- *Corresponding Author:
- P. Farishta
Department of Botany, Cotton University, Guwahati, Assam 781001, India
E-mail: peenazricha@gmail.com
| Date of Received | 17 July 2023 |
| Date of Revision | 12 August 2024 |
| Date of Accepted | 11 October 2024 |
| Indian J Pharm Sci 2024;86(5):1810-1820 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Lindernia crustacea (L.) F Muell is one of the most popular medicinal plants used in the traditional medicinal system throughout the world. The aim of the present study was to detect the phytochemical constituents, estimate the quantity of important phytochemicals in the methanolic, aqueous and acetone extract of the plant. The methanol extract was subjected to gas chromatography-mass spectrometry technique to identify the bioactive compounds present in the extract. Standard procedures were used to detect the preliminary phytochemical constituents along with the quantitative estimation of total phenols, total flavonoids, total tannins and total alkaloids content for all the three extracts. The qualitative analysis revealed the presence of important phytochemicals such as phenols, flavonoids, saponins, tannins, glycosides and alkaloids. The quantitative estimation affirmed considerable amount of total phenolic, flavonoid, tannins and alkaloid content in the extracts. The gas chromatography-mass spectrometry analysis of the methanolic extract showed the presence of biologically active compounds such as 1-ethyl2,2,4a,7,7-pentamethyl-1,2,3,4,4a,5,6,7-octahydro (1,8) naphthyridine; 2-allyl-3,6-dimethoxybenzyl alcohol; isoquinolin,1,2,3,4-tertahydro-2-(1-phenethyl) carbanyl-6,7-dimethoxy; pentanedioic acid, 2-((trimethylsilyl)oxy), dimethyl ester; valerophenone, 4´-(trimethylsiloxy) and (-)-cis-myrtanol. These compounds were detected for the first time in the gas chromatography-mass spectrometry analysis of Lindernia crustacea plant. Each of the major compounds revealed various therapeutic properties that are beneficial for treating different human diseases. Further studies for the isolation of the bioactive compounds may be useful in the pharmaceutical sector for the development of novel drugs.
Keywords
Lindernia crustacea, medicinal plants, phytochemical, antioxidant, gas chromatographymass spectrometry, bioactive
Nature has provided mankind a repository of remedies to cure human ailments in the form of medicinal plants. Medicinal plants are the richest source of drugs and have a long-standing use in the traditional medicine system, practiced for thousands of years, by the indigenous people of China, India and many other countries. Based on their use in traditional medicinal system, a significant number of drugs have been isolated from these plants. The therapeutic properties of medicinal plants are attributed to the presence of a broad spectrum of chemical compounds present in their different parts such as leaves, flowers, stem, roots, fruits and bark[1]. These chemical constituents include the phytochemicals which are biologically active such as tannins, alkaloids, terpenoids, steroids, phenols, flavonoids and glycosides and are enriched with various pharmacological properties[2].
The application of various chemicals obtained from medicinal plant parts such as seeds, flowers, roots or leaves for therapeutic purposes are referred as phytomedicine or herbal medicine[3]. In the recent years there has been an increasing demand for phytomedicine or herbal medicine due to their easy availability, lesser cost and higher efficacy with minimal side effects as compared to modern medicines[4]. Lately, the significance of medicinal plants has expanded due to their therapeutic potential[5]. Approximately 20 % of the known plants have shown vital impact in treating various harmful human diseases and are extensively being used in pharmaceutical industries. The bioactive compounds commonly known as phytochemicals present in medicinal plants produce specific physiological action on the human body, thereby, aiding in the treatment of many human ailments. Thus, medicinal plants and their phytochemicals play a significant role as valuable entities in the synthesis of novel drugs[6].
In the recent years phytochemicals have been widely investigated as a source of medicinal agents which could be used for the treatment of many diseases. The most important among the substances present in medicinal plants include flavonoids, tannins, steroids, alkaloids and phenolic compounds[7]. Plants containing important phytochemicals exhibited potent cytotoxic effect against human diseases[8]. Phenolic compounds act as natural antioxidants and play a major role in scavenging reactive oxygen species thereby, inhibiting damages caused by oxidative stress in the human body[9]. Flavonoids are responsible for pharmacological activities such as anti-diabetic, antitumor, antioxidant, anaesthetic, anti-proliferative and immuno-stimulatory effects[10]. They also act as anti-cancer agents[11]. Tannins exhibit therapeutic properties such as anti-inflammatory, antioxidant, wound-healing and anti-cancer activities[12]. Alkaloids possess anti-inflammatory and analgesic[13] and act against chronic diseases such as cancer and neurological disorders[14]. The potentiality of these phytochemicals has been globally recognized, thus, increasing the global interest in commercializing the production of therapeutic drugs obtained from natural resources. The versatile application of the plant-derived substances have urged many researchers to carry out extensive scientific studies on the therapeutic properties of medicinal plants and utilize them in the production of new drugs for the treatment of human diseases.
In India, thousands of plants are used by the traditional medicinal healers belonging to different ethnic communities for the purpose of primary healthcare and nutrition. Though, many of the plant species which possess convincing therapeutic and pharmacological efficacy are still to be explored scientifically. One such plant is Lindernia crustacea (L. crustacea) (L.) F. Muell which belongs to the family Scrophulariaceae. Later it was segregated as a member of the new family Linderniaceae as accepted by Angiosperm Phylogeny Group (APG) III in the year 2009[15]. The plant is distributed all over India including Assam. It mainly habituates in moist places like river beds, rice fields and open grassy places[16]. L. crustacea is an annual herb with prostrate and diffused branches. Roots develop at the nodes and stems are 4-angled with slightly winged on angles. Pedicels are 1-1.2 cm in length. Leaves are found to be ovate with truncate base and serrated margins and subacute apex. Flowers are axillary and usually solitary or 2-per node on terminal racemes, corolla are rose or purple in colour (fig. 1).
L. crustacea is regarded as a popular ethno medicinal plant and has been used in the traditional system of medicine all over the world[17]. It is used to treat many diseases such as ear ache, fever and thrush[18] and ring worm[19]. It is also reported to show anticancer and antioxidant activities[20]. The whole plant in the form of poultice is applied externally on the affected areas to cure skin diseases[21]. Decoction of whole herb is used for the treatment of asthma, rheumatism and wounds, whereas roots are chewed to relieve throat irritation[22]. Juice of the plant is used in the treatment of tonsil[23]. Phytochemical screening of leaf and stem extracts of L. crustacea have showed the presence of various phytochemicals such as alkaloids, carbohydrates, flavonoids, tannins and glycosides[24]. Previous reports have showed the presence of flavonoid in the benzene, ethyl acetate and ethanol extract of the plant[17]. The use of L. crustacea as a medicinal plant possessing therapeutic properties may be attributed to the important phytocompounds present in different solvent extracts of the plant.
Since, scientific investigation regarding the pharmacological studies and phytochemical analysis of L. crustacea (L.) F. Muell are very limited further investigation concerning the biological activities of important phytochemicals is essential. Therefore, the present endeavour was taken up to study further, the presence and identification of important phytochemicals in the methanol extract using Gas Chromatography-Mass Spectrometry (GC-MS) technique along with the quantification of compounds present in different extracts of L. crustacea whole plant. Thus, this study might validate the utilization of the plant in the traditional system of medicine.
Materials and Methods
Collection of plant material:
The whole plant of L. crustacea were collected from Balijan Shyam village (26°, 34°, 57° N and 94°, 16°, 6° E), Titabor in Jorhat district of Assam, India. The collection was done during the month of July 2021. The plant was identified and authenticated at the Botanical Survey of India, Eastern Circle, Shillong, Meghalaya, India. The identification and accession number for the specimen was given in Table 1.
| S. No. | Name of the species | Family | Collection No. | Accession No. |
|---|---|---|---|---|
| 1 | L. crustacea (L.) F. Muell. | Scrophulariaceae | 28 | 98160 |
Table 1: Scientific Name and Family of the Selected Plant Species
A voucher specimen of the plant was also deposited at the herbarium of Botanical Survey of India, Shillong and Department of Botany, Cotton University, Guwahati, Assam, India.
Preparation of different extracts:
The fresh plant materials were collected and washed in running tap water and dried in the shade. After washing, the dried materials were crushed into fine powder by a mechanical grinder. The crushed plant material was then subjected to successive extraction in Soxhlet’s apparatus in three different solvent water/Aqueous (LCW), Methanol (LCM) and Acetone (LCA) using Soxhlet extraction method. Soxhlet method was used for the extraction process as it can be utilized to extract larger volumes of drugs using lower volume of solvents and it filtration is not required in this method[25]. 10 g of plant sample was treated with 250 ml of the three solvents used, separately and extracted continuously in Soxhlet apparatus for 24 h until the solvent shows no colour. After that the extract was taken in a beaker and kept on a water bath and heated at 30° to 40°. Heating was continued till all the solvent gets evaporated. The dried extracts were then kept in refrigerator at 4° for using further in phytochemical analysis.
Qualitative assessment of phytochemicals:
The plant extracts (methanol, water and acetone) were used to test for detection of different phytochemicals viz. alkaloids, tannins, flavonoids, saponins, cardiac glycosides, carbohydrates, terpenoids and amino acids using standard procedures[26-28].
Phenols and tannins:
Ferric chloride (FeCl3) solution (2 %) of 2 ml was added to 2 ml of plant extract. Appearance of blue-green or black colour of the mixture showed the presence of phenols and tannins.
Flavonoids:
Shinoda test: In this test the plant extract was mixed with few pieces of magnesium ribbon. To this mixture concentrated Hydrochloric Acid (HCl) was added drop by drop. After few minutes appearance of pink scarlet colour indicated the presence of flavonoids.
Proteins:
Millon’s test: 2 ml of extract when mixed with 2 ml of Millon’s reagent, white precipitate appeared. Upon gentle heating the white precipitate turned red that confirmed the presence of proteins.
Ninhydrin test: 2 ml of extract was boiled with 2 ml 0.2 % ninhydrin solution. The violet colour of the mixture indicated the presence of proteins.
Carbohydrates:
Fehling’s test: Equal amounts of Fehling A and Fehling B reagents were mixed together. From this mixture 2 ml was mixed with to 1 ml of plant extract and heated till boiling occurs. Precipitate of brick red occurred at the base of the test tube. This indicated the presence of reducing sugars.
Molisch’s test: 2 ml of Molisch’s reagent was added to 3 ml of plant extract. The mixture was then shaken vigorously. 2 ml of concentrated sulphuric acid was added slowly to the mixture. At the interphase of both the solutions a violet ring occurred which detected that the extract contains carbohydrates.
Saponins:
5 ml distilled water was added to 2 ml plant extract in a test tube and then shaken thoroughly. Formation of stable foam takes place which persists for some time. This stable foam showed the presence of saponins.
Glycosides:
Keller-Killani test: Glacial acetic acid (2 ml) with 1-2 drops of 2 % FeCl3 solution was mixed with 2 ml plant extract. 2 ml concentrated sulphuric acid was added to the mixture slowly. A brown ring appeared at the interphase of both the solutions which detected cardiac glycosides are present in the extract.
Salkowski’s test: Chloroform of 2 ml was added to 2 ml of crude extract. To this mixture 2 ml concentrated sulphuric acid was added slowly and shaken mildly. Appearance of red brown colour indicated the presence of glycosides.
Terpenoids:
2 ml chloroform was added to 2 ml of plant extract. The mixture was then evaporated until dried. About 2 ml of concentrated sulphuric acid was added to the mixture and heated for about 2 min. A greyish colour of the mixture detected for the presence of terpenoids.
Alkaloids:
Plant extract of 2 ml was mixed with 2 ml of 1 % HCl and the mixture was gently heated. Mayer’s reagent was added to the mixture in one test tube and Wagner’s reagent was added separately in another test tube. A turbid precipitate occurred which detected the presence of alkaloids.
Quantitative assessment of phytochemicals:
Determination of Total Phenol Content (TPC): The determination of TPC of the plant extracts was done by the Folin-Ciocalteu method[29]. In this method 10 mg gallic acid used as a standard was dissolved in 100 ml distilled water. From this solution a set of five reference standard solutions of gallic acid (20, 40, 60, 80 and 100 μg/ml) were prepared in separate test tubes. 1 ml of each extract was taken in separate test tubes. Then to each test tube of references and samples 2 ml of distilled water was added. To this 0.3 ml Folinciocalteau phenol reagent was added. After 5 min, 0.8 ml of 20 % sodium carbonate solution was added to each test tube and finally the volume was made to 5 ml with distilled water. After 30 min the absorbance was measured at 765 nm using Ultraviolet-Visible (UVVis) spectrophotometer. Calibration curve was drawn between gallic acid concentration vs. absorbance. Each reading was taken in triplicates.
Total Flavonoids Content (TFC):
The TFC was determined by aluminium chloride method[30]. In this method quercetin was used as a standard. 0.1 g of quercetin was added to 100 ml distilled water. From this solution five different concentrations (20, 40, 60, 80 and 100 μg/ml) of quercetin were prepared in distilled water in separate test tubes. After this 0.5 ml of each solution was diluted with 1.5 ml methanol and then mixed with 0.5 ml of 10 % aluminium chloride. To each mixture 0.1 ml of sodium acetate was added and the final volume of was made 5 ml with distilled water. The mixture was shaken thoroughly. Sample solution was prepared at a concentration of 1 mg/ml in methanol. To 0.5 ml of each sample solution was mixed with the same reagents as described above. After keeping at room temperature for 30 min the absorbance of each sample and standard was measured at 415 nm using a UV-Vis spectrophotometer.
Total Tannin Content (TTC):
The TTC of the plant extracts was determined by method described by Sudha et al.[31]. In this method tannic acid was used as the standard. Stock solution of the standard was prepared by dissolving 1 mg tannic acid in 1 ml distilled water. A diluted solution of the stock solution was prepared by mixing 1 ml stock and 9 ml distilled water. From this solution five different concentrations of 20, 40, 60, 80 and 100 μl reference standards were prepared. For sample preparation 50 mg of each extract was added to 50 ml distilled water in a conical flask. The mixture was then shaken for 1 h using a mechanical shaker and filtered into a 50 ml volumetric flask and made up to the final volume by adding distilled water. Now 1 ml of each of the filtrate was added to 4 ml distilled water in separate test tubes and 1 ml of each reference was taken in separate test tubes and treated with 2 ml of 0.1 M FeCl3 solution in 0.1 M HCl and 0.008 M potassium ferrocyanide. The resultant solution was mixed thoroughly and allowed to stay for 30 min in room temperature. Absorbance for sample and standard solutions was measured at 700 nm using UV-Vis spectrophotometer. Results were expressed as Tannic Acid Equivalent (TAE) in mg/g of dry plant material.
Total alkaloids:
Total alkaloids in the plant extracts were estimated by spectrophotometric method using Dragendorff’s reagent[32]. Berberine hydrochloride was used as a standard and its solution was prepared in warm distilled water at a concentration of 1 mg/ml. Stock solutions of berberine were prepared from five different concentrations (20, 40, 60, 80 and 100 μg/ml). Plant samples were prepared at a concentration of 1 g/ml in distilled water. 0.5 ml of each of the extract/standard was taken in separate test tubes and the pH was maintained at 2-2.5 with dilute HCl. Then 2 ml of Dragendorff’s reagent was added and the precipitate formed was centrifuged. The centrifugation was decanted completely with care. The precipitate was further washed with alcohol and then filtered. The filtrate was discarded and the residue was then treated with 2 ml disodium sulphide solution. The precipitate formed was again centrifuged. The residue was dissolved in 2 ml concentrated nitric acid, and heated gently. This solution was again diluted to 10 ml in a standard flask with distilled water. Then 1 ml of this was pipetted out and 5 ml thiourea solution was added to it. The absorbance of the samples and the standards was measured at 435 nm using a UV-VIS spectrophotometer. The standard curve of berberine was drawn from the stock solutions of five different concentrations prepared above against the absorbance measured for each concentration.
GC-MS analysis of methanolic extract:
The GC-MS analysis of the plant extracts (methanol) was done at the GC-MS analysis Lab, Institute of Advanced Study in Science and Technology, Gorchuk, Guwahati, Assam. For carrying out GC-MS analysis of the methanolic extract of the whole plant of L. crustacea (L.) F. Muell., was performed in a Shimadzu make GC-MS 2010 model, Plus/TQ8030 of 30.0 m length, 0.25 mm diameter and 0.25 μm thick column DB-5-MS. The carrier gas helium was passed through a pressure of 65.9 kPa at a flow rate of ml/min. The volume of the sample injected was 1 μl and used in a split less mode. The injection temperature was 260° and ion source temperature was 200°; mass scan range was covered from 50-1000 m/z. The details of the programmed temperature were as follows-starting column temperature was 80° held for 2 min; increased by 5° and raised to 230°, held for 5 min; further increased by 3° and raised to 280°, held for 1 min. The total running time of the program was 54.67 min.
The data acquired were processed through GC-MS software post-run analysis. For identification of the compounds present in the extract was obtained through the National Institute of Standard and Technology (NIST 11) library and PESTEI-3 library.
Statistical analysis:
The results were expressed in terms of mean±standard deviation. All the data are presented in the form of mean values of triplicate measurements obtained from three separate readings.
Results and Discussion
The results of preliminary screening of phytochemicals of the acetone, methanol and water extracts of L. crustacea whole plant extracts are presented in Table 2. The results of the present study revealed the presence of important phytoconstituents such as phenols, tannins, flavonoids, proteins, glycosides and alkaloids in all the three extracts (methanol, water and acetone) of the L. crustacea plant. This is in agreement with previous study conducted by Ghori et al.[24] and Das et al.[17] which reported the presence of major group of compounds such as alkaloids, carbohydrates, flavonoids, tannins and glycosides, phenols in various extracts of the plant. In the present study, carbohydrates were detected only in the aqueous extract while saponins were found to be present in the acetone extract. On the other hand, terpenoids were absent in the aqueous extract and present in both the methanol and acetone extracts (Table 2).
| S. No. | Secondary metabolites | Tests | Methanol | Aqueous | Acetone |
|---|---|---|---|---|---|
| 1 | Phenols and tannin | FeCl3 test | + | + | + |
| 2 | Flavonoids | Shinoda test | - | - | + |
| Alkaline reagent test | + | - | - | ||
| Sulphuric acid test | + | + | - | ||
| 3 | Carbohydrates | Fehling’s test | - | - | - |
| Molisch’s test | - | + | - | ||
| 4 | Proteins | Millon’s test | - | + | + |
| Ninhydrin test | + | - | - | ||
| 5 | Saponins | Foam test | - | - | + |
| 6 | Glycosides | Keller-Killani test | - | + | + |
| Salkowski test | + | - | - | ||
| 7 | Terpenoids | Chloroform test | + | - | + |
| 8 | Alkaloids | Mayer’s test | + | - | + |
| Wagner’s test | - | - | + |
Note: (+): Present and (-): Absent
Table 2: Preliminary Phytochemical Analysis of Methanol, Aqueous and Acetone Extract of I. Crustacea
The results of quantitative evaluation of total phenols, total flavonoids, total tannins and total alkaloids in the methanol, aqueous and acetone extract of L. crustacea are presented in Table 3. The highest phenol content was found in the acetone extract with (165±3.25) mg Gallic Acid Equivalent (GAE)/g of dry extract followed by the methanol extract with (91.50±2.70) mg GAE/g of dry extract. Regarding the TFC, methanol extract showed the maximum yield with (84.51±2.39) mg QE/g dry weight while least amount was seen in the aqueous extract with (15.07±0.97) mg QE/g. Similar trend was followed in the total alkaloids content where the methanol extract exhibited highest quantity of alkaloids followed by the aqueous extract with (87.12±1.55) g and (55.59±0.94) g BE/ml of the plant extract respectively. While maximum yield of tannin content was found in the acetone extract with (10.73±0.42) mg QE/g of dry extract (Table 3).
| Parameters | Methanolic extract | Aqueous extract | Acetone extract |
|---|---|---|---|
| TPC (mg GAE/g) | 91.50±2.70 | 54.54±2.00 | 165±3.25 |
| TFC (mg QE/g) | 84.51±2.39 | 15.07±0.97 | 52.48±2.36 |
| TTC (mg TAE/g) | 5.29±0.29 | 7.55±0.11 | 10.73±0.42 |
| Total alkaloid content (mg BE/ml) | 87.12±1.55 | 55.59±0.94 | 32.05±1.51 |
Table 3: Quantitative Estimation of Total Phenol, Flavonoids, Tannin and Alkaloid Contents in Methanolic, Aqueous and Acetone Extract of l. crustacea (l.)F. Muell. Plant
In the present study, the quantification of total phenols, total flavonoids, total tannins and total alkaloids in methanol, water and acetone extract of L. crustacea (L.) F. Muell. whole plant was conducted for the first time. The results of the quantitative estimation of potential phytochemicals revealed that in all the three extracts of L. crustacea, maximum content of phenols, flavonoids, tannins and alkaloids were present. Phenolic compounds such as flavonoids, phenolic acids and tannins are the major group of compounds that act as strong free radical scavengers contributing to the antioxidant activities of medicinal plants[33]. Flavonoids are reported to possess several biological activities such as anti-inflammatory, anti-hepatotoxic, antiulcer, anti-allergic, antiviral and anti-cancer[34]. Tannins are a group of polymeric phenolic substances exhibiting anticancer, antioxidant[35], antiviral, antibacterial and antitumour activities[36]. While, alkaloids possess analgesic, antispasmodic and anti-bacterial properties[37]. Thus, the presence of elevated amounts of these compounds in L. crustacea whole plant shows that the plant may be used in the treatment of several harmful human diseases including cancer.
The present study revealed higher amounts of potential bioactive compounds such as phenolic, flavonoids, tannins and alkaloids in L. crustacea whole plant extracts which possibly validates the global use of L. crustacea in the traditional medicine system as each of these compounds exhibits numerous therapeutic properties.
The results of GC-MS analysis of the methanol extract of L. crustacea whole plant had accounted for altogether 47 phytochemicals that are presented in Table 4 along with their respective retention time, area and height percentage (Table 4).
| Peaks | Retention time | Compound name | Area (%) | Height (%) |
|---|---|---|---|---|
| 1 | 22.04 | 3,4-O-Isopropylidene-d-galactose | 2.44 | 2.26 |
| 2 | 22.11 | Methyl 2,3-di-O-acetyl-4-O-methyl-alpha-D-xylopyranoside | 3.21 | 2.99 |
| 3 | 22.16 | Hydroperoxide, 1,4-dioxan-2-yl | 1.97 | 3.37 |
| 4 | 22.195 | Beta-Ethoxypropionic acid | 2.4 | 3.57 |
| 5 | 22.24 | 2-Deoxy-D-galactose | 3.64 | 4.08 |
| 6 | 22.296 | 2-Butenoic acid, 3-ethoxy-ethyl ester | 1.9 | 4.43 |
| 7 | 22.325 | Pentanoic acid, 1-methylethyl ester | 2.33 | 4.4 |
| 8 | 22.414 | 3-O-Methyl-d-glucose | 11.31 | 4.85 |
| 9 | 22.52 | Pentanoic acid, 3-mercaptohexyl ester | 1.27 | 3.29 |
| 10 | 22.555 | Silane, (2-(2-methoxyethoxy) ethoxy) trimethyl | 1.54 | 2.4 |
| 11 | 22.605 | 2H-thiopyran, tetrahydro | 0.79 | 1.71 |
| 12 | 22.695 | o-Dithiane-3-carboxylic acid | 0.79 | 0.75 |
| 13 | 22.74 | Pentanoic acid, 3-mercaptohexyl ester | 0.21 | 0.37 |
| 14 | 25.174 | 3-Hexadecyne | 2.31 | 2.83 |
| 15 | 26.091 | 3-Hexadecyne | 0.54 | 0.74 |
| 16 | 27.152 | Benzenepropanoic acid, 3,5-bis (1,1-dimethylethyl)-4-hydroxy, methyl ester | 0.82 | 0.93 |
| 17 | 27.905 | 1,1,2,2,3,3-Hexamethyltrigermane | 0.15 | 0.29 |
| 18 | 27.995 | 5-Isoxazolol, 3-(2-furanyl)-4,5-dihydro-5-(trifluoromethyl) | 0.16 | 0.2 |
| 19 | 28.095 | Butanoic acid, 2-nitro-4-(phenylamino)-, 2,6-bis(1,1-dimethylethyl)-4-methoxyphenyl ester | 0.16 | 0.33 |
| 20 | 30.685 | Phytol | 0.8 | 1.1 |
| 21 | 31.069 | 2,8,9-Trioxa-5-aza-1-silabicyclo (3.3.3) undecane, 1-methoxy | 0.33 | 0.51 |
| 22 | 38.265 | 1-Ethyl-2,2,4a,7,7-pentamethyl-1,2,3,4,4a,5,6,7-octahydro (1,8) naphthyridine | 1.28 | 1.53 |
| 23 | 38.29 | Benzophenone, 2-(methylamino)-5-methyl | 0.79 | 2.14 |
| 24 | 38.37 | Pyrazine, 2,5-bis (1,1-dimethylethyl), 1,4-dioxide | 3.54 | 3.89 |
| 25 | 38.527 | 2-Allyl-3,6-dimethoxybenzyl alcohol | 21.86 | 7.15 |
| 26 | 38.63 | 3,3-Di-p-tolyl-thietan-2-one | 2.68 | 4.88 |
| 27 | 38.665 | Isoquinoline, 1,2,3,4-tetrahydro-2-(1'-phenethyl) carbamyl-6,7-dimethoxy | 1.14 | 4.06 |
| 28 | 38.69 | Stannane, methyltris (1-methylethyl) | 1.32 | 3.74 |
| 29 | 38.715 | Pentanedioic acid, 2-((trimethylsilyl) oxy), dimethyl ester | 1.63 | 3.3 |
| 30 | 38.76 | Valerophenone, 4'-(trimethylsiloxy) | 3.09 | 2.86 |
| 31 | 38.84 | (13-(2-Methoxyphenyl) tricycle (8.2.2.24,7) hexadeca-1 (13), 4, 6, 10 (14), 11, 15-hexaen-5-yl) methanol | 0.64 | 1.43 |
| 32 | 38.875 | Propane-1,3-diol 3-nitro-4-carboxy-benzeneboronate | 0.42 | 1.01 |
| 33 | 38.95 | Methyl 4-acetyl-2-(methoxycarbonyl)-5-methyl-1H-pyrrole-3-propanoate | 0.49 | 0.42 |
| 34 | 45.32 | Tricyclo (4.3.0.0 (7,9)) non-3-ene, 2,2,5,5,8,8-hexamethyl, (1 alpha, 6 beta, 7 alpha, 9 alpha) | 0.84 | 0.27 |
| 35 | 45.49 | Propanoic acid, 2-methyl-, (dodecahydro-6a-hydroxy-9a-methyl-3-methylene-2,9-dioxoazuleno (4,5-b) furan-6-yl) methyl ester, 3aS | 0.54 | 0.94 |
| 36 | 45.627 | 2-Allyl-3,6-dimethoxybenzyl alcohol | 15.06 | 7.4 |
| 37 | 47.722 | 1,4-Benzenedimethanethiol, S,S'-bis (tert-butyldimethylsilyl) | 0.4 | 0.32 |
| 38 | 48.065 | Hexasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11-dodecamethyl | 0.15 | 0.46 |
| 39 | 48.098 | (-)-cis-Myrtanol | 0.65 | 0.79 |
| 40 | 48.2 | 1-(2-Methylbutoxy)-7-isohexyl-2,2,4,4,6,6-hexamethyl-1,3,5,7-tetraoxa-2,4,6-trisilaheptane | 0.19 | 0.27 |
| 41 | 49.385 | Tartronic acid, 4-(dimethylethylsilyl) phenyl, dimethyl ester | 0.29 | 0.35 |
| 42 | 49.975 | N-methyl-1-adamantaneacetamide | 0.3 | 0.24 |
| 43 | 50.115 | Benzoic acid, 3-((trimethylsilyl)oxy)-trimethylsilyl ester | 0.08 | 0.18 |
| 44 | 50.155 | Sulfide, bis (2-cyano-3,4-dihydro-2,3,3-trimethyl-2H-pyrrol-5-yl)- | 0.11 | 0.19 |
| 45 | 50.21 | Sulfide, bis (2-cyano-3,4-dihydro-2,3,3-trimethyl-2H-pyrrol-5-yl)- | 0.17 | 0.29 |
| 46 | 51.694 | 3-Hydroxy-4-methoxybenzyl alcohol, bis (trimethylsilyl) ether | 0.08 | 0.36 |
Table 4: PhytochemicalsDetected in gc-ms Analysis of Methanol Extract of l. crustacea
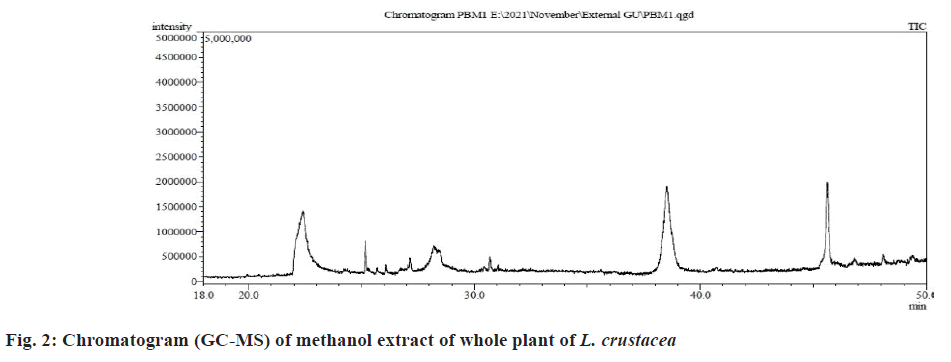
The chromatograms depicting the peaks are presented in fig. 2. The chromatogram of the GC-MS analysis of L. crustacea have detected some major phytochemicals representing higher area percentage belonging to naphthyridine, alcohol, ketone and terpene group of bioactive compounds. The major phytocompounds are presented in Table 5 along with their peak number, retention time, molecular weight, molecular formula and biological activities exhibited by each compound. The major phytochemical compounds are 2-allyl-3,6- dimethoxybenzyl alcohol-(area percentage=21.90 %; retention time=38.527 min)?valerophenone, 4´-(trimethylsiloxy)-(area percentage=3.09 %; retention time=38.760 min)?pentanedioic acid, 2-((trimethylsilyl) oxy)-, dimethyl ester (area percentage=1.63 %; retention time=38.715 min)?stannane, methyltris (1-methylethyl) (area percentage=1.32 %; retention time=38.690 min)?1-ethyl-2,2,4a,7,7-pentamethyl 1,2,3,4,4a,5,6,7-octahydro (1,8) naphthyridine (area percentage=1.28 %; retention time=38.265 min)?isoquinolin, 1,2,3,4-tertahydro-2-(1-phenethyl) carbanyl-6,7-dimethoxy (area percentage=1.14 %; retention time=38.665 min)?(-)-cis-myrtanol (area percentage=0.65 %; retention time=48.098 min) (fig. 2).
| Peak No. | Retention time | Compound name | Peak area (%) | Molecular weight (g/mol) | Molecular formula | Biological activity |
|---|---|---|---|---|---|---|
| 25 | 38.527 | 2-Allyl-3,6-dimethoxybenzyl alcohol | 21.9 | 208.25 | C12H16O3 | Antioxidant (34) |
| 30 | 38.76 | Valerophenone, 4´-(trimethylsiloxy) | 3.09 | 250.41 | C14H22O2Si | Antioxidant (36) |
| 29 | 38.715 | Pentanedioic acid, 2-((trimethylsilyl) oxy), dimethyl ester | 1.63 | 248.35 | C10H20O5Si | Antifungal (32) |
| 28 | 38.69 | Stannane, methyltris (1-methylethyl) | 1.32 | 206.901 | C6H16Sn | Not reported yet |
| 22 | 38.265 | 1-Ethyl-2,2,4a,7,7-pentamethyl-1,2,3,4,4a,5,6,7-octahydro (1,8) naphthyridine | 1.28 | 208.34 | C13H24N2 | Antimicrobial, antiviral, anticancer, anti-inflammatory, analgesic, anti-allergic, antioxidant, anti-malarial and protein kinase inhibitory properties (32, 33) |
| 27 | 38.665 | Isoquinolin, 1,2,3,4-tertahydro-2-(1-phenethyl) carbanyl-6,7-dimethoxy | 1.14 | 297.4 | C19H23NO2 | Antibacterial, antifungal, antitumor, anti-tubercular and cardiovascular activities (35) |
| 39 | 48.098 | (-)-cis-myrtanol | 0.65 | 154.25 | C10H18O | Antifungal (37) and antioxidant (38) |
Table 5: Major Bioactive Compounds Identified in the gc-ms Chromatogram of Methanol Extract of Whole Plant of l. Crustacea
In the present study the important phytochemical compounds identified by GC-MS assay mainly belongs to alcohol, ketone, terpenes and naphthyridine groups, each of which exhibits various biological activities. In a study conducted previously by Ghori et al.[24], the presence of five active compounds in the leaf extracts of L. crustacea identified by LC-MS analysis was reported. These compounds were found to be derivatives of chloro and nitro group of compounds. In contrary, in another study Das et al.[17], reported the presence of a flavonoid in the benzene, ethyl acetate and ethanol extract of L. crustacea with comparatively better pharmacological activities of the benzene extract.
In our study, the major phytocompounds revealed in the methanol extract of L. crustacea plant were detected for the first time in the GC-MS analysis. All these compounds are bioactive in nature and possess important therapeutic properties (Table 5).
The first major compound identified is 1-ethyl- 2,2,4a,7,7-pentamethyl 1,2,3,4,4a,5,6,7-octahydro (1,8) naphthyridine is a naphthyridine derivative compound that exhibits biological activities such as antioxidant, anti-inflammatory, anti-malarial and anti-cancer[38,39].
The second compound revealed in the assay is 2-allyl- 3,6-dimethoxybenzyl alcohol which have been reported earlier in the GC-MS analysis of methanol extract of Eichhornia crassipes[40]. It displayed potent antioxidant activity. On the other hand, another major compound isoquinolin, 1,2,3,4-tertahydro- 2-(1-phenethyl) carbanyl-6,7-dimethoxy possess antibacterial, antifungal, anti-tumour, anti-tubercular and cardiovascular activities[41]. The next compound identified as valerophenone, 4´-(trimethylsiloxy) is an aromatic ketone and have been reported to play an important role as a natural antioxidant in the essential oil extracted from the leaves of Apium graveolens[42].
Another important compound detected is (-)-cismyrtanol. It is a terpene which shows strong antifungal and antioxidant activity as detected previously in the essential oil of Thymus tosevii L.[43] and Polygonum minus respectively[44]. The next phytochemical revealed is pentanedioic acid, 2-((trimethylsilyl) oxy)-dimethyl ester which is a fatty acid derivative and have been reported to possess antifungal properties[45]. Thus, the results of the GC-MS analysis of the methanol extract of L. crustacea whole plant showed that the plant consists of many important phytochemical constituents each depicting important biological properties that may be responsible for the medicinal attributes of the plant. Medicinal plants are reservoir of important bioactive compounds exhibiting various therapeutic properties. The use of the L. crustacea in traditional medicine may be validated by the present study resulting in higher amounts of phytochemicals such as flavonoids, tannins and phenols in the different extracts of the plant. The potentiality of L. crustacea as a medicinal plant was understood rationally from the present study and may be attributed to the presence of the important phytocompounds detected and identified in the GCMS analysis. In our study, the phytochemicals revealed were detected for the first time by GC-MS assay in the methanolic extract of L. crustacea plant. Thus, the bioactive compounds identified may be responsible for the multi-therapeutic uses of L. crustacea in curing various harmful health disorders including cancer. The significance of these compounds lies in the fact, that each of these phytocompounds expends specific physiological effect on the human body that may be responsible for the prevention and treatment of several human diseases. The presence of naphthyridines, esters, ketones and terpenes may be accountable for advancement of pharmacological activities and aid in the manufacture of new drugs. Thus, detailed study of the biologically active compounds is most essential. Further, isolation and investigation of these phytocompounds may provide a scope for the development of novel drugs with improved efficacy and superior traits serving as advanced therapeutic agents.
Acknowledgements:
The authors would like to extend their sincere gratitude to the people of Balijan Shyam village, Titabor, Jorhat for allowing us to collect the plant species from their locality. The authors are also grateful to the head of the Department of Botany, Cotton University, Guwahati for providing the necessary facilities to carry out the research work. The authors also express their gratitude to CIF IASST, Guwahati, Assam for providing the GCMS facility.
Conflict of interest:
The authors declare no conflict of interests.
References
- Rahmati E, Khoshtaghaza MH, Banakar A, Ebadi MT. Decontamination technologies for medicinal and aromatic plants: A review. Food Sci Nutr 2022;10(3):784-99.
[Crossref] [Google Scholar] [PubMed]
- Afzal I, Habiba U, Yasmeen H. Review on therapeutic potential of phytochemicals from medicinal plants. J Bioresource Manag 2023;10(4):7.
- Nair SS, Nithyakala CM, Rozario RV, Jennifer J, Somashekharaiah BV. Biochemical characterization of selected plant species and investigation of phytochemicals for in vitro antioxidant activity. Int J Pharmacogn Phytochem Res 2012;4(3):127-33.
- Yadav RN, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol 2011;3(12):10-4.
- Aye MM, Aung HT, Sein MM, Armijos C. A review on the phytochemistry, medicinal properties and pharmacological activities of 15 selected Myanmar medicinal plants. Molecules 2019;24(2):293.
[Crossref] [Google Scholar] [PubMed]
- Oladeji OS, Odelade KA, Oloke JK. Phytochemical screening and antimicrobial investigation of Moringa oleifera leaf extracts. African J Sci Technol Innov Dev 2020;12(1):79-84.
- Gillani SW, Ahmad M, Zafar M, Haq SM, Waheed M, Manzoor M, et al. An insight into indigenous ethnobotanical knowledge of medicinal and aromatic plants from Kashmir Himalayan region. Ethnobotany Res Appl 2024;28:1-21.
- Chowdhury S, Poddar SK, Zaheen S, Noor FA, Ahmed N, Haque S, et al. Phytochemical screening and evaluation of cytotoxic and hypoglycemic properties of Mangifera indica peels. Asian Pac J Tropic Biomed 2017;7(1):49-52.
- Akinmoladun AC, Obuotor EM, Farombi EO. Evaluation of antioxidant and free radical scavenging capacities of some Nigerian indigenous medicinal plants. J Med Food 2010;13(2):444-51.
[Crossref] [Google Scholar] [PubMed]
- Kumar Singh A, Cabral C, Kumar R, Ganguly R, Kumar Rana H, Gupta A, et al. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019;11(9):2216.
[Crossref] [Google Scholar] [PubMed]
- Ballard CR, Junior MR. Health benefits of flavonoids. In: Bioactive compounds 2019. p. 185-201.
- Ayinde BA, Omogbai EK, Amaechina FC. Pharmacognosy and hypotensive evaluation of Ficus exasperata Vahl (Moraceae) leaf. Acta Pol Pharm 2007;64(6):543-6.
[Google Scholar] [PubMed]
- Abdallah MH, Lila AS, Unissa R, Elsewedy HS, Elghamry HA, Soliman MS. Preparation, characterization and evaluation of anti-inflammatory and anti-nociceptive effects of brucine-loaded nanoemulgel. Colloids Surf B Biointerfaces 2021;205:111868.
[Crossref] [Google Scholar] [PubMed]
- Tripathi AK, Ray AK, Mishra SK. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni Suef Univ J Basic Appl Sci 2022;11(1):16.
[Crossref] [Google Scholar] [PubMed]
- Umakrithika S. A comprehensive overview of plant genus: Lindernia. J Pharmacogn Phytochem 2021;10(5):42-8.
- Joshi SK, Sharma BD, Bhatia CR, Singh RV, Thakur RS. The wealth of India raw materials. Council of Scientific and Industrial Research Publication, New Delhi. 1992;3:270-1.
- Das SR, Ahmed AB, Shil DI, Chanda IN. Isolation and in vitro antioxidant activity of flavonoid from Lindernia crustacea (L.) F. Muell. Asian J Pharm Clin Res 2019;12(6):289-93.
- Silalahi M, Supriatna J, Walujo EB. Local knowledge of medicinal plants in sub-ethnic Batak Simalungun of North Sumatra, Indonesia. Biodiversitas 2015;16(1):44-54.
- Swapna MM, Prakashkumar R, Anoop KP, Manju CN, Rajith NP. A review on the medicinal and edible aspects of aquatic and wetland plants of India. J Med Plants Res 2011;5(33):7163-76.
- Smriti Rekha CD, Ahmed AB, Saha D, Chanda I. Scientific evidence of Lindernia crustacea (L) F. Muell., an indigenous plant: Folklore medicine used traditionally. Int Res J Pharm 2019;10(1):176-83.
- Madhu V, Rajesh Yarra RY. Investigations on ethno-medicinal plants used to cure skin diseases in Adilabad District, Andhra Pradesh, India. Int J Pharm Life Sci 2011;2(5):742-5.
- Meenu M, Krishnendra SN, Kiran C. Economic and ethnomedicinal importance of the floral diversity on ancient walls of Kota District, Rajasthan, India. Indian J Pure Appl Sci 2016;4(4):167-73.
- Sarmah R, Saikia A. Folklore medicine practiced by traditional healers of Fringe villages of Gibbon Wildlife Sanctuary, Assam, India. Acta Biomed Scientia 2016;3(4):227-33.
- Ghori SS, Tehseen F, Sultana SS, Fatima N, Ahmed MM. Phytochemical investigation, liquid chromatography-mass spectrometry analysis, antibacterial and anthelminthic activity of Lindernia crustacea (L.) F. Muell. Indian J Pharm Sci 2021;83(6):1314-9.
- Peiris DS, Fernando DT, Senadeera SP, Ranaweera CB. Phytochemical screening for medicinal plants: Guide for extraction methods. Asian Plant Res J 2023;11:13-34.
- Harborne JB. Phytochemical methods. Chapman and Hall Ltd., London; 1973. p. 49-188.
- Trease GE, Evans WC. Pharmacognosy. 11th ed. Bailliere Tindall, London; 1989. p: 49-188.
- Sofowora A. Medicinal plants and traditional medicine in Africa. Spectrum Books Ltd. Ibadan, Nigeria; 1993. p. 191-289.
- McDonald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem 2001;73(1):73-84.
[Crossref] [Google Scholar] [PubMed]
- Har LeeWei HL, Intan Safinar Ismail IS. Antioxidant activity, total phenolics and total flavonoids of Syzygium polyanthum (Wight) Walp leaves. Int J Med Aromatic Plants 2012;2(2):219-28.
- Sudha BR, Remakanthan A, Hareesh KH, Aryakrishna UK. A comparative study of the phytochemicals, antioxidant and antibacterial potential of methanolic extracts of Trichosanthes cucumerina (L.) Var. Cucumerina under in vitro culture and natural conditions. Int J Pharm Pharm Sci 2018;10(1):147-54.
- Sreevidya N, Mehrotra S. Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff's reagent in plant materials. J AOAC Int 2003;86(6):1124-7.
- Mohan VR, Lincy MP, Devi GS. Evaluation of phenolic and flavonoid contents and antioxidant activity of various solvent extracts of Cadaba indica lam. Int J Adv Pharm Sci 2015;6(3):2849-53.
- Paulsamy S, Karthika K. Screening of in vitro antioxidant activity of methanolic leaf and root extracts of Hypochaeris radicata L. (Asteraceae). J Appl Pharm Sci 2012;2(7):149-54.
- Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther 2002;96(2-3):67-202.
[Crossref] [Google Scholar] [PubMed]
- Aiyelaagbe OO, Osamudiamen PM. Phytochemical screening for active compounds in Mangifera indica leaves from Ibadan, Oyo State. Plant Sci Res 2009;2(1):11-3.
- Sowjanya M, Kiran Kumar M, Sandeep BV. Assessment of phytochemicals and antioxidant activities of Leucas indica aerial parts-A comparative study. Int J Life Sci 2016;4:29-43.
- Madaan A, Verma R, Kumar V, Singh AT, Jain SK, Jaggi M. 1,8-naphthyridine derivatives: A review of multiple biological activities. Archiv der Pharmazie 2015;348(12):837-60.
[Crossref] [Google Scholar] [PubMed]
- Ojha M, Yadav D, Kumar A, Dasgupta S, Yadav R. 1,8-naphthyridine derivatives: A privileged scaffold for versatile biological activities. Mini Rev Med Chem 2021;21(5):586-601.
[Crossref] [Google Scholar] [PubMed]
- Lenora LM, Senthilkumar J, Murugesan S, Senthilkumar N. GC-MS-MS analysis of alien invasive aquatic weed, Eichhornia crassipes (Mart.) Solms. Der Chem Sin 2016;7:48-52.
- Tiwari RK, Singh D, Singh J, Chhillar AK, Chandra R, Verma AK. Synthesis, antibacterial activity and QSAR studies of 1,2-disubstituted-6,7-dimethoxy-1,2, 3, 4-tetrahydroisoquinolines. Eur J Med Chem 2006;41(1):40-9.
- Nagella P, Ahmad A, Kim SJ, Chung IM. Chemical composition, antioxidant activity and larvicidal effects of essential oil from leaves of Apium graveolens. Immunopharmacol Immunotoxicol 2012;34(2):205-9.
[Crossref] [Google Scholar] [PubMed]
- Sokovic MD, Vukojevic J, Marin PD, Brkic DD, Vajs V, van Griensven LJ. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 2009;14(1):238-49.
[Crossref] [Google Scholar] [PubMed]
- Ahmad R, Baharum SN, Bunawan H, Lee M, Mohd Noor N, Rohani ER, et al. Volatile profiling of aromatic traditional medicinal plant, Polygonum minus in different tissues and its biological activities. Molecules 2014;19(11):19220-42.
[Crossref] [Google Scholar] [PubMed]
- Pohl CH, Kock JL, Thibane VS. Antifungal free fatty acids: A review. Sci Against Microb Pathogens Curr Res Technol Adv 2011;1:61-71.