- Corresponding Author:
- Sarasija Suresh
Department of Pharmaceutics, H. K. E. Society′s College of Pharmacy, Sedam Road, Gulbarga-585 105, 1Pharmaceutics, Al-Ameen College of Pharmacy, Near Lal Bagh Main Gate, Hosur Road, Bangalore-560 027, India
E-mail sarasija_s@hotmail.com
| Date of Submission | 15 July 2010 |
| Date of Revision | 1 August 2011 |
| Date of Acceptance | 14 August 2011 |
| Indian J Pharm Sci, 2011, 73 (5): 491-496 |
Abstract
Fast dissolving tablets of clonazepam were prepared by sublimation method with a view to enhance patient compliance. A 3² full factorial design was applied to investigate the combined effect of two formulation variables: amount of croscarmellose sodium and camphor. Croscarmellose sodium (2-8% w/w) was used as superdisintegrant and camphor (20-40% w/w) was used as subliming agent, to increase the porosity of the tablets, since it helps water to penetrate into the tablets, along with directly compressible mannitol to enhance mouth feel. The tablets were evaluated for hardness, friability, thickness, drug content uniformity, in vitro dispersion time, wetting time and water absorption ratio. Based on in vitro dispersion time (approximately 11 s); the formulation containing 5% w/w croscarmellose sodium and 40% w/w camphor was found to be promising and tested for in vitro drug release pattern (in pH 6.8 phosphate buffer). Short-term stability (at 40º/75% relative humidity for 3 mo) and drug-excipient interaction. Surface response plots are presented to graphically represent the effect of independent variables on the in vitro dispersion time. The validity of the generated mathematical model was tested by preparing two extra-design checkpoints. The optimized tablet formulation was compared with conventional commercial tablet formulation for drug release profiles. This formulation showed nearly nine-fold faster drug release (t50% 1.8 min) compared to the conventional commercial tablet formulation (t50% 16.4 min). Short-term stability studies on the formulation indicated that there are no significant changes in drug content and in vitro dispersion time (P<0.05).
Keywords
32 full factorial design, camphor, clonazepam, croscarmellose sodium, fast dissolving tablets.
Introduction
We often experience inconvenience in swallowing conventional tablets where water is not available. Dysphagia is a common problem encountered in all age groups in concern to solid dosage forms, which results in high incidence of non-compliance and ineffective therapy [1]. Recent advances in novel drug delivery systems (NDDS) aim to enhance safety and efficacy of drug molecule by formulating a convenient dosage form for administration and to achieve better patient compliance i.e., one, which will rapidly disintegrate in the mouth without need of water (fast dissolving tablet). Advantages of this drug delivery system include administration without water, anywhere, anytime, accuracy of dosage, easy portability, alternative to liquid dosage forms, ideal for pediatric and geriatric patients, rapid onset of action, increased bioavailability and good stability make these tablets popular as a dosage form of choice in the current market [2-4]. Clonazepam (CZ) is a benzodiazepine derivative with marked antiepileptic properties. It may be used in the treatment of all types of epilepsy and seizures, practically insoluble in water, half life of about 20 h [5]. Since epileptic patients have to strictly follow the dosage regimen for preventing sub-therapeutic concentration, FDT will avoid missing out of a dose even during travelling or other situations, where there is no access to water; offers a suitable and practical approach in serving desired objective of faster disintegration and dissolution characteristics with increased bioavailability. Aim of the present study was to develop such a NDDS for CZ by simple and costeffective sublimation method.
In sublimation method, the rapid disintegration of the tablets is achieved by creation of pores in the tablets up on sublimation of volatile components added in the tablets. The saliva will enter these pores and cause the rapid disintegration of the tablets in the oral cavity. The porous structure is responsible for the faster water uptake, Hence it facilitates wicking action in bringing about faster disintegration.
Optimization techniques provide both a depth of understanding and ability to explore and defend ranges for formulation and processing factors. With a rational approach to the selection of several excipients and manufacturing steps for a given product, one quantitatively selects a formulation. It is at this point that optimization can become a useful tool to quantitate a formulation that has been qualitatively determined.
Materials and Methods
CZ and croscarmellose sodium (CCS) were gift samples from Torrent Pharma, Ahmedabad, India and Wockhardt Research Centre, Aurangabad, India respectively. Directly compressible mannitol (Pearlitol SD 200), sodium stearyl fumarate (SSF) were generous gifts from Strides Arco Labs, Bangalore, India, Glenmark Ltd., Nashik, India and Alkem Labs Pvt Ltd, Mumbai, India. All other chemicals were of analytical reagent grade.
Experimental design
Preliminary trial formulations of CZ were designed by sublimation method using three superdisintegrants (crosspovidone, CCS, sodium starch glycollate) in different ratios (2-10% w/w) along with camphor (20- 60% w/w) as subliming agent. Aerosil (lubricant) was added to impart the hardness to the tablets, mannitol as a diluent to enhance the mouth feel.
Based on the results of the preliminary studies the formulations were designed from 15 batches of three superdisintegrants in different ratios (2-10% w/w), the best screened super disintegrant (CCS) was used for the final optimization of the formulation, according to the 32 full factorial design, allowing a simultaneous evaluation of the two formulation variables and their interaction. The experimental design with the corresponding formulation are outlined in Table 1. The effect of the independent variables, viz., CCS (X1) and camphor (X2) on the dependent variable (Y1), In vitro dispersion time was evaluated. The preliminary studies indicated that the superdisintegrant CCS was found to be superior compared to the other two superdisintegrants (crospovidone and sodium starch glycolate); and hence it was selected as the superdisintegrant of choice for the present investigation.
Preparation of fast dissolving tablets of CZ
Fast dissolving tablets (FDT) of CZ were prepared by sublimation method [6] according to the formulae given in Table 1. Camphor as subliming agent and mannitol was used as diluent. Aerosil was added to impart hardness to the tablet. All the ingredients were passed through #60 mesh separately, weighed and mixed in geometrical order. Then lubricant and glidant (# 200 mesh) were added and mixed for further 5 min. The blend thus obtained was directly compressed using 7 mm flat round punches into tablets of 150 mg on a 10-station rotary tablet machine (Clit, Ahmedabad, India). The compressed tablets were then subjected to sublimation at 600 for six hours in hot air oven.
| Ingredients* (mg) | Formulationcode | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SF1 | SF2 | SF3 | SF4 | SF5 | SF6 | SF7 | SF8 | SF9 | SF0 | C1 | C2 | |
| Clonazepam | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Croscarmellose sodium | 3 | 3 | 3 | 7.5 | 7.5 | 7.5 | 12 | 12 | 12 | -- | 5.2 | 9.7 |
| Camphor | 30 | 45 | 60 | 30 | 45 | 60 | 30 | 45 | 60 | 30 | 37 | 52 |
| Aerosil | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Aspartame | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Sodium streaylfumarate | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Flavor (pineapple) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Talc | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Directly compressible | 103 | 88 | 73 | 98.5 | 83.5 | 68.5 | 94.0 | 79.0 | 64.0 | 106.0 | 93.25 | 73.75 |
| mannitol | ||||||||||||
| *All the quantities expressed are in mg/tablet. SF formulation prepared by sublimation method using camphor as subliming agent at three different levels and croscarmellose sodium as super disintegrant. SF0 is control formulation; C1 and C2 are extra design check-point formulations. | ||||||||||||
Table 1 Factorial design formulations of clonazepam prepared by sublimation method
Optimization by 32 full factorial design
A 32 randomised full factorial design was adopted to optimize the variables. In this design 2 factors were evaluated, each at 3 levels, and experimental trials were performed at all nine possible combinations [7].As reflected from Table 2, the amounts of CCS (X1), and camphor (X2), were selected as independent variables. In vitro dispersion time was selected as dependent variable/response (Y1).
| Formulation | Variable levels in | In vitro dispersion | |
| code | coded form* | time (s) | |
| 1 | X2 | ||
| SF1 | -1 | -1 | 27.13±1.80 |
| SF2 | -1 | 0 | 27.13±1.80 |
| SF3 | -1 | +1 | 27.13±1.80 |
| SF4 | 0 | -1 | 27.13±1.80 |
| SF5 | 0 | 0 | 27.13±1.80 |
| SF6 | 0 | +1 | 27.13±1.80 |
| SF7 | +1 | -1 | 27.13±1.80 |
| SF8 | +1 | 0 | 27.13±1.80 |
| SF9 | +1 | +1 | 27.13±1.80 |
| C1 | -0.5 | -0.5 | 21.13±0.63 |
| C2 | +0.5 | +0.5 | 11.76±0.49 |
Table 2 Formulation and evaluation of 32 full factorial design
Evaluation of tablets
For the weight variation twenty tablets were selected at random and assessed individually. The individual weights were compared with the average weight for determination of weight variation [8]. Hardness and friability of the tablets were determined by using Monsanto hardness tester and Roche friabilator respectively. For content uniformity test, ten tablets were weighed and powdered, a quantity of powder equivalent to 2 mg of CZ was extracted into methanol and liquid was filtered. The CZ content was determined by measuring the absorbance at 308 nm after appropriate dilution with methanol. The drug content was determined using the standard calibration curve. The mean percent drug content was calculated as an average of three determinations [9]. For determination of In vitro dispersion time, one tablet was placed in a beaker containing 10 ml of pH 6.8 phosphate buffer at 37±0.5º and the time required for complete dispersion was determined [10]. For determination of wetting time and water absorption ratio [11], a piece of tissue paper folded twice was placed in a small petridish (internal diameter of 5 cm) containing 6 ml of water. A tablet was placed on the paper and the time required for complete wetting was measured. The wetted tablet was then weighed. Water absorption ratio ‘R’ was determined using the equation, R=100(Wb-Wa)/Wa; where Wa is weight of tablet before water absorption and Wb is weight of tablet after water absorption. The results are shown in Table 3. IR spectra of the pure drug and its formulations were obtained by potassium bromide pellet method using Perkin-Elmer FTIR series (model 1615) spectrophotometer in order to rule out drug-carrier interactions.
| Parameter | Formulation code | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SF0 | SF1 | SF2 | SF3 | SF4 | SF5 | SF6 | SF7 | SF8 | SF9 | |
| Hardness* | 2.06±0.05 | 2.36±0.05 | 2.40±0.10 | 2.35±0.152 | 2.53±0.05 | 2.50±0.01 | 2.46±0.05 | 2.50±0.01 | 2.40±0.10 | 2.33±0.152 |
| (kg/cm²) | ||||||||||
| ±SD | ||||||||||
| Friability | 0.73 | 0.42 | 0.64 | 0.61 | 0.55 | 0.48 | 0.56 | 0.60 | 0.52 | 0.57 |
| (%) | ||||||||||
| Thickness | 3.5 | 3.20 | 3.46 | 3.37 | 3.50 | 3.63 | 3.70 | 3.40 | 3.37 | 3.62 |
| (mm) | ||||||||||
| In vitro | 225.08±4.82 | 27.13±1.80 | 23.48±0.71 | 17.74±1.13 | 18.50±1.01 | 15.49±0.91 | 11.59±0.70 | 12.24±0.95 | 9.83±0.74 | 7.67±1.18 |
| dispersion | ||||||||||
| time* | ||||||||||
| (seconds) | ||||||||||
| ±SD | ||||||||||
| Wetting | 237.56±2.23 | 29.24±1.37 | 24.56±0.38 | 18.0±0.85 | 20.43±0.85 | 16.60±0.85 | 12.38±0.79 | 14.39±0.56 | 11.0±0.75 | 9.35±0.44 |
| time* | ||||||||||
| (seconds) | ||||||||||
| ±SD | ||||||||||
| Water | 51.06±1.06 | 72.0±1.32 | 78.0±1.20 | 81.40±1.30 | 76.46±1.85 | 80.40±1.20 | 82.49±1.54 | 82.78±2.17 | 84.49±1.54 | 86.49±1.54 |
| absorption | ||||||||||
| ratio* (%) | ||||||||||
| ±SD | ||||||||||
| Percent | 99.25±0.53 | 97.29±0.64 | 96.79±1.42 | 98.92±1.42 | 102.56±0.84 | 101.71±1.32 | 98.09±0.62 | 97.20±2.24 | 101.34±2.02 | 98.09±0.62 |
| drug | ||||||||||
| content* | ||||||||||
| ±SD | ||||||||||
| *Average of three determinations. Weight variation (147 ? 154 mg) within the IP limits of ±7.5% | ||||||||||
Table 3 Evaluation of factorial design fdt formulations
In vitro drug release study
In vitro dissolution of the formulated fast dissolving tablets of CZ and one commercial conventional tablet was studied in USP XXIII type-2 dissolution apparatus (Electrolab, Model-TDT 06N) employing a paddle stirrer at 50 rpm using 900 ml of pH 6.8 phosphate buffer at 37±0.5º as dissolution medium [12]. One tablet was used in each test. Aliquots of dissolution medium (5 ml) were withdrawn at specific intervals of time and analyzed for drug content by measuring the absorbance at 307.5 nm. The volume withdrawn at each time interval was replaced with fresh quantity of dissolution medium. Cumulative percent of CZ released was calculated and plotted against time.
Stability testing
Short-term stability studies on the optimized promising formulation (SF6) were carried out by storing the tablets (in amber colored rubber stoppered vials) at 40º/75% RH for 3 mo period (as per ICH guidelines). At intervals of 1 mo, the tablets were visually examined for any physical changes, changes in drug content and In vitro dispersion time.
Results and Discussion
A 32 full factorial design was used in the present study. In this deign 2 factors are evaluated, each at 3 levels, and experimental trials are performed at all 9 possible combinations. The amount of superdisintegrant, CCS (X1), and the amount of subliming agent, camphor (X2), selected as independent variables. The in-vitro dispersion time were selected as dependent variables.
Fast dissolving tablets of CZ were prepared by sublimation method using CCS as superdisintigrant and camphor as subliming agent along with directly compressible mannitol (Pearlitol SD 200), which was used to enhance the mouth feel. A total of nine formulations and a control formulation (SF0, without super-disintegrant) were designed.
As the material was free flowing (angle of repose value <30º and Carr′s index <15%), tablets obtained were of uniform weight (due to uniform die fill), with acceptable variation as per IP specifications (±7.5%).Drug content was found to be in the range of 96.79- 102.56%, which is within acceptable limits (as per USP the drug content limit is not less than 90% and not more than 110%). Hardness of the tablets was found to be 2.00 to 2.50 kg/cm2. Friability below 1% was an indication of good mechanical resistance of the tablets (Table 2). Formulation SF6 was found to be promising and displayed an In vitro dispersion time of 11 s, which facilitates faster dispersion in the mouth.
In order to investigate the factors systematically, a factorial design was employed in the present investigation. Formulation optimization has been done by using 32 full factorial design, preparing nine batches of formulations (SF1 to SF9). A polynomial equation was derived for In vitro dispersion time, by backward stepwise linear regression analysis, using PCP Disso 2000 V3 software. Formulation SF6 containing 5% w/w CCS, 40% w/w camphor was found to be promising with an In vitro dispersion time of 11 s against the 225 s displayed by control formulation (SF0), which does not contain the superdisintegrant (CCS).
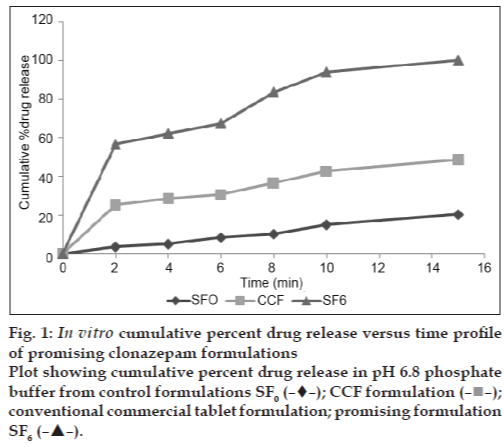
In vitro dissolution studies on the promising formulation (SF6), the control (SF0) and commercial conventional tablet formulation (CCF) were carried out in pH 6.8 phosphate buffer and the various dissolution parameter values, viz., percent drug dissolved in 5 min (D5), 10 min (D10), dissolution efficiency [13] at 10 min (DE10min), t50%, t70% and t90% are shown in Table 4 and the dissolution profiles are depicted in fig. 1. This data reveals that overall, the formulation SF6 has shown nearly nine-fold faster drug release (t50% 1.8 min) when compared to CCF (t50% 16.4 min).
Fig. 1 In vitro cumulative percent drug release versus time profile
of promising clonazepam formulations
Plot showing cumulative percent drug release in pH 6.8 phosphate
buffer from control formulations SF0 (?![]() ?); CCF formulation (?■?);
conventional commercial tablet formulation; promising formulation
SF6 (?
?); CCF formulation (?■?);
conventional commercial tablet formulation; promising formulation
SF6 (?![]() ?).
?).
| Formulation code | D5 (%) | D10 (%) | D15 (%) | DE10min (%) | t50% (min) | t70% (min) | t90% (min) |
|---|---|---|---|---|---|---|---|
| SF0 | 6.5 | 15 | 20.3 | 6.78 | >15 | >15 | >15 |
| SF6 | 64.5 | 93.91 | 100 | 63.05 | 1.8 | 6.2 | 9.4 |
| CCF | 29.5 | 42.5 | 48.5 | 33.41 | 16.4 | >15 | >15 |
| SF0 is control formulation, SF6 is promising fast dissolving tablet formulation, CCF is conventional commercial tablet formulation, D5 is percent drug released in5 min, D10 is percent drug release in 10 min, D15 is percent drug release in 15 min, DE10min is dissolution efficiency at 10 min, t50% is time for 50% drug dissolution, t70% is time for 70% drug dissolution, t90% is time for 90% drug dissolution | |||||||
Table 4 In vitro dissolution parameters in ph 6.8 phosphate buffer
Rapid dissolution of the tablets may be due to improvement in the ability of water to penetrate into the tablets due to the high porosity obtained by increase in the number of pores after sublimation of camphor, hence it facilitates wicking action in bringing about faster disintegration. Since they are prepared from water soluble materials these tablets have the advantage of not causing a feeling of roughness in the mouth.
IR spectroscopic studies indicated that the drug is compatible with all the excipients. The IR spectrum of SF6 showed all the characteristic peaks of CZ, thus confirming that no interaction of drug occurred with the components of the formulation. The IR spectrum of pure drug shows characteristics peaks at 3185, 1692, 1580, 1057 and 844 cm-1 due to NH-stretching, carbonyl stretching, aromatic ring (-CH-bending), chlorine substituted benzene and ?CH bending (aryl & alkyl) groups respectively. Formulation SF6 also exhibited similar peaks at 3181, 1601, 1395, 1076 and 876 cm-1. This confirms undistrubed structure of drug in the formulation. Short-term stability studies of the above formulation indicated that there are no significant changes in drug content and In vitro dispersion time at the end of 3 mo period (P<0.05) (Table 5 and 6).
| Time (Days) | Physical | % drug | In vitro dispersion |
|---|---|---|---|
| change | content±SD* | time* (Sec) | |
| 1st day (Initial) | -- | 98.09±0.62 | 11.59±0.70 |
| 30th day | No change | 99.67±0.03 | 11.70±0.60 |
| 60th day | No change | 99.87±0.37 | 11.85±0.06 |
| 90th day | No change | 100.10±0.63 | 11.95±0.43 |
| *Average of three determinations | |||
Table 5 Stability data of promising sf6 formulation of clonazepam at 40°C / 75% RH
| Sl. no. | Trial | First day (A) | 90th day (B) | A-B |
|---|---|---|---|---|
| 1 | 01 | 98.22 | 97.84 | 0.38 |
| 2 | 02 | 97.41 | 97.70 | 0.29 |
| 3 | 03 | 98.64 | 97.60 | 1.04 |
| 4 | Mean | 98.09 | 97.71 | 0.38 |
| 5 | SD | 0.62 | 0.12 | 0.50 |
| t = 1.35 (P<0.05) | ||||
Table 6 Statistical analysis of drug content data for stability of sf6 formulation
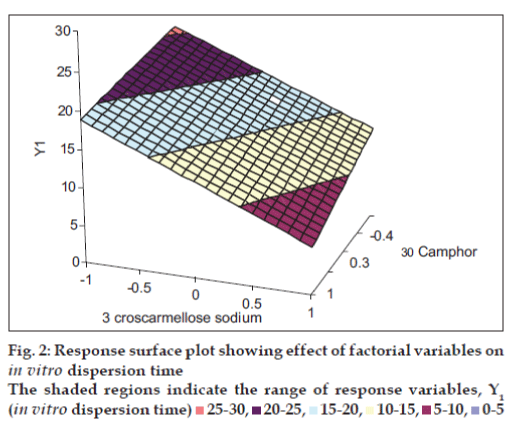
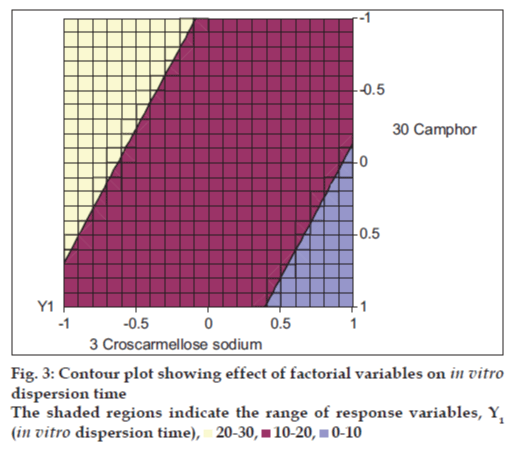
From the data of In vitro dispersion time of the factorial formulations SF1 to SF9, polynomial equation for In vitro dispersion time has been derived using ?PCP Disso 2000 V3 software?. Polynomial equation for 32 full factorial design with two independent variables i.e., proportion of CCS (X1) and proportion of camphor (X2), at three levels is [14]: Y=b0+ b1X1 + b2X2+ b12X1X2 + b11X12 + b22X22, where, Y is dependent variable, b0 arithmetic mean response of nine batches, and b1 estimated coefficient for factor X1. The main effects (X1 and X2) represent the average results of changing one factor at a time from its low to high value. The interaction term (X1X2) shows how the response changes when two factors are simultaneously changed. The polynomial terms X12 and X22 are included to investigate non-linearity. The equation derived for In vitro dispersion time of the factorial formulations is: Y1 = 15.96 ? 3.21 X1 ? 1.73X2. The negative sign for coefficients of X1 and X2 indicate that as the concentration of disintegrants increases, In vitro dispersion time decreases.
Validity of the above equation was verified by designing two extra design check point formulations (C1 and C2) and determining the In vitro dispersion time. The In vitro dispersion time values predicted from the equation for these formulations are 21.13 and 11.76 s, whereas those observed from experimental results are 20.90 and 11.02 s respectively.
The closeness of the predicted and observed values for C1 and C2 in the method indicates validity of derived equation for the dependent variable (In vitro dispersion time). The computer generated response surface and contour plots for the dependent variable are shown in figs. 2 and 3, respectively.
The results of a 32 full factorial design revealed that the amount of CCS and camphor significantly affect the depended variable, In vitro dispersion time. It is thus concluded that, by adopting a systematic formulation approach, an optimum point can be reached in the shortest time with minimum efforts. Sublimimation technique would be an effective alternative approach compared with the use of more expensive adjuvants in the formulation of fast dissolving tablets.
References
- Sreenivas SA, Dandagi PM, Hiremath SP. Orodispersible tablets: Newfangled drug delivery system ? A review. Indian J Pharm Educ Res 2005;39:177-80.
- Mahajan HS, Patil SB, Gattani SG. Rapidly disintegrating tablets for elderly patients.Pharma Rev 2005;3:49-51.
- Kuchekar BS, Arumugam V. Design of fast dissolving tablets. Indian J Pharm Educ 2001;35:50-2.
- Reddy LH, Ghosh BR. Fast dissolving drug delivery systems: A review of the literature. Indian J Pharm Sci 2002;64:331-6.
- Sweetman SC. Martindale: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002. p. 347-8.
- Kaushik D, Saini TR, Dureja H. Development of Melt in mouth tablets by sublimation method. J Pharm Res 2004;3:35-37.
- Bolton S. Pharmaceutical Statistics. 2nd ed. New York: Marcel Decker Inc; 1990. p. 234-6.
- Banker GS, Anderson NR. In: Lachman L, Libermann HA, Kanig JL, editors. The Theory and Practice of Industrial Pharmacy. 3rd ed. New Delhi: Varghese Publishing House; 1987. p. 293-9.
- Indian Pharmacopoeia. New Delhi: Controller of Publications; 1996. p. 735.
- Bi YX, Sunada H, Yonezawa Y, Danjo K. Evaluation of rapidly disintegrating tablets by direct compression method. Drug DevInd Pharm 1999;25:571-81.
- Chaudhari PD, Chaudhari SP, Kolhe SR, Dave KV, More DM. Formulation and evaluation of fast dissolving tablets of famotidine. Indian Drugs 2005;42:641-9.
- Bhagwati ST, Hiremath SN, Sreenivas SA. Comparative evaluation of disintegrants by formulating cefixime dispersible tablets. Indian J Pharm Educ Res 2005;39:194-7.
- Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol 1975;27:48-9.
- Gohel M, Patel M, Amin A, Agarwal A, Dave R, Bariya N. Formulation design and optimization of mouth dissolve tablets of nimesulide using vacuum drying technique. AAPS PharmSciTech 2004;5:e36.