- *Corresponding Author:
- Linyuan Wang
Department of Periodontics, School of Stomatology of Jinzhou Medical College, China
E-mail: wang_linyuan@outlook.com
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “284-293” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the role of gallic acid in lipopolysaccharide/interferon-gamma induced macrophage polarization toward M1. Macrophages were isolated from peritoneal wash fluid and exposed to gallic acid prepared at the concentrations of control, 6.25, 12.5, 25 and 37.5 μg/ml, in presence of lipopolysaccharide and interferon-gamma, for establishment of M1 polarization. Adenosine 5’-monophosphate-activated protein kinase inhibitor compound C was used to pre-treat the macrophages; cell viability was determined by flow cytometry with Annexin V-PE/7-amino actinomycin D staining, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide and trypan blue assays; flow cytometry was applied to evaluate the expression of F4/80+ inducible nitric oxide synthase+ M1 macrophages; real-time polymerase chain reaction was used to assess the messenger ribonucleic acid expression level of inducible nitric oxide synthase, tumor necrosis factor-alpha and interleukin-1beta. Protein expression levels of cyclooxygenase-2, adenosine 5‘-monophosphate-activated protein kinase, signal transducers and activators of transcription 1 and phosphorylation levels of adenosine 5’-monophosphate-activated protein kinase and signal transducers and activators of transcription 1 were evaluated by Western blotting. Gallic acid at the concentrations of 6.25-37.5 μg/ml showed no noticeable effects on macrophages viability, but significantly decreased F4/80+ inducible nitric oxide synthase+ M1 macrophages polarization and the expression levels of inducible nitric oxide synthase, tumor necrosis factoralpha, interleukin-1 beta, cyclooxygenase-2 and phospho-signal transducers and activators of transcription 1, meanwhile increased the expression levels of p-adenosine 5’-monophosphate-activated protein kinase contents in a concentration-dependent manner. Adenosine 5’-monophosphate-activated protein kinase inhibitor compound C pre-treatment can reverse the effects of gallic acid on M1 macrophage polarization. Gallic acid may inhibit macrophage polarization toward M1 via adenosine 5’-monophosphate-activated protein kinase/signal transducers and activators of transcription 1 signaling pathway.
Keywords
Gallic acid, macrophage polarization, M1-type macrophages, adenosine 5‘-monophosphateactivated protein kinase, homeostasis
Materials and Methods
As heterogeneous and versatile cells, macrophage can acquire distinct morphological and functional properties, which contributes to inflammatory response[1], autoimmunity and damage repair[2,3]. The polarization of macrophages plays a crucial role in eliminating the pathogens or maintaining tissue homeostasis. Nowadays, it is generally believed that the development of various immune-related diseases is influenced by the polarization of macrophages[4,5].
Under the complex and changeable microenvironment, macrophages can be transformed into different phenotypes. In general, macrophages have at least two different polarizations, namely M1-type macrophages and M2-type macrophages[6]. Macrophages were stimulated by Lipopolysaccharide (LPS), Interferon- Gamma (IFN-γ) and other substances to be transformed into M1-type macrophages. These macrophages produce inducible Nitric Oxide Synthase (iNOS), Interleukin-1 Beta (IL-1β), Tumor Necrosis Factor- Alpha (TNF-α), which responses to participate in the promotion of inflammation response. On the other hand, under the stimulation of IL-4 and other substances, macrophages can be transformed into M2-type macrophages that highly express Arginase-1 (Arg-1), thus Transforming Growth Factor-β (TGF-β) and so on. These macrophages promote inflammatory breakdown and wound repair. However, the mechanism of macrophage polarization is still unclear[7,8].
Gallic Acid (GA) is a polyphenolic compound with a wide range of bioactive properties, including antioxidant activity, protecting from infections, exerting immunological effects and inducing metabolic changes[9,10]. Numerous recent studies have demonstrated the anti-inflammatory effects of GA through the regulation of macrophage-related functions. Huang et al. have proved that GA can reduce the overexpression of TNF-α, IL-6, IL-1 and other inflammatory cytokines by down-regulating the Toll- Like Receptor 4 (TLR4)/Nuclear Transcription Factor Kappa B (NF-κβ) signaling pathway, thereby protecting against tissue damage caused by LPS[11]. Moreover, Ahn et al. have discovered that GA can down-regulate the TLR4 pathway in LPS-stimulated macrophages via 67 Laminin Receptor (67LR) signaling, thus leading to the Mitogen-Activated Protein Kinase (MAPK) inhibition and reduced the production of pro-inflammatory cytokines[12]. These results indicated that GA had potential anti-inflammatory activity.
In this study, we investigate the role of GA in LPS/IFN-γ induced M1 polarization and its related mechanism. The results from this study would not only expand our knowledge of mechanisms underlying the effects of GA on macrophage polarization, but also provide new clues for gene-targeted therapies at M1 macrophage-related inflammatory disease in the future.
Materials and Methods
Reagents and antibodies:
GA (cat: T0877, TargetMol, United States of America (USA)); LPS (cat: L2880, Sigma, USA)[13,14]; IFN-γ (cat: 315-05, Peprotech, USA); anti-mouse F4/80 (cat: 123108, BioLegend, USA); anti-mouse iNOS (cat: 61-5920-82, Invitrogen, USA); Compound C (cat: 1219168-18-9, AbMole, USA); penicillin and streptomycin (cat: 15140-122), Roswell Park Memorial Institute (RPMI) 1640 medium (cat: 31800-022) and Fetal Bovine Serum (FBS) (cat: 16000-044) were purchased from Gibco (USA).
Peritoneal macrophage isolation:
The mouse peritoneal macrophages were prepared as stationary phenotype according to the protocol described previously[15]. Briefly, 8 w old female C57BL/6 mice (Specific Pathogen-Free (SPF), Animal Experimental Center, Jinzhou Medical University) received 3 ml peritoneal injection of 2 ml, 3 % thioglycollate medium (Solarbio). Mice were sacrificed by Carbon dioxide (CO2) suffocation 3 d and further received peritoneal injection of 3 ml 0.05 % ethylenediaminetetraacetic acid which was dissolved in Phosphate-Buffered Saline (PBS). Then thioglycollate-elicited peritoneal macrophages were harvested. Cell pellet were collected after centrifugation at 1200 g for 5 min at 4° and then washed by PBS. Then the resulted cells were cultured with RPMI-1640 medium supplemented with 10 % FBS at 37° for 2 h. Then the unattached cells were removed after washed by PBS three times. All animal experimental protocol were reviewed and approved by Institutional Animal Care and Use Committee of Jinzhou Medical University.
Cell treatments:
For GA exposure, the cultured macrophages were respectively treated with serially diluted GA at final concentrations of 6.25, 12.5, 25 and 37.5 μg/ml for 24 h. For LPS and IFN-γ stimulation, macrophages were treated with LPS and IFN-γ at final concentration of 100 and 20 ng/ml for 24 h[16]. Several macrophages were pre-treated with specific Adenosine 5‘-Monophosphate- Activated Protein Kinase (AMPK) inhibitor compound C at final concentration of 5 μm 1 h prior to GA administration.
Annexin V-PE/7-Amino Actinomycin D (7-AAD) flow cytometry analysis:
Cell apoptosis was measured using an Annexin V-PE/7- AAD apoptosis detection kit (cat: A213-02, Vazyme, China). Briefly, cells were collected and re-suspended in 1× binding buffer. Then, the solution (suspension containing 1×105 cells) was supplemented with 5 μl of PE Annexin V and 7-AAD and incubated in the dark for 15 min at room temperature. Apoptotic cells were identified by flow cytometry. All the experiments were performed in triplicate.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2Htetrazolium bromide (MTT) assay:
Peritoneal macrophages in the exponential growth phase were seeded in 96-well plates at a density of 5×103 cells/well. GA was added at control, 6.25, 12.5, 25 and 37.5 μg/ml and the cells were incubated at 37° for 24 h. Cell viability was determined using the MTT reduction assay (CellTiter 96® Aqueous One Solution Cell Proliferation Assay kit; cat: G3582, Promega, USA), which measures mitochondrial respiration in living cells. Reduction of MTT to formazan within the cells was quantified by measuring absorbance at 490 nm using a molecular device microplate reader (Sunnyvale, CA, USA). Results are expressed as mean percent viability±Standard Deviation (SD) of 3 independent experiments.
Trypan blue exclusion assay:
Peritoneal macrophages were cultured at a density of 50 000 cells per well in 96-well culture plates and contaminated culture media contained different concentrations of GA were added to the respective wells. After 24 h, the cells were washed with PBS and using trypsin/Ethylenediaminetetraacetic Acid (EDTA) and the cells were detached from culture plates and subsequently collected by centrifugation at 1700 rpm for 5 min. Following centrifugation, the cells were resuspended in fresh culture media and 50 μl of the cell suspension was mixed with an equal volume of trypan blue (cat: T8154, Sigma, USA) and incubated for 2 min at 37°. Using hemocytometer, the percentage of viable and dead cells was determined. Trypan blue is a dye that enters the cell when the cell membrane is damaged cell appears to be blue in color.
Flow cytometry analysis of cell markers in vitro:
The following fluorochrome-conjugated antibodies were used, Fluorescein Isothiocyanate (FITC)-labeled rat F4/80 isotype control and Phycoerythrin (PE)- labeled mouse iNOS isotype control. The stained cells were analyzed by flow cytometer (Becton Dickinson (BD) Biosciences) using specialized software (FlowJo 7.6.1 Software; tree star Inc., Ashland, OR, USA).
Total Ribonucleic Acid (RNA) extraction and quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR):
Cells in each group were collected for messenger RNA (mRNA) analysis. Total RNA was extracted using RNAiso reagent (TaKaRa Biotechnology Co. Ltd., Dalian, China) and complementary Deoxyribonucleic Acid (cDNA) synthesized by Reverse Transcription (RT) using PrimeScript RT system (TaKaRa Biotechnology Co. Ltd.), according to the manufacturer's instructions. The expression levels of iNOS, TNF-α and IL-1β transcripts were quantified using qRT-PCR with SYBR Premix Ex Taq II (TaKaRa Biotechnology Co. Ltd.) and a LightCycler® system (Roche molecular bio chemicals, Mannheim, Germany), in accordance with the manufacturers protocol. Primers for iNOS, TNF-α, IL-1β and β-actin were used. Primer sequences (TaKaRa Biotechnology Co. Ltd.), predicted amplicon sizes and annealing temperature are shown in Table 1. Relative target gene quantification was carried out using the 2−ΔΔCt method, as described previously.
Western blotting analysis:
Peritoneal macrophages total proteins were extracted using Radio Immunoprecipitation Assay (RIPA) with phosphatase inhibitor and Phenylmethylsulfonyl Fluoride (PMSF). Protein concentrations were measured using a Bicinchoninic Acid (BCA) protein assay kit (cat: P0012S, Beytime, China), total proteins were separated by Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE). Following electrophoresis, blotted onto Polyvinylidene Difluoride (PVDF) (cat: IPVH00010, Millipore, USA) membranes and blocked with Tris Buffered Saline with Tween-20 (TBST) and 5 % milk for 2 h. The membranes were then incubated with primary antibodies against AMPK (1:1000, cat: A17290, ABclonal, China), phospho- AMPK (1:1000, cat: AP1171, ABclonal, China), Signal Transducer and Activator of Transcription 1 (STAT1) (1:1000, cat: A0027, ABclonal, China), phospho- STAT1 (1:1000, cat: AP0054, ABclonal, China) and Cyclooxygenase-2 (COX-2) (1:1000, cat: A12796, ABclonal, China) at 4° overnight. β-actin (1:50 000, cat: AC026, ABclonal, China) expression was used as an internal control. After washed with TBST, membranes were incubated with Horse Radish Peroxidase (HRP) goat anti-rabbit Immunoglobulin G (IgG) (1:2000, cat: AS014, ABclonal, China) for 1 h at room temperature. The protein samples were visualized using the Enhanced Chemiluminescence (ECL) kit (cat: E412-01, Vazyme, China) and analyze by ImageJ software (NIH, USA).
Statistical analysis:
All data were presented in a (mean±SD) manner in this study which was then analyzed by using Statistical Package for the Social Sciences (SPSS) (version 23.0) software. One-way Analysis of Variance (ANOVA) was used to analyze the differences between groups. Difference were considered statistically significant when p<0.05.
Results and Discussion
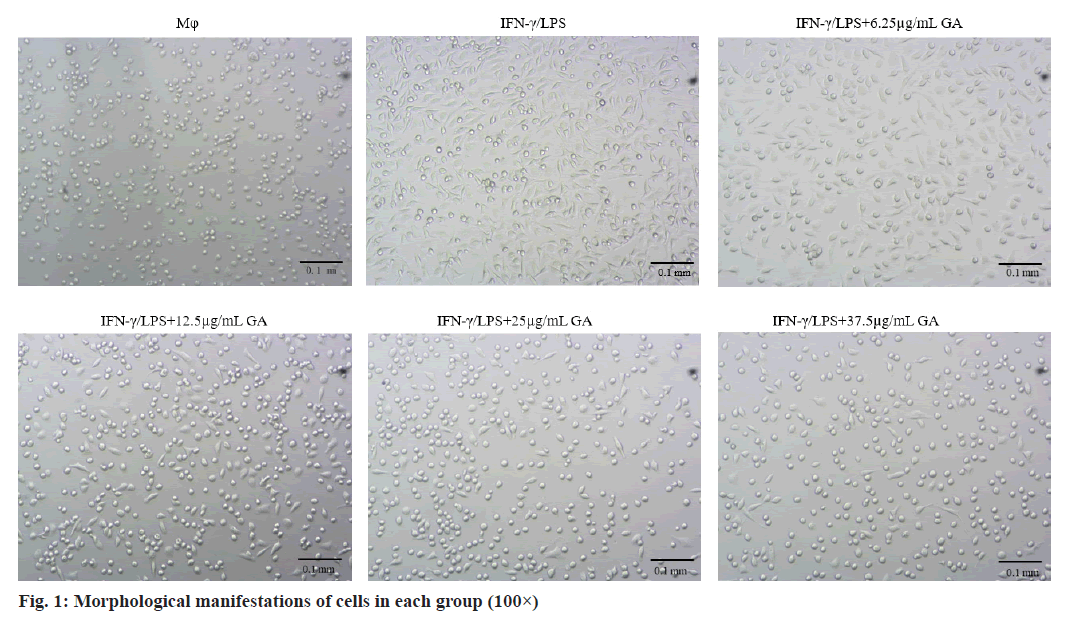
Morphological changes of macrophages were examined under an inverted microscope (100× magnification) as shown in fig. 1. Cells in the macrophages group appear circular or elliptical in shape, with clear cell boundaries and full cytosol. Addition of IFNγ/LPS caused apparent changes in morphology including the emergence of elongated and spindle-shaped cells, pseudopodia formation and increased cell scattering. Treatment of cells with GA caused decreased cell volume, changes in cell shape from the original spindle gradient shape to a round shape and increases in cell gap when compared with the IFNγ/LPS group.
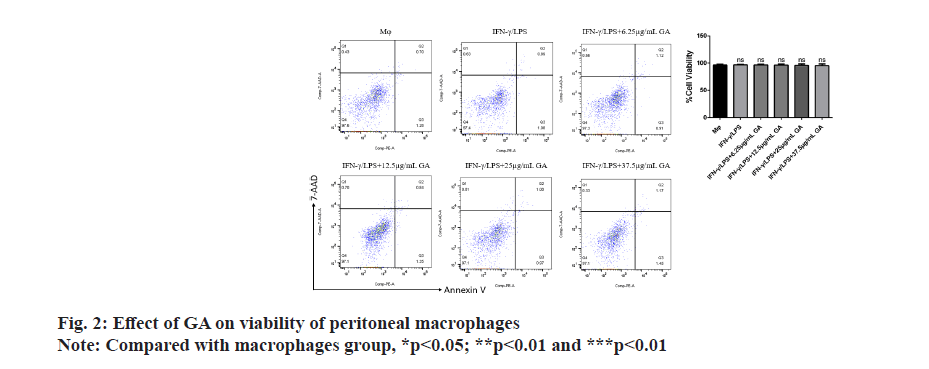
Apoptosis of cells in each group was assessed by flow cytometry as shown in fig. 2. There were no significant differences in the rates of apoptosis in either the IFNγ/ LPS group or the GA-treated group when compared with the macrophage controls. There was also no significant difference between rates of apoptosis in the IFNγ/LPS group compared with the GA-treated.
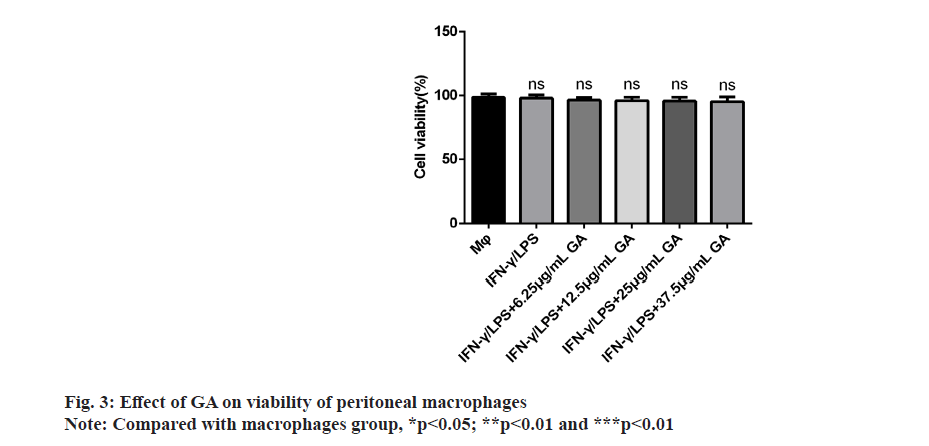
We examined the effect of GA on viability of peritoneal macrophages in vitro by incubating cells with control, 6.25, 12.5, 25 and 37.5 μg/ml of GA for 24 h. The results of the MTT assay showed that GA was not cytotoxic to primary macrophages at concentrations up to 37.5 μg/ml as shown in fig. 3. Therefore, we selected concentrations of 6.25, 12.5, 25 and 37.5 μg/ml for further study.
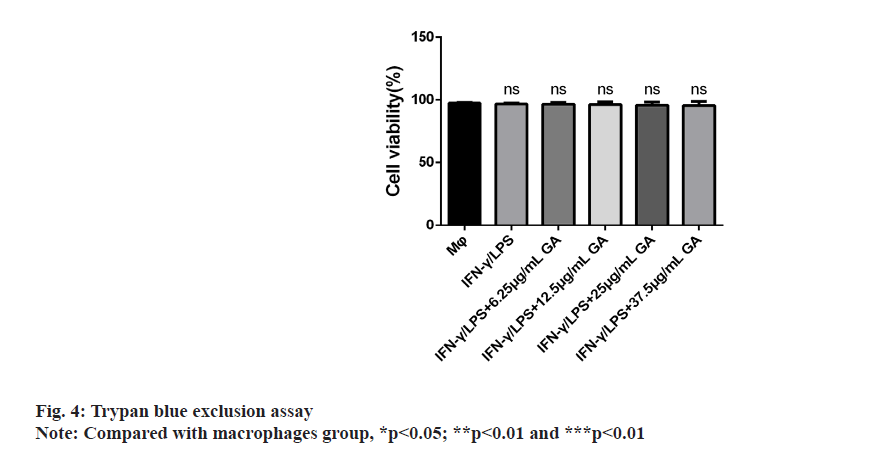
The results of cellular viability are shown in fig. 4. The mean cellular viability was >97 % in all the macrophage cultures, regardless of exposure to GA. There was no difference in the percentages of live cells between groups.
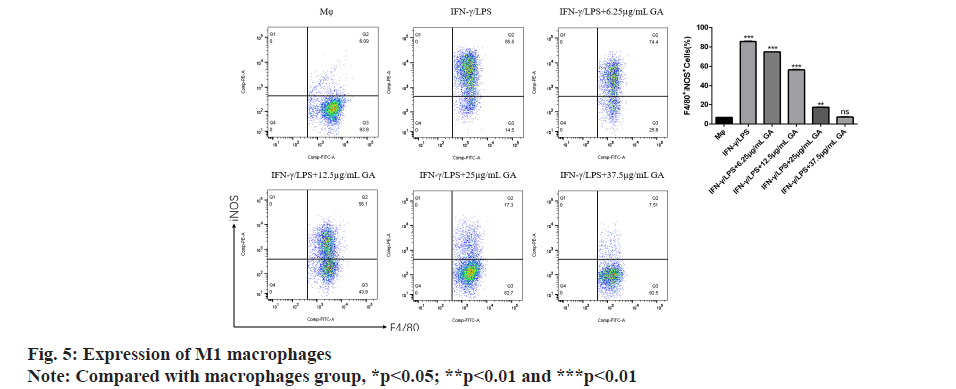
Using flow cytometry, we detected the proportion of M1 macrophages expressing F4/80 and iNOS in each group as shown in fig. 5. The proportion of F4/80+ iNOS+ M1 macrophages in the IFNγ/LPS group was significantly higher (p<0.05) than in the macrophages control group. The proportion of F4/80+ iNOS+ M1 macrophages was significantly decreased in a concentration-dependent manner by GA treatment (p<0.05).
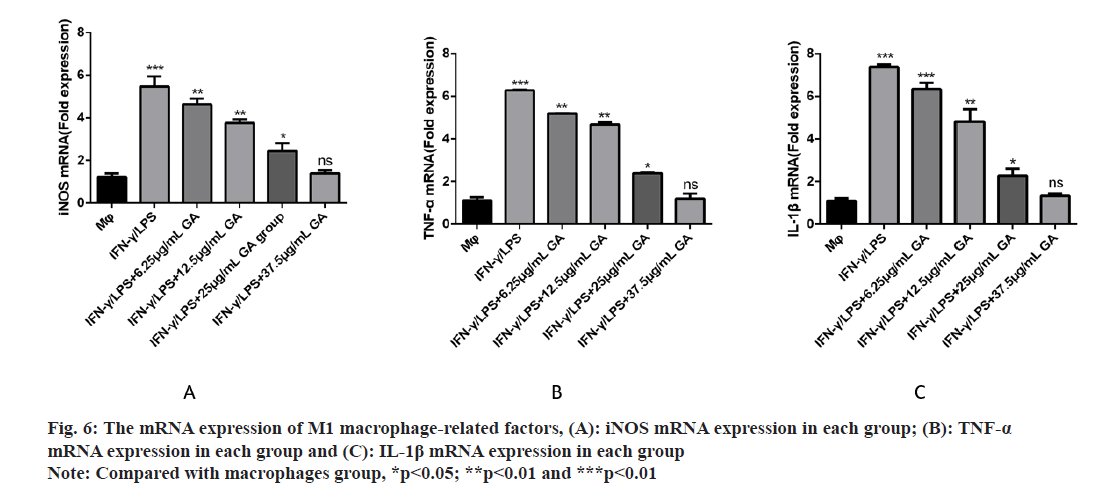
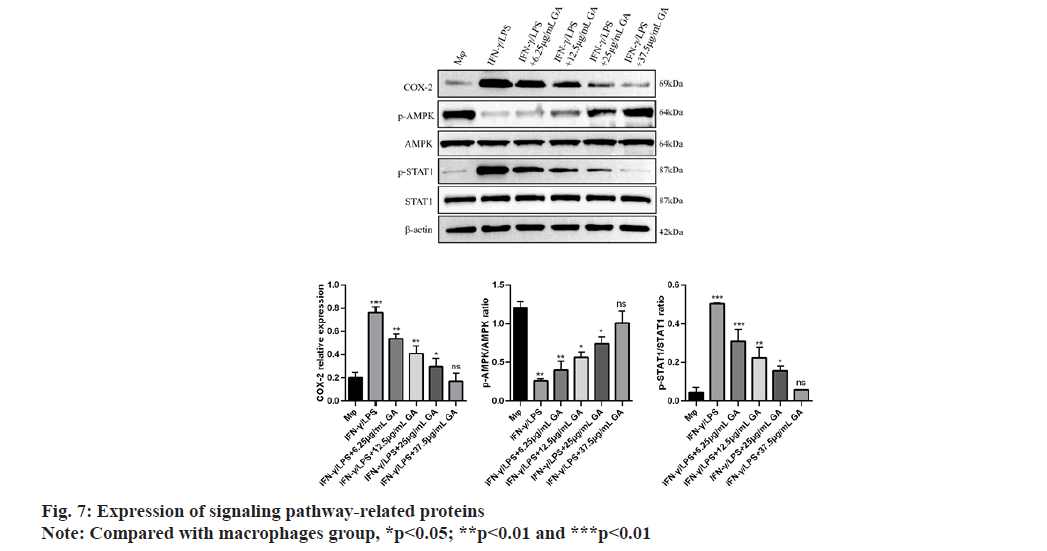
We used qRT-PCR to investigate the expression of M1-related cytokines (fig. 6A-fig. 6C). iNOS, TNF-α and IL-1β, biomarkers of M1-activated macrophages, were significantly up regulated in the IFNγ/LPS group compared with the macrophages control group (5.71, -6.35 and -7.16) fold, respectively, p<0.01, as shown in fig. 6A-fig. 6C. Moreover, treatment with GA reduced expression of the proinflammatory cytokines, iNOS,TNF-α and IL-1β, in a concentration-dependent manner. In order to further detect the expression of signaling pathway-related factors, we used Western blot analysis as shown in fig. 7. The IFNγ/LPS group showed increased expression of p-STAT1 and COX-2 and decreased expression of p-AMPK (p<0.05) compared with the macrophages control group. GA up-regulated p-AMPK expression in a dose-dependent manner at concentrations ranging from 6.25 to 37.5 μg/ml, p<0.05 and down regulated p-STAT1 and COX-2 expression (p<0.05).
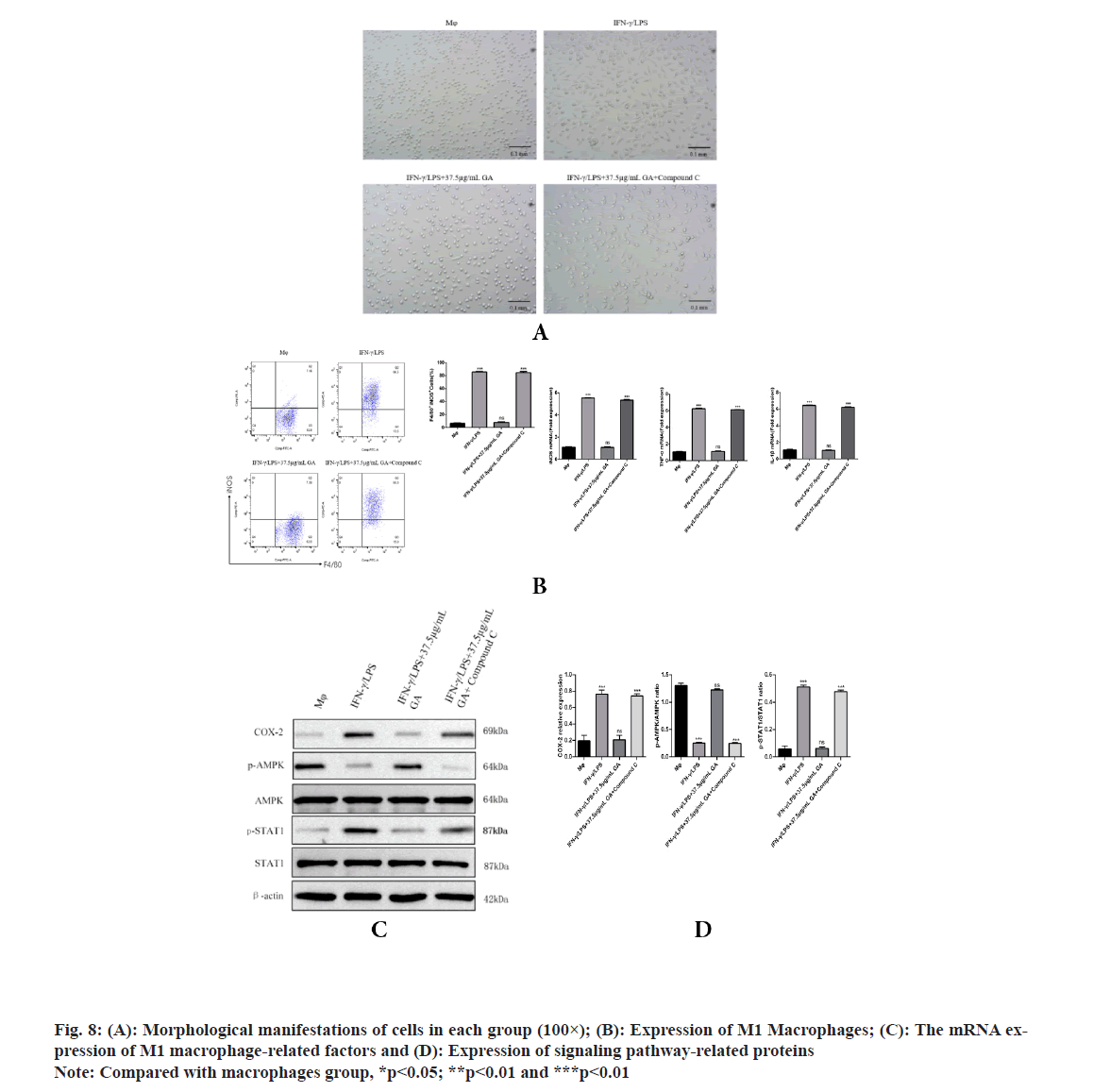
To further explore whether AMPK is responsible for the GA induced inhibition of M1 macrophage polarization, AMPK channel was blocked with compound C (an AMPK inhibitor) in GA treatment cells. As shown in fig. 8, GA significantly reduced M1 macrophage polarization, but the pretreatment with compound C significantly inhibited the decrease in M1 macrophage polarization by GA. Morphological changes of macrophages were examined under an inverted microscope, cells in the GA group appear circular or elliptical in shape, with clear cell boundaries and full cytosol, pretreatment with compound C caused apparent changes in morphology including the emergence of elongated and spindle-shaped cells, pseudopodia formation and increased cell scattering; compared with GA group, pretreatment with compound C significantly increased F4/80+ iNOS+ M1 macrophages polarization and the expression levels of iNOS, TNF-α, IL-1β, COX-2 and p-STAT1, while decreased the expression levels of p-AMPK contents. These results revealed that AMPK activation accounts for the GA-induced inhibition of M1 macrophage polarization.
The mouse peritoneal macrophage is widely used in medical research, especially as an inflammatory model. Although many previous studies have used the cellline, RAW264.7, such research has been hampered by frequent mutations after multiple passages during culture which lead to changes in the genotype and phenotype of the cell-line. By contrast, primary cells retain many more of the characteristics of cells in vivo. In current study we used primary extracts of mouse peritoneal macrophages in order to induce M1 macrophage polarization in vitro.
Macrophages are vital for the immune response and switch between two phenotypes in order to fulfill their role[17,18]. M1 macrophages secrete high levels of proinflammatory cytokines (TNF-α, IL-6, IL-1β) and produce reactive oxygen species through activation of iNOS. An experimental model of LPS induced inflammation is most commonly used. Cells must express the cell-surface protein, Cluster of Differentiation 14 (CD14), in order to respond to LPS and stimulate the following sequence of events[16]. CD14 interacts with TLR4 to activate TNF Receptor-Associated Factor 6 (TRAF6) which stimulates TGF β-Activated Kinase 1 (TAK1) leading to phosphorylation of IκB Kinase (IKK). These events promote IκB degradation and activation of the Nuclear Factor Kappa B (NF- κB) signaling pathway, contributing to the release of inflammatory factors, NO production by iNOS and M1 macrophage polarization. Interferon gamma (IFN-γ), a cytokine primarily produced by NK cells, increases the sensitivity of macrophages to LPS activation by triggering the cells’ inflammatory potential, thereby promoting polarization[19].
GA is a polyphenolic organic compound found in a number of newly-identified medicinal plants which has a range of clinical applications[20]. For example, GA inhibits tumorigenesis and induces apoptosis in tumor cells. Moreover, GA has a protective effect on cartilaginous joints which ameliorates chronic osteoarthritis and has a potentially beneficial effect for the prevention and treatment of neurodegenerative diseases[21]. Numerous recent studies have demonstrated an anti-inflammatory effect of GA via the regulation of macrophage-related functions. Huang et al. have shown that GA can reduce overexpression of TNF-α, IL-6 and IL-1 and other inflammatory cytokines by down-regulating the TLR4/NF-κβ signaling pathway, thereby protecting against tissue damage caused by LPS[11]. Moreover, Ahn et al. have found that GA can down-regulate the TLR4 pathway in LPS-stimulated macrophages via 67LR signaling, leading to MAPK inhibition and reduced production of pro-inflammatory cytokines[12]. Furthermore, GA alone, may inhibit production of pro-inflammatory cytokines, IL-1β, TNKα and iNOS by macrophages via an effect on AMPK[22]. Thus, indications are that GA can exert its regulatory effect on macrophages via multiple signaling pathways. Currently, the studies on GA and macrophages are mostly limited to the effect of GA on the expression of LPS-induced macrophage-related inflammatory factors, while the targeted research of GA on M1 macrophage polarization has not been carried out. In this study, mouse peritoneal macrophages were used as the research object and the polarization model of M1 macrophages was established to observe the proportion of M1 macrophages and the expression of related inflammatory cytokines and protein in each group. The study showed that during the polarization of M1 macrophages, macrophages gradually changed from round or elliptical to spindle-shaped. Meanwhile, there are protruding pseudopodia. After GA treatment, the microscopic morphology of macrophages changed again, spindle cells and irregular cells decreased, the increase of round cells and the decrease of protruding pseudopodia indicated that GA could affect the polarization process of M1 macrophages. The results of apoptosis rate in this study showed that the apoptosis rate of M1 macrophages induced differentiation system had no significant change after adding 37.5 mg/l GA.
The results of cell phenotype, related cytokine mRNA and protein expression of M1 macrophages showed that, GA significantly decreased F4/80+ iNOS+ M1 macrophages polarization and the expression levels of iNOS, TNF-α, IL-1β, COX-2 and p-STAT1, while increased the expression levels of p-AMPK contents in a concentration-dependent manner. F4/80+ is the surface marker of peritoneal macrophages in mouse[23] and the expression levels of iNOS, TNF-α, IL-1β, COX-2 and p-STAT1 can reflect the polarization degree of M1 macrophages[24,25].
Production of IL-1β and TNF-α by macrophages is crucial for regulating the inflammatory response[26,27]. IL-1β is not only a hallmark of many human chronic inflammatory states but it is also associated with the acute-phase reaction[23,28]. TNF-α also plays significant roles in inflammatory diseases[24]. Indeed, high levels of IL-1β and TNF-α have been associated with increased risk of cardiovascular disease. Therefore, identification and investigation of natural products, such as GA, that could reduce the secretion of these cytokines may have significant consequences for treatment and prevention of chronic inflammatory states. The current study found that GA treatment reduced levels of IL-1β and TNF-α, mRNA in a lysate of IFN-γ/LPS-activated Murine Peritoneal Macrophages (MPM). Our data demonstrated that GA may suppress the inflammatory response in IFN-γ/LPS-activated MPM macrophages through down regulation of expression of inflammatory genes at the transcriptional level. This mode of action is consistent with the effects of GA on iNOS expression previously reported in activated RAW 264.7 macrophages[12]. IFN-γ/LPS-stimulated MPM macrophages display increased COX-2 production which can be significantly reduced by GA treatment. Phospho (p)-STAT1 is acknowledged to be primarily responsible for COX-2 production in inflammatory processes[25]. The present study found that elevated p-STAT1 expression following from IFNγ/LPS-stimulation of macrophages could be significantly reduced by GA treatment, with consequent reduction of COX-2 expression. Thus, the inhibition of STAT1 activity and subsequent COX-2 production may explain the anti-inflammatory effects of GA. LPS is the best-studied M1 macrophage activation signal. It is recognized by TLR4, resulting in decreased AMPK activation via the MyD88 and MaL/Tirap (Toll-IL 1 receptor domain containing adaptor protein)-dependent pathways. Inhibition of AMPK activation may account for M1 macrophage polarization and subsequent pro-inflammatory effects[29]. It is well known that phosphorylation of AMPK is an essential step in the AMPK/STAT1 signaling cascade and is required for nuclear translocation and subsequent transcriptional activation. AMPK signaling pathway plays a role in the process of macrophage polarization, but whether GA plays a role in M1 macrophage polarization through AMPK signaling pathway has not been reported. Therefore, compound C (AMPK inhibitor) was used to pretreat macrophages to observe whether GA can inhibit the polarization of M1 macrophages after blocking AMPK signaling pathway in this experiment, we found that after being pretreated with compound C, cells in the GA group exhibited obvious dendritic, fusiform and radial changes in appearance and compound C pretreatment significantly increased the proportion of F4/80+ iNOS+ M1 macrophages and the expression levels of M1 macrophage-related factors such as iNOS, TNF-α and p-STAT1, and decreased the expression level of p-AMPK protein, suggesting that GA may inhibit the polarization of M1 macrophages through AMPK signaling pathway[30]. Previous studies have shown that macrophages can activate IKK through TLR4 or IL-1 signaling and thus regulate M1 macrophage activation through the key inflammation signaling pathway, TLR2/NF-κB[31,32]. GA can prevent IκB degradation through competitive antagonism, thereby inhibiting NF-κB activation and blocking expression of the TLR4/NF-κβ signaling pathway[33]. GA, as a polyphenolic organic anti-inflammatory drug, may also affect the polarization of M1 macrophages through a variety of other mechanisms and further indepth studies are required.
Acknowledgements:
We thank laboratory technicians in central laboratory and all Department of Jinzhou Medical College for assistance with animal caretaking and laboratory analyses.
Funding:
This work was supported by grant from the Specialized Research Fund for the National Youth Fund (No. 81600870), Project of Liaoning Natural Science Foundation Guiding Plan (No. 2019-ZD-0824) and General Project Supported by Natural Science Foundation of Liaoning Province (No. 2021-MS-324).
Conflict of interests:
The authors declared no conflict of interests.
References
- Olukitibi TA, Ao Z, Mahmoudi M, Kobinger GA, Yao X. Dendritic cells/macrophages-targeting feature of Ebola glycoprotein and its potential as immunological facilitator for antiviral vaccine approach. Microorganisms 2019;7(10):402.
[Crossref] [Google Scholar] [PubMed]
- Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci 2018;19(6):1801.
[Crossref] [Google Scholar] [PubMed]
- Lee WJ, Tateya S, Cheng AM, Rizzo-DeLeon N, Wang NF, Handa P, et al. M2 macrophage polarization mediates anti-inflammatory effects of endothelial nitric oxide signaling. Diabetes 2015;64(8):2836-46.
[Crossref] [Google Scholar] [PubMed]
- Li J, Diao B, Guo S, Huang X, Yang C, Feng Z, et al. VSIG4 inhibits proinflammatory macrophage activation by reprogramming mitochondrial pyruvate metabolism. Nat Commun 2017;8(1):1322.
[Crossref] [Google Scholar] [PubMed]
- Chen Z, Zhuo R, Zhao Y, Yang L, Zhou Y, Cheng X, et al. Oleoylethanolamide stabilizes atherosclerotic plaque through regulating macrophage polarization via AMPK-PPARα pathway. Biochem Biophys Res Commun 2020;524(2):308-16.
[Crossref] [Google Scholar] [PubMed]
- Koller A, Brunner SM, Bianchini R, Ramspacher A, Emberger M, Locker F, et al. Galanin is a potent modulator of cytokine and chemokine expression in human macrophages. Sci Rep 2019;9(1):7237.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Ma Y, Cui Q, Xu J, Tang Z, Wang Y, et al. Toll-like receptor 4 plays a key role in advanced glycation end products-induced M1 macrophage polarization. Biochem Biophys Res Commun 2020;531(4):602-8.
[Crossref] [Google Scholar] [PubMed]
- Tian Y, Yang C, Yao Q, Qian L, Liu J, Xie X, et al. Procyanidin B2 activates PPARγ to induce M2 polarization in mouse macrophages. Front Immunol 2019;10:1895.
[Crossref] [Google Scholar] [PubMed]
- Tanaka M, Sato A, Kishimoto Y, Mabashi-Asazuma H, Kondo K, Iida K. Gallic acid inhibits lipid accumulation via AMPK pathway and suppresses apoptosis and macrophage-mediated inflammation in hepatocytes. Nutrients 2020;12(5):1479.
[Crossref] [Google Scholar] [PubMed]
- Agrawal V, Jaiswal MK, Pamarthy S, Katara GK, Kulshrestha A, Gilman Sachs A, et al. Role of Notch signaling during lipopolysaccharide-induced preterm labor. J Leukoc Biol 2016;100(2):261-74.
[Crossref] [Google Scholar] [PubMed]
- Huang L, Hou L, Xue H, Wang C. Gallic acid inhibits inflammatory response of RAW264. 7 macrophages by blocking the activation of TLR4/NF-κB induced by LPS. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016;32(12):1610-4.
- Ahn CB, Jung WK, Park SJ, Kim YT, Kim WS, Je JY. Gallic acid-g-chitosan modulates inflammatory responses in LPS-stimulated RAW264. 7 cells via NF-κB, AP-1 and MAPK pathways. Inflammation 2016;39(1):366-74.
[Crossref] [Google Scholar] [PubMed]
- Tanaka Y, Obinata H, Konishi A, Yamagiwa N, Tsuneoka M. Production of ROS by gallic acid activates KDM2A to reduce rRNA transcription. Cells 2020;9(10):2266.
[Crossref] [Google Scholar] [PubMed]
- Lin Y, Luo T, Weng A, Huang X, Yao Y, Fu Z, et al. Gallic acid alleviates gouty arthritis by inhibiting NLRP3 inflammasome activation and pyroptosis through enhancing Nrf2 signaling. Front Immunol 2020;11:580593.
[Crossref] [Google Scholar] [PubMed]
- Zięba P, Kała K, Włodarczyk A, Szewczyk A, Kunicki E, Sękara A, et al. Selenium and zinc biofortification of Pleurotus eryngii mycelium and fruiting bodies as a tool for controlling their biological activity. Molecules 2020;25(4):889.
[Crossref] [Google Scholar] [PubMed]
- Sakharwade SC, Mukhopadhaya A. Vibrio cholerae porin OmpU induces LPS tolerance by attenuating TLR-mediated signaling. Mol Immunol 2015;68(2):312-24.
[Crossref] [Google Scholar] [PubMed]
- Lin R, Deng C, Li X, Liu Y, Zhang M, Qin C, et al. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics 2019;9(21):6300-13.
[Crossref] [Google Scholar] [PubMed]
- Xue J, Cheng Y, Hao H, Gao J, Yin Y, Yu S, et al. Low-dose decitabine assists human umbilical cord-derived mesenchymal stem cells in protecting β cells via the modulation of the macrophage phenotype in type 2 diabetic mice. Stem Cells Int 2020;2020:4689798.
[Crossref] [Google Scholar] [PubMed]
- Shapouri Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization and function in health and disease. J Cell Physiol 2018;233(9):6425-40.
[Crossref] [Google Scholar] [PubMed]
- Kawada M, Ohno Y, Ri Y, Ikoma T, Yuugetu H, Asai T, et al. Anti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice. Anticancer Drugs 2001;12(10):847-52.
[Crossref] [Google Scholar] [PubMed]
- Sakalauskas A, Ziaunys M, Smirnovas V. Gallic acid oxidation products alter the formation pathway of insulin amyloid fibrils. Sci Rep 2020;10(1):14466.
[Crossref] [Google Scholar] [PubMed]
- Chan KC, Yu MH, Lin MC, Huang CN, Chung DJ, Lee YJ, et al. Pleiotropic effects of acarbose on atherosclerosis development in rabbits are mediated via upregulating AMPK signals. Sci Rep 2016;6(1):38642.
[Crossref] [Google Scholar] [PubMed]
- Wedell Neergaard AS, Krogh Madsen R, Petersen GL, Hansen ÅM, Pedersen BK, Lund R, et al. Cardiorespiratory fitness and the metabolic syndrome: Roles of inflammation and abdominal obesity. PloS One 2018;13(3):e0194991.
[Crossref] [Google Scholar] [PubMed]
- Yang S, Xie C, Chen Y, Wang J, Chen X, Lu Z, et al. Differential roles of TNFα-TNFR1 and TNFα-TNFR2 in the differentiation and function of CD4+ Foxp3+ induced Treg cells in vitro and in vivo periphery in autoimmune diseases. Cell Death Dis 2019;10(1):27.
[Crossref] [Google Scholar] [PubMed]
- Blazanin N, Cheng T, Carbajal S, DiGiovanni J. Activation of a protumorigenic IFNγ/STAT1/IRF-1 signaling pathway in keratinocytes following exposure to solar ultraviolet light. Mol Carcinog 2019;58(9):1656-69.
[Crossref] [Google Scholar] [PubMed]
- Shen J, Yang T, Xu Y, Luo Y, Zhong X, Shi L, et al. δ-Tocotrienol, isolated from rice bran, exerts an anti-inflammatory effect via MAPKs and PPARs signaling pathways in lipopolysaccharide-stimulated macrophages. Int J Mol Sci 2018;19(10):3022.
[Crossref] [Google Scholar] [PubMed]
- Eom T, Kim E, Kim JS. In vitro antioxidant, antiinflammation and anticancer activities and anthraquinone content from Rumex crispus root extract and fractions. Antioxidants 2020;9(8):726.
[Crossref] [Google Scholar] [PubMed]
- Ge D, Yuan H, Xiao J, Zhu N. Insight into the enhanced sludge dewaterability by tannic acid conditioning and pH regulation. Sci Total Environ 2019;679:298-306.
[Crossref] [Google Scholar] [PubMed]
- Wu Y, Feng Z, Jiang S, Chen J, Zhan Y, Chen J. Secreting-lux/pT-ClyA engineered bacteria suppresses tumor growth via interleukin-1β in two pathways. AMB Express 2019;9(1):189.
[Crossref] [Google Scholar] [PubMed]
- Feng Y, Li F, Yan J, Guo X, Wang F, Shi H, et al. Pan-cancer analysis and experiments with cell lines reveal that the slightly elevated expression of DLGAP5 is involved in clear cell renal cell carcinoma progression. Life Sci 2021;287:120056.
[Crossref] [Google Scholar] [PubMed]
- Liu YL, Hsu CC, Huang HJ, Chang CJ, Sun SH, Lin AM. Gallic acid attenuated LPS-induced neuroinflammation: Protein aggregation and necroptosis. Mol Neurobiol 2020;57(1):96-104.
[Crossref] [Google Scholar] [PubMed]
- Adhya D, Dutta K, Kundu K, Basu A. Histone deacetylase inhibition by Japanese encephalitis virus in monocyte/macrophages: A novel viral immune evasion strategy. Immunobiology 2013;218(10):1235-47.
[Crossref] [Google Scholar] [PubMed]
- De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013;23(3):277-86.
[Crossref] [Google Scholar] [PubMed]