- Corresponding Author:
- J. Ali
Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard, Hamdard Nagar, New Delhi - 110 062, India.
E-mail: jali@jamiahamdard.ac.in
| Date of Submission | 22 March 2006 |
| Date of Revision | 2 December 2006 |
| Date of Acceptance | 12 May 2007 |
| Indian J. Pharm. Sci., 2007, 69 (3): 360-364 |
Abstract
In the present study a hydrodynamically balanced system of celiprolol hydrochloride was developed as single unit floating capsule. Various grades of low density polymers were used for formulation of this system. They were prepared by physical blending of celiprolol hydrochloride and the polymer in varying ratios. The formulation was optimized on the basis of in vitro buoyancy and in vitro release in citrate phosphate buffer at pH 3.0. Effect of various release modifiers was studied to ensure the delivery of drug from the hydrodynamically balanced system capsule over a period of 12 h. Capsule prepared with HPMC K4 M and liquid paraffin gave the best in vitro percentage release and was taken as optimized formulation. By fitting the data in zero order, first order and Higuchi model it could be concluded that the release followed zero order release, as the correlation coefficient was higher for zero order release. It could be concluded from R 2 value for Higuchi model and K-Peppas model that drug release followed fickian diffusion mechanism.
Keywords
Hydrodynamically balanced system, celiprolol hydrochloride, in vitro buoyancy studies, stability studies.
Drug absorption from gastrointestinal tract is a complex procedure and is subject to many variables. It has been reported that the extent of gastrointestinal tract drug absorption is related to contact time with the small intestinal mucosa. Gastroretentive system can remain in the gastric region for several hours and therefore significantly prolong the gastric residence time of drugs. Many approaches have been reported in the literature for the formulation of gastroretentive system viz. mucoadhesion [1-2], floatation, sedimentation [3-5], expansion [6-7] and modified shape systems [8-9]. Both single and multiple systems have been reported in the literature [10]. Floating drug delivery offers a number of applications for drugs having poor bioavailability because of narrow absorption window in the upper part of gastrointestinal tract. It retains the dosage form at the site of absorption and thus enhances the bioavailability
Celiprolol hydrochloride (CEH) is an antihypertensive agent that antagonizes the action of β1-receptor. It has been reported that the absolute bioavailability of celiprolol when given orally is 30-70%. The biological half life of celiprolol hydrochloride is 4-6 h and the main site of absorption is proximal small intestine. A hydrodynamically balanced system (HBS) is planned for CEH, as such a system when administered would remain buoyant on the gastric fluids for a prolonged period of time and the drug would be available at the main site of its absorption i.e. proximal small intestine resulting in enhanced bioavailability.
Materials and Methods
Celiprolol hydrochloride, different grades of the HPMC (K4M, K15M, and K100M), ethyl cellulose, and polyethylene oxide WSR 60K were obtained as gift samples from Ranbaxy Research Laboratories, New Delhi, India. Liquid paraffin was obtained from S. D. Fine Chemicals, Mumbai. All other reagents were of analytical reagent grade.
Preparation of capsules
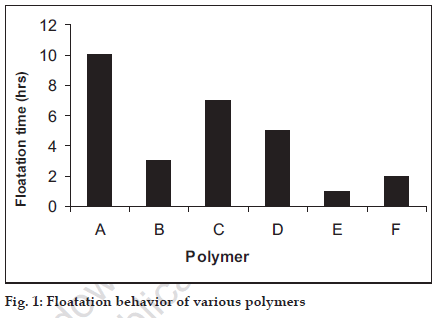
Single unit capsules were formulated with the help of different low density polymers, which upon administration would attain a density of less than that of the gastric fluids and therefore would float (Table 1). On the basis of floatation HPMC K4M was selected for further studies. 200 mg of celiprolol and different ratios of the polymer were accurately weighed and were physically blended in a glass mortar and pestle and filled in a hard gelatin capsule (size 00). Total weight of capsule was kept 400 mg. The formula of various formulations is given in the Table 2.
| Formulation code | Content of capsule (200 mg) |
|---|---|
| A | HPMC K4M |
| B | HPMC K15M |
| C | HPMC K4M: HPMC 15M (1:1) |
| D | HPMCK100 M |
| E | Guar gum |
| F | Sodium carboxy methyl cellulose |
Table 1: Formulae For Floating Capsule.
In vitro buoyancy studies
The capsules were immersed in 900 ml of citrate buffer pH 3, simulating the fed state of stomach, in USP paddle apparatus at 100 rpm. The time for which the capsule remained buoyant was observed and was taken as the floating time. The polymer that showed the best floating behavior was taken for in vitro release studies (fig. 1).
In vitro release studies
Based on the in vitro buoyancy studies, formulations showing good floatation were subjected to in vitro release studies. In vitro studies were carried out by carrying out the dissolution studies in USP paddle type apparatus at 100 rpm using 900 ml of citrate buffer pH 3.0. The samples were withdrawn at regular intervals and replaced with the blank buffer. Sample were analyzed by spectrophotometrically (Shimadzu UV 1601) at 232 nm.
Effect of release modifiers
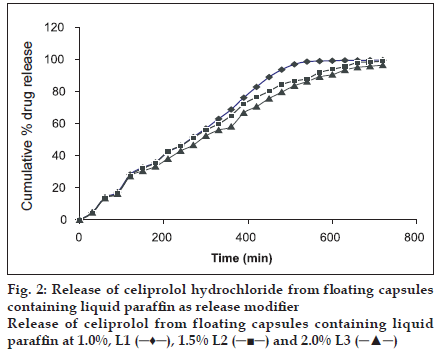
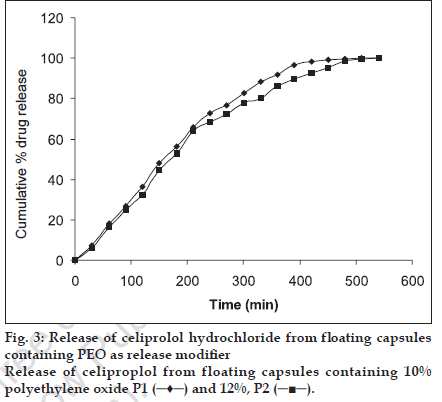
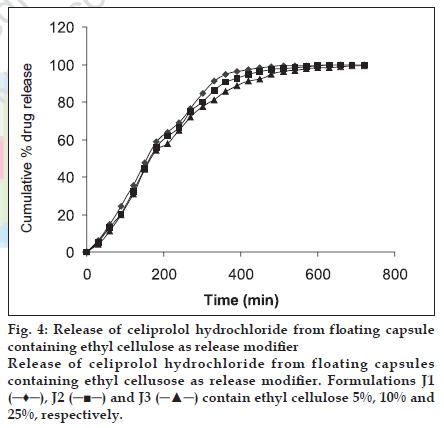
Ethylcellulose (EC), Polyethylene oxide (PEO) WSR 60K and liquid paraffin were used at different concentration as shown in Table 2. For addition of ethyl cellulose (5-25%) to the formulation corresponding solution of ethyl cellulose was made in acetone and granulated with weighed drug content and HPMC K4 M and were allowed to dry at 30°. The granules were passed through sieve no 44 and 400 mg of granules equivalent to 200 mg of the drug were filled in capsule. PEO was added in dry form. Liquid paraffin (1%, 1.5% and 2%) was added by making a solution in isopropanol, granules were prepared, dried and passed through the sieve no 25 mesh and filled in to capsules.
| Formulation Code |
Celiprolol HCl (mg) |
HPMC K4M (mg) |
Ethyl cellulose (mg) |
Polyethylene Oxide (mg) |
Liquid ParafÞn (mg) |
|---|---|---|---|---|---|
| L1 | 200 | 198 | - | - | 2 |
| L2 | 200 | 197 | - | - | 3 |
| L3 | 200 | 196 | - | - | 4 |
| P1 | 200 | 180 | - | 20 | - |
| P2 | 200 | 176 | - | 24 | - |
| J1 | 200 | 190 | 10 | - | - |
| J2 | 200 | 176 | 24 | - | - |
| J3 | 200 | 150 | 50 | - | - |
Table 2: Working Formulae For Floating Capsules.
Analysis of in vitro drug release
To analyze the mechanism of drug release from the capsules, the in vitro dissolution data was fitted to zero order, first order [11], Higuchi release model [12] and Korsemeyer and Peppas model [13-14].
Stability studies
Stability studies were carried out according to ICH guidelines by storing the optimized formulation at 40°C/75±5% RH for a period of three months. The samples were withdrawn at 30, 60 and 90 d and analyzed for drug content and dissolution study as given in in vitro release studies by an HPLC method. The chromatographic column used was C18. Mobile phase used was acetonitrile:phosphate buffer pH 3.0 (30:70), with 0.1% triethylamine, a stability indicating method. Flow rate was kept at 0.8 ml/min [15].
Results and Discussion
The in vitro release studies revealed that the formulation remained buoyant but probably because of the high aqueous solubility of (CEH), the release could not be sustained. Only formulation containing HPMC K4M could sustained the drug release up to 9 h therefore it is decided to add release modifier to these formulations (Table 2) so as to aid the sustained release of the drug from the formulation.
It was observed that best result was obtained with formulation set I, (L2) containing HPMC K4M, CEH and liquid paraffin at 1.5% level as the drug release could be sustained for 12 h (fig. 2). But the formulation set II (P1, P2) containing polyethylene oxide 10%, 12% were able to sustain the release only for up to 9 h (fig. 3). Formulation set III (J1, J2, J3) containing ethyl cellulose 5%, 10% and 25% could not sustain the drug release for up to 12 h (fig. 4). HPMC K4 M formed a firm matrix and could control the drug release for a longer duration time. Liquid paraffin has only been used as an inert material for enhancing the buoyancy of the formulation16. However based on release profile we can conclude that besides imparting the buoyancy to the formulation it also modify the release significantly. Among the release modifiers used to control the release of celiprolol from the formulation, formulation containing liquid paraffin (1.5%) gave the best in vitro release as it could control the release for 12 h and hence it was the optimized formulation.
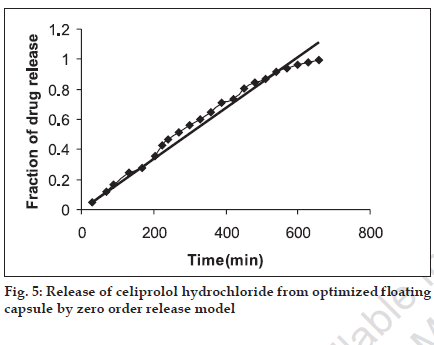
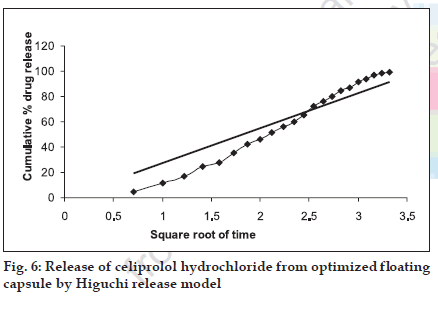
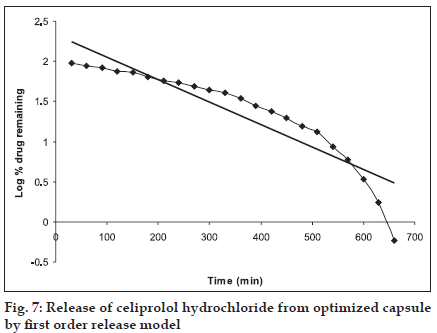
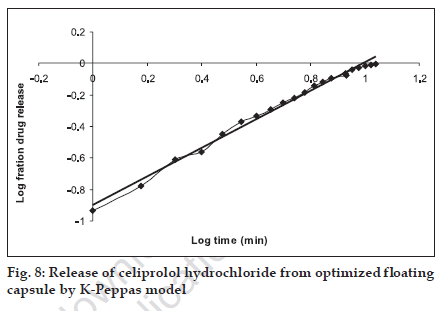
The in vitro release pattern of the optimized formulation was analyzed by fitting the dissolution data in to various kinetics models. It was observed that R2 value was higher when fitted to zero order equation (fig. 5), Table 3, followed by Higuchi model (fig. 6), as compared to first order equation (fig. 7), which indicated a zero order release from the optimized HBS capsule for celiprolol hydrochloride. As the n value for the k-Peppas model was found to be less than 0.5 (0.438) it suggests that release from the optimized formulation follows fickian diffusion mechanism (fig. 8).
Figure 4:Release of celiprolol hydrochloride from ß oating capsule containing ethyl cellulose as release modiÞ er Release of celiprolol hydrochloride from floating capsules containing ethyl cellusose as release modiÞ er. Formulations J1 ( ), J2 (
), J2 ( ) and J3 (
) and J3 ( ) contain ethyl cellulose 5%, 10% and 25%, respectively.
) contain ethyl cellulose 5%, 10% and 25%, respectively.
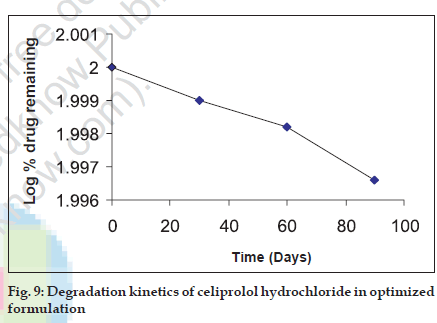
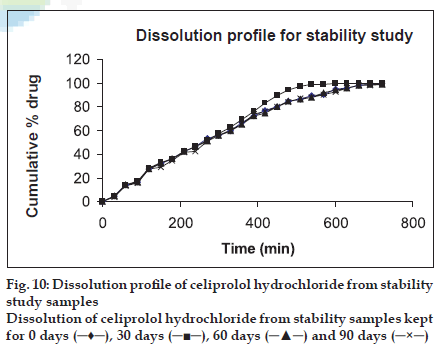
Stability studies revealed that the drug content in HBS capsule degraded by 0.76% in a period of 90 d. (fig. 9). Hence a shelf life of 1.9 years could be assigned to the above formulation. Dissolution profile for stability sample showed that there was no significant change in the release rate of the drug from optimized capsule kept on stability studies (fig. 10).
| Release order/model | R (CoefÞcient of correlation) |
|---|---|
| Zero-order release model | 0.976 |
| First -order release model | 0.841 |
| Higuchi model | 0.886 |
Table 3: Release Of Celiprolol Hydrochloride From Optimized Floating Capsule.
Acknowledgements
The authors are grateful to Department of science and Technology, Govt. of India for financial support to the project and Jamia Hamdard for providing facilities to carry out the experiments.
References
- Arora S, Ali J, Ahuja A, Khar RK, Baboota S. Floating drug delivery system: A review. AAPS Pharm Sci Tech 2005;6:372-90.
- Sheth PR, Tossounian JL. Sustained release pharmaceutical capsules. US Patent No. US 4126672; 1978.
- Ponchel G, Irache JM. SpeciÞc and nonspeciÞc bioadhesive particulate system for oral delivery to the gastrointestinal tract. Adv Drug Del Rev 1998;34:191-219.
- Akiyama Y, Nagahara N. Novel Formulation approaches to oral mucoadhesive drug delivery systems. In: Mathiowitz E, Chickering DE, Lehr CM, editors. Bioadhesive Drug Delivery Systems. 1st ed Bocaraton. New York; Marcel Dekker Inc: 1999. p. 477-506.
- Deshpande A, Shah NH, Rhodes CT, Malick W. Development of novel controlled release system for gastric retention. Pharm Res 1997;14:815-9.
- Rednick AB, Tucker SJ. Sustained release bolus for animal husbandry. US Patent No. US3 507 952; 1970.
- Davis SS, Stockwel AF, Taylor MJ, Hardy JG, Whalley DR, Wilson CG, et al. The effect of density on the gastric emptying of single and multiple unit dosage forms. Pharm Res 1986;3:208-13.
- Urguhart J, Theeuwes F. Drug delivery system comprising a reservoir containing a plurality of tiny pellets. US Patent No. US4 434 153; 1994.
- Mamajek RC, Moyer ES. Drug dispensing devices and method. US4 207 890, 1980.
- Fix JA, Cargil R, Engle K. Controlled gastric emptying III gastric residence time of a non disintegrating geometric shape in human volunteers. Pharm Res 1993;10:1087-9.
- Chen GL, Hao WH. In vitro performance of ßoating sustained release capsule of verapamil. Drug Develop Ind Pharm 1998;24:1067-72.
- Higuchi T. Mechanism of sustained action medication: Theoretical analysis of rate of release of solid drug. J Pharm Sci 1961;50:874-5.
- Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and nonÞckian release from swellable devices in the form of slabs spheres cylinder or disc. J Control Release 1987;5:23-36.
- Korsmeyer RW, Deolkar G, Peppas NA. Mechanism of release from controlled hydrophilic polymeric matrices: Effect of entrapped air. J Pharm Sci 1983;72:1189-91.
- Lipka E. Celiprolol double peak occurrence and gastric motility: Nonlinear mixed effects modeling of bioavailability data obtained dogs. J Pharm Biopharm 1995;23:267-86.
- Bolton S, Desai S. Floating sustained release therapeutic composition. U S Patent No. US4 814 179; 1989.