulation kept at room temperature were found to be 241 nm and 87.66%, respectively, while it was found to be 210 nm and 96.72%, respectively for sample kept as 5±3°. It indicated that the particle size of the rehydrated lyophilised GEM HCl-loaded f-CNTs was not altered significantly and also no significant reduction in the percent drug retained after rehydration of lyophilised f-CNTs when stored at 5±3° temperature, as compared with 30±2°/ 60±5% RH over a period of 3 months. Hence after lyophilisation storage at 5±3° provides the better stability to the f-CNTs.
GEM HCl-loaded functionalised MWCNTs were formulated and PEGylated. The drug loading capacity of 3 mg of GEM HCl per mg of f-CNTs was obtained. In acidic pH media, higher GEM HCl release was obtained, which confirmed suitability of using such formulation for tumour targeting.
However, attachment of specific ligands or antibodies can still further improve the targeting efficiency. Extended research on the f-CNTs is expected to yield promising results in tumour bearing animals. Biodistribution and pharmacokinetic studies can still give us a better idea on the performance of the f-CNTs in vivo.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Globalcancer statistics. CA Cancer J Clin 2011;61:69-90.
- Sherwood R. Disadvantages of chemotherapy. In: eHow Health.Available from: http://www.ehow.com/about_4707268_disadvantagesof-chemotherapy.html. [Last accessed on 2012 Mar. 8].
- Dimberu P, Leonhardt R. Cancer immunotherapy takes a multi-facetedapproach to kick the immune system into gear. Yale J Biol Med2011;84:371-80.
- Bianco A. Carbon nanotubes for the delivery of therapeutic molecules.Expert Opin Drug Deliv 2004;1:57-65.
- Nagayasu A, Uchiyama K, Kiwada H. The size of liposomes: A factorwhich affects their targeting efficiency to tumors and therapeutic activityof liposomal antitumor drugs. Adv Drug Deliv Rev 1999;40:75-87.
- Duan C, Zhang D, Wang F, Zheng D, Jia L, Feng F, et al. Chitosang-poly(N-isopropylacylamide) based nanogels for tumor extracellulartargeting. Int J Pharm 2011;409:252-9.
- Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A. Poly-epsiloncaprolactonemicrospheres and nanospheres: An overview. Int J Pharm2004;278:1-23.
- Cheng C, Wei H, Zhu JL, Chang C, Cheng H, Li C, et al. Biotinylatedthermoresponsivemicelle self-assembled from double-hydrophilicblock copolymer for drug delivery and tumor target. Biomaterials2008;29:497-505.
- Mitra A, Nan A, Papadimitriou JC, Ghandehari H, Line BR. Polymer–peptide conjugates for angiogenesis targeted tumor radiotherapy. NuclMed Biol 2006;33:43-52.
- Danhier F, Feron O, Preat V. To exploit the tumor microenvironment:Passive and active targeting of nanocarriers for anticancer drug delivery.J Control Release 2010;148:135-46.
- Gabizon A, Isacson R, Libson E, Kaufman B, Uziely B, Catane R,et al. Clinical studies of liposome-encapsulated doxorubicin. ActaOncol1994;33:779-86.
- Pastorin G. Crucial Functionalizations of carbon nanotubes forimproved drug delivery: A valuable approach? Expert Opin Pharm Res2009;26:746-69.
- Hilder TA, Hill JM. Modeling the loading and unloading of drugs intonanotubes. Small 2009;5:300-8.
- Sinha N, Yeow J. Carbon nanotubes for biomedical applications. IEEETrans Nanobiosci 2005;4:180-95.
- Beg S, Rizwan M, Sheikh A, Hasnain M, Anwer K, Kohli K.Advancement in carbon nanotubes: Basics, biomedical applications andtoxicity. J Pharm Pharmacol 2011;63:141-63.
- Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes indrug design and discovery. AccChem Res 2008;41:60-8.
- Liu Z, Davis C, Cai WB, He L, Chen XY, Dai HJ. Circulation andlong-term fate of functionalized biocompatible single-walled carbonnanotubes in mice probed by raman spectroscopy. ProcNatlAcadSciUSA 2008;105:1410-5.
- Gemzar (Gemcitabine HCl) for injection. Available from: http://pi.lilly.com/us/gemzar.pdf. [Last accessed on 2006 May 6].
- Noble S, Goa KL. Gemcitabine A review of its pharmacology andclinical potential in non-small cell lung cancer and pancreatic cancer.Drugs 1997;54:447-72.
- Osorio AG, Silveira IC, Bueno VL, Bergmann CP. H2SO4/HNO3/HCl-Functionalization and its effect on dispersion of carbon nanotubes in aqueous media. Appl Surf Sci2008;255:2485-9.
- Vaisman L, Wagner HD, Marom G. The role of surfactants in dispersion of carbon nanotubes.Adv Colloid Interface Sci 2006;128-130:37-46.
- Liu Z, Sun X, Nakayama-Ratchford N, Dai H. Supramolecularchemistry on water soluble carbon nanotubes for drug loading anddelivery. ACS Nano 2007;1:50-6.
- Dang Z, Wang L, Zhang L. Surface functionalization of multiwalledcarbon nanotube with trifluorophenyl. J Nanomaterials 2006:Article ID83583:1-5.
- Liu Z, Chen K, Davis C. Drug delivery with carbon nanotubes for invivo cancer treatment. Cancer Res 2008;68:6652-60.
- Shah VP, Elkins JS, Williams RL. In vitro drug release measurementof topical glucocorticoid creams. Pharmacopeial Forum 1993;19:5048.
- Hurt RH, Monthioux M, Kane A. Toxicology of carbon nanomaterials:Status, trends, and perspectives on the special issue. Carbon2006;44:1028-33.
- Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D,Murray AR, Gandelsman VZ, et al. Exposure to carbon nanotubematerial: Assessment of nanotube cytotoxicity using human keratinocytecells. J Toxicol Environ Health A 2003;66:1909-26.
- ICH, Q1A (R2), Stability testing of new drug substances and products, In: Proceedings of the International Conference on Harmonization,Geneva, 2003.
- Meng L, Fu C, Lu Q. Advanced technology for functionalization ofcarbon nanotubes. Prog Nat Sci 2009;19:801-10.
- Abuilaiwi F, Laoui T, Al-Harthi M, Atieh M. Modification andfunctionalization of multiwalled carbon nanotube (MWCNT) via fischeresterification. Arab J SciEng 2010;35:37-48.
- Coates J. Interpretation of infrared spectra, A practical approach. In:Meyers RA, editors. Encyclopedia of analytical chemistry.Chichester:John Wiley and Sons Ltd.; 2000. p. 10815-37.
- Liu Z, Chen K, Davis C. Drug delivery with carbon nanotubes for invivo cancer treatment. Cancer Res 2008;68:6652-60.
- Arsawang U, Saengsawang O, Rungrotmongkol T, Sornmee P,Wittayanarakul K, Remsungnen T, et al. How do carbon nanotubesserve as carriers for gemcitabine transport in a drug delivery system? J Mol Graph Model 2011;29:591-6.
 ) for 36 h (n=3).
) for 36 h (n=3).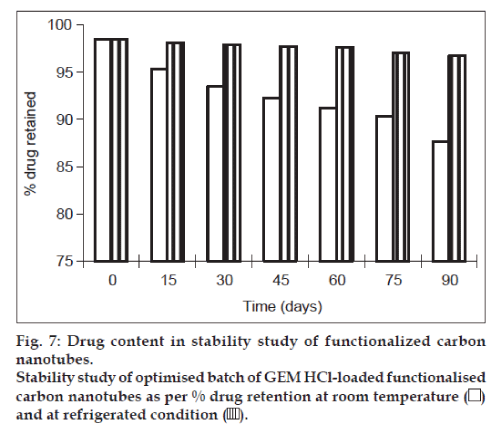
 ).
).
 ).
).



