- *Corresponding Author:
- M. C. Gohel
Department of Pharmaceutics and Pharmaceutical Technology, L. M. College of Pharmacy, Navrangpura, Ahmedabad - 380 009, India
E-mail: mukeshgohel@hotmail.com
| Date of Submission | 7 June 2006 |
| Date of Revision | 28 June 2007 |
| Date of Acceptance | 18 September 2007 |
| Indian J Pharm Sci, 2007, 69 (5): 640-645 |
Abstract
The objective of present work was to develop modified release tablets of isoniazid using hydroxylpropylmethylcellulose as a release-controlling agent. The low-viscosity grade hydroxylpropylmethylcellulose, medium-viscosity grade hydroxylpropylmethylcellulose, and high-viscosity grade hydroxylpropylmethylcellulose were used to prepare the matrix tablets. The tablets, prepared by direct compression, were subjected to physical characterization and in vitro drug release studies. The in vitro drug release was carried out using USP apparatus 1 at 50 rpm in 900 ml of acidic dissolution medium (pH 1.2) for 2 h, followed by 900 ml alkaline dissolution medium (pH 6.8). The polymer type did not affect the flow of powder blend and crushing strength of isoniazid tablets. The drug release rate was strongly influenced by the type of polymer and the concentration of polymer. The viscosity grade of hydroxylpropylmethylcellulose and the drug release was inversely correlated. To analyze the release mechanism Higuchi, zero-order, first-order, Hixson-Crowell, Weibull, and Korsmeyer-Peppas model were used. The optimized formulation followed the Weibull model. The use of simplified optimization methodology is demonstrated to evolve unified mathematical model.
Keywords
Direct compression, isoniazid, modifi ed release, tablet
Hydrophilic polymers have been given considerable attention in the formulation of controlled release drug delivery systems for various drugs. Hydroxypropylcellulose, hydroxypropylmethylcellulose, and sodiumcarboxymethylcellulose, Carbopols® and polyvinyl alcohol are a few representative examples of the hydrophilic polymers that have been extensively used in the formulation of controlled release systems.
Hydroxypropylmethylcellulose (HPMC), a semisynthetic derivative of cellulose, is popular as a swellable and hydrophilic polymer. Its non-toxic nature and ease of handling makes it an excellent release retardant material. On exposure to aqueous fluids, the polymer in tablet hydrates to form a viscous gel layer through which the drug is released by diffusion and/or erosion of the matrix. The formulation factors influencing the drug release from hydrophilic matrices are polymer viscosity, polymer particle size, drug to polymer ratio, drug solubility, drug particle size, drug loading, compression force, tablet shape, formulation excipients, coatings, and processing techniques [1-4]. The dissolution can be either disentanglement or diffusion controlled, depending on the polymer molecular weight and the thickness of the diffusion boundary layer [5].
Tuberculosis (TB), a rampant infectious disease, is considered to be the foremost cause of death from a single microorganism. Annually worldwide, of the 8 million people who actively manifest the disease, 3 million die [6]. Chemotherapy of TB is complicated by the need of multi-drug regimens given over a long period. Development of delivery system that releases drug in a sustained manner at therapeutic concentration over a period of time can ensure patient compliance and may also minimize the risk of emergence of drug resistant mutants and potential toxicity.
Isoniazid (300 mg) is a first-line drug recommended by World Health Organization (WHO) for the treatment of tuberculosis. The high solubility in the aqueous medium, shorter half-life (1.5 to 4.5 h) and absorption throughout gastrointestinal tract, indicates that it is a better candidate for sustained drug delivery system. During the last few years, various carrier systems, like microparticles, stealth liposome and microspheres have been developed for the sustained delivery of isoniazid for better chemotherapeutic efficacy against tuberculosis [7-9]. However, these formulations have to be injected either subcutaneously or intravenously. The parenteral route is not suitable for long-term treatment. Hence, there is a need to develop an oral drug delivery system that is convenient for the patients.
Jain and co-workers formulated controlled released tablet of isoniazid using guar and karaya gums alone and in combination with HPMC. The tablets exhibited non-Fickian release profile with rapid initial release and showed better in vitro and in vivo correlation (r = 0.9794) [10]. Bulutoner and co-workers investigated polymethylacrylate, polyvinylchloride and Carbomer® as a matrixing agent for isoniazid formulations. They reported Carbomer® is the best matrixing agent for isoniazid [11]. Lucinda-Silva and Evangelist encapsulated isoniazid in the microspheres of alginate and chitosan. Half of the drug was released within 2.5 h [12]. Sharma and co-workers fabricated lectin-functionalized poly (lactide-co-glycolide) nanoparticles as oral/ aerosolized antitubercular drug carriers for treatment of tuberculosis [13]. The natural matrixing agents are less preferred at industry due to non-consistency in quality and compression problems. Scale-up problems are experienced with microspheres and nonoparticles. The major advantages of HPMC are directly compressible, gelling on exposure to aqueous media, releases the drug by the mechanism of erosion and/or diffusion. The objective of the present work was to fabricate sustained release formulation of isoniazid using a single synthetic excipient (HPMC).
Materials and Methods
Isoniazid (INH) IP was received as a gift sample from Sunij Pharma Pvt. Ltd., Ahmedabad. Various grades of hydroxypropylmethylcellulose (Methocel K4M, Methocel K15M and Methocel K100M) were obtained as gift from Colorcon Asia Pvt. Ltd., Goa. Dibasic calcium phosphate (DCP) and magnesium stearate were purchased from Laser Chemicals, Ahmedabad. Cab-O-Sil M5 was received as gift from Cabot Sanmar, Chennai.
Preparation of tablets
The modified release monolithic tablet formulation consisting of Methocel, isoniazid and DCP were prepared. The polymer concentration was varied while the drug content (300 mg) and the total tablet weight (650 mg) were kept constant. The composition of various tablet formulations is given in Tables 1-3. The ingredients were individually passed through 40# sieve and mixed for 15 min. The mixture was lubricated with magnesium stearate (1%) and Cab-O-Sil (1%) and compressed into tablets using a single punch tablet machine (Cadmach Machinery, Ahmedabad). The compressed tablets were evaluated for physical parameters such as crushing strength, friability, uniformity of weight and in vitro drug dissolution study.
| Ingredient (mg/tablet) | I-1 | I-2 | I-3 | I-4 | I-5 |
|---|---|---|---|---|---|
| INH* | 300 | 300 | 300 | 300 | 300 |
| Methocel K4M | 65 | 130 | 195 | 260 | 325 |
| DCP** | 272 | 207 | 142 | 77 | 12 |
| Cab-O-Sil M5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 |
| Magnesium stearate | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 |
| Angle of repose (o) | 26.36±0.09 | 25.23±0.08 | 25.22±0.14 | 25.59±0.14 | 25.18±0.15 |
| Crushing strength(kilopascal) | 10.36±0.12 | 10.33±0.17 | 10.37±0.22 | 10.32±0.13 | 10.25±0.19 |
| %Friability | 0.30±0.02 | 0.19±0.02 | 0.24±0.01 | 0.33±0.02 | 0.47±0.03 |
| Uniformity of weight (mg) | 644.0±3.60 | 643.6±3.05 | 645.6±2.51 | 646.7±2.52 | 646.3±2.08 |
*INH = isoniazid, **DCP = Dibasic calcium phosphate
Table 1: Compositions And Evaluation Of Inh Tablets Contaning Methocel K4m
| Ingredient (mg/tablet) | I-6 | I-7 | I-8 | I-9 | I-10 |
|---|---|---|---|---|---|
| INH* | 300 | 300 | 300 | 300 | 300 |
| Methocel K15M | 65 | 130 | 195 | 260 | 325 |
| DCP** | 272 | 207 | 142 | 77 | 12 |
| Cab-O-Sil M5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 |
| Magnesium stearate | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 |
| Angle of repose (o) | 26.97±0.19 | 25.74±0.07 | 24.89±0.1 | 23.98±0.07 | 27.28±0.14 |
| Crushing strength(kilopascal) | 10.30±0.21 | 10.19±0.17 | 10.27±0.16 | 10.29±0.07 | 10.28±0.12 |
| %Friability | 0.38±0.01 | 0.23±0.01 | 0.18±0.02 | 0.24±0.03 | 0.33±0.02 |
| Uniformity of weight (mg) | 646.6±2.08 | 646.3±3.05 | 647.6±1.53 | 644.6±2.51 | 645.6±3.05 |
*INH = Isoniazid, **DCP = Dibasic calcium phosphate
Table 2: Compositions And Evaluation Of Inh Tablets Contaning Methocel K15m
| Ingredient (mg/tablet) | I-11 | I-12 | I-13 | I-14 | I-15 |
|---|---|---|---|---|---|
| INH* | 300 | 300 | 300 | 300 | 300 |
| Methocel K100M | 65 | 130 | 195 | 260 | 325 |
| DCP** | 272 | 207 | 142 | 77 | 12 |
| Cab-O-Sil M5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 |
| Magnesium stearate | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 |
| Angle of repose (o) | 25.95±0.11 | 25.37±0.18 | 24.64±0.1 | 24.59±0.1 | 24.02±0.08 |
| Crushing strength(kilopascal) | 10.28±0.12 | 10.26±0.13 | 10.32±0.13 | 10.28±0.1 | 10.31±0.11 |
| %Friability | 0.45±0.02 | 0.32±0.02 | 0.28±0.01 | 0.22±0.02 | 0.27±0.01 |
| Uniformity of weight (mg) | 646.7±2.08 | 646.3±3.06 | 647.7±1.53 | 644.7±2.52 | 645.7±3.06 |
*INH= Isoniazid, **DCP= Dibasic calcium phosphate, kp= Kilo Pascal
Table 3: Compositions And Evaluation Of Inh Tablets Contaning Methocel K100m
Angle of repose
A glass funnel was secured with its tip at a given height (h) above a graph paper placed on a horizontal surface. Powder was poured through the funnel until the apex of conical pile touched the tip of the funnel and then the angle of repose (Ø) was calculated using the following formula, tan Ø=h/r, where r is the radius of conical pile.
Tablet dimensions, crushing strength and friability
The diameter, thickness, and crushing strength of ten randomly selected tablets per batch were determined using Dr. Scheleuniger® (Pharmatron 8M, Germany) hardness tester. Twenty tablets were rotated in a friabilator (Model EF2, Electrolab, India) at 25 rpm for 4 min. The tablets were then dedusted, and the loss in weight due to fracture or abrasion was recorded as percentage weight loss (% friability).
Drug release study
The dissolution study was performed in a calibrated dissolution test apparatus (Electrolab, TDT 06-T, Mumbai) equipped with a basket employing 900 ml of pH 1.2 (0.1 N HCl) solution for first 2 h and pH 6.8 buffer (Phosphate buffer) solution for additional 22 h. The baskets were rotated at 50 rpm and the dissolution media were maintained at 37±2°. Five ml of samples were withdrawn and analyzed spectrophotometrically at 263 nm using a UV/Vis spectrophotometer (Model 1700, Shimadzu, Kyoto, Japan) after suitable dilution of the samples. The fresh dissolution medium was replaced after each withdrawal.
Analysis of the in vitro data
The method of Bamba et al. was adopted to ascertain kinetics of drug release14. Data from the in vitro drug release were analyzed by different kinetic models (Table 4) in order to evaluate the release mechanism of INH from the matrices. A Fortran software, developed in-house, was used. The F-value was employed to select the most appropriate kinetic model.
| Function | Equation |
|---|---|
| Zero-order | |
| First-order | |
| Hixson-Crowell | |
| Higuchi | |
| Korysmeyer-Peppas | |
| Weibull |
*%diss, percent dissolved at time t; k, dissolution rate constant; n, release component which is indicative of drug release mechanism; β shap parameter Td, time at which 63.2% of the drug is dissolved.
Table 4: Applied Mathematical Models To The Dissolution Data Of Isoniazid Tablet*
Results and Discussion
The angle of repose of the formulations containing different grades of Methocel were comparable (Tables 1-3). As per USP15, the powder blend of all the batches exhibited excellent flow. The crushing strength of all the tablets was in the range of 9 kp to 11 kp. The loss in total weight of the tablets due to friability was less than 0.5%. The high value of crushing strength and low friability indicated that the compressibility of INH and adjuvants was good.
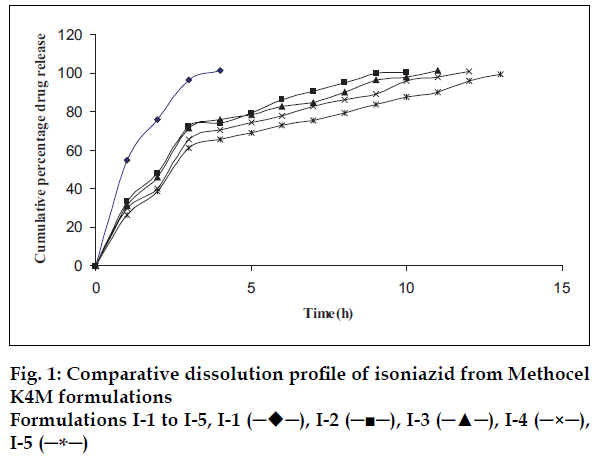
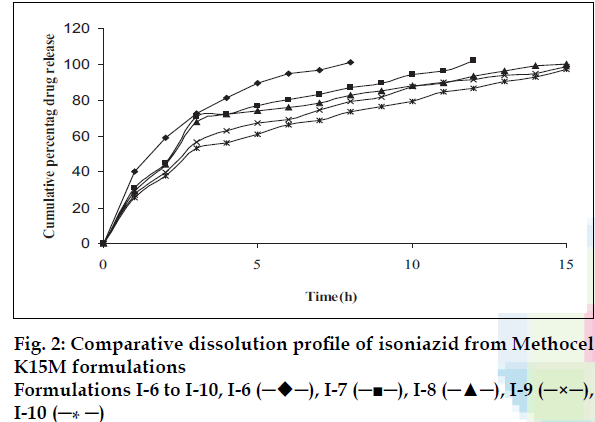
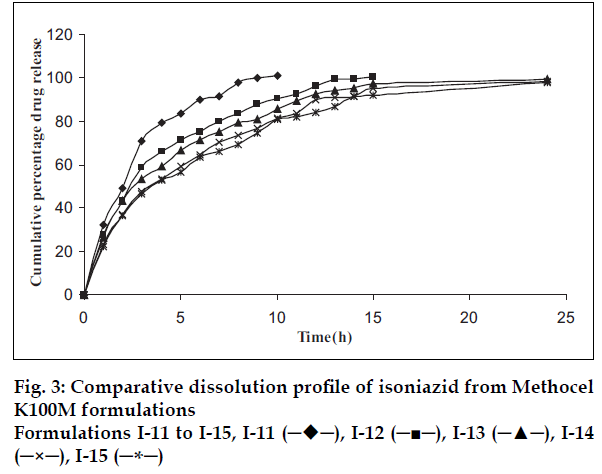
Plots of percent cumulative drug released versus time for all tablet formulations are shown in figs. 1-3. The drug release profiles were characterized by an initial burst effect (more than 20% drug release in first hour and slow release thereafter). The biphasic release is often observed from hydrophilic matrix systems. As the release-rate-limiting polymer changes from a glassy state to a rubbery state, a gel structure is formed around the tablet matrix, which considerably decreases the release of the drug since the drug has to diffuse through this gel barrier into the bulk phase. The strength of the gel depends on the chemical structure and molecular size of the polymer [16-19].
It is known that higher viscosity grade polymer (Methocel K100M) hydrates at a faster rate and, therefore, it is capable of forming a gel structure faster than a medium viscosity grade (Methocel K15M) and low viscosity grade (Methocel K4M) polymer. The drug release rate is significantly dependent on the proportion and type of the polymer used [20].
The results in fig. 1 show that the low viscosity grade (Methocel K4M) was not able to extend the drug release up to 24 h. Batches I-4 and I-5 showed the extended release up to 12 h with the initial burst release. The DCP is non-gelling acid soluble excipient. The results shown in figs. 1-3 reveal that as the concentration of DCP was increased, the drug release rate was enhanced. Fast drug release from batches may be attributed to dissolution of DCP in acidic dissolution medium and subsequent increase in porosity of tablets. The size and surface area of the tablets were kept constant by adding required quantity of DCP, as it is well known fact that the drug release is also dependent on the size of matrix tablets. Dibasic calcium phosphate also contributes compressibility to the powder blend due to its high fragmentation propensity. While with medium viscosity grade polymer (Methocel K15M), the release was extended further (beyond 12 h) with burst release. Fig. 2 shows that more than 20% drug was released from all the batches in first hour. The required release profile with suitable drug release in 1 h (approximately 20%) was obtained with 40-50% Methocel K100M as shown in fig. 3.
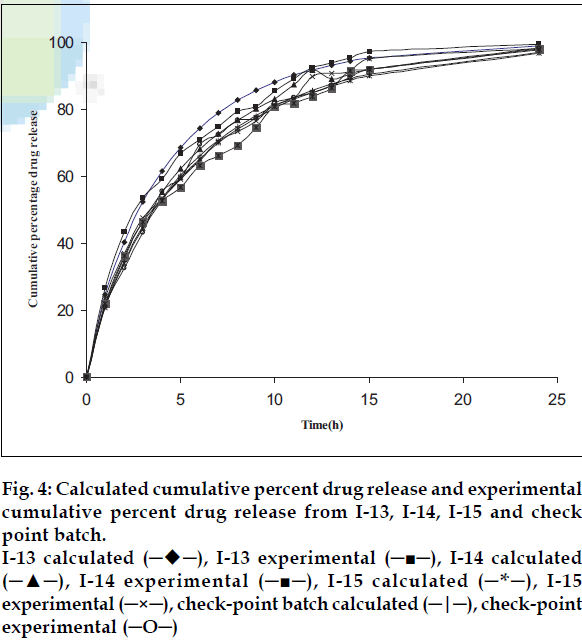
The batches I-13, I-14 and I-15 showed desirable drug release (nearly 20% drug release in the 1 h and complete drug release in 24 h) and hence, the results of these batches were used for data mining. Kirilmaz levent procedure was adopted for data mining [21]. Briefly, three modified release formulations of INH were prepared using various amounts of Methocel K100 M. The in vitro drug dissolution data was analyzed for establishing kinetics of drug release. Zero-order, first-order, Higuchi, Hixson-Crowell, Korsmeyer-Peppas and Weibull model were tested for selected best batches. Weibull model showed the least sum of square of residuals (103.73, 88.07 and 79.53 respectively for batches I-13 to I-15) and F value (7.4,6.29 and 5.68, respectively for batches I-13 to I-15) than other models (SSR was >150 and F was > 30 for all other models in batches I-13 to I-15) and, therefore, it was used for further data analysis. The slope and intercept values for the three formulations (I-13 to I-15) were 0.8791, 0.8683, 0.8457 and - 0.5494, -0.6184, -0.6331, respectively.
A characteristic drug release profile of the matrix type drug delivery systems is represented by the Weibull’s equation [22,23].
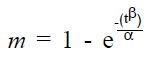 (1),
(1),
where, m is the cumulative amount of drug released after time t, β is shape parameter and α is the scale parameter. The term α is replaced by the term Td (α = (Td) β). The parameter Td is the time necessary to dissolve 63.2% of the drug.
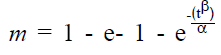 (2).
(2).
The equations for slope and intercept were evolved by the method reported by Kirilmaz [21]. The values of correlation coefficient (r) for the equations of slope and intercept were 0.996 and 0.887, respectively. The high value of ‘r’ indicated good correlation. Hence, an unified equation was evolved by combing the equations of slope and intercept, by employing the steps given in Table 5.
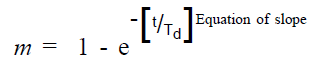 (3)
(3)
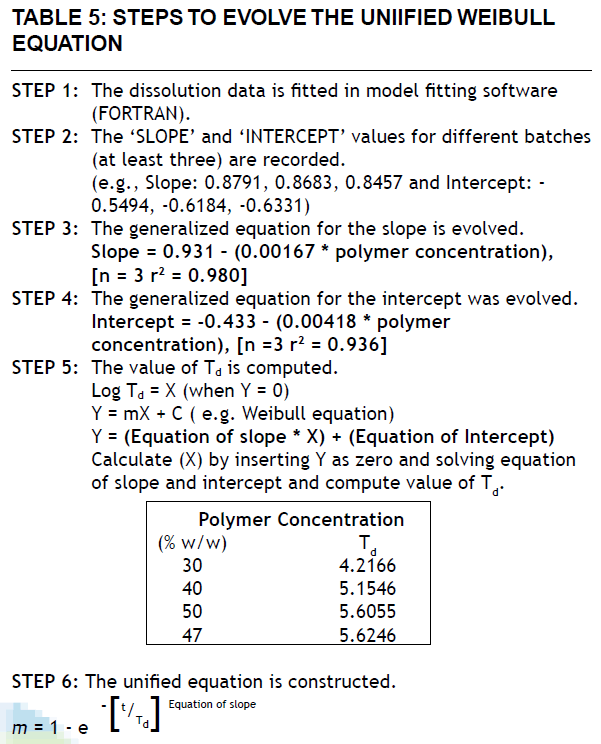
Model validation was done by comparing the values of observed and predicted drug release for three formulations as well as for a check-point batch containing 46.15% drug and 47% matrixing agent. The observed and predicted values for check-point batch are depicted in fig. 4. The theoretical and experimental release profiles showed insignificant difference. The results revealed that the matrix system fabricated with 47% Methocel K100M was able to provide required release in first hour and a modified release profile thereafter.
Fig 4: Calculated cumulative percent drug release and experimental
cumulative percent drug release from I-13, I-14, I-15 and check
point batch.
I-13 calculated (─♦─), I-13 experimental (─■─), I-14 calculated
(─▲─), I-14 experimental (─■─), I-15 calculated (─*─), I-15
experimental (─×─), check-point batch calculated (─|─), check-point
experimental (─O─)
The results of this investigation enabled us to fabricate hydrophilic matrices containing isoniazid (a first line antitubercular drug). It was also demonstrated that the release of isoniazid from directly compressed matrix tablets could be modified by changing the type and amount of polymer in the matrix tablets. Fine tuning of formulation could be done by adopting a simple optimization procedure.
References
- Vazquez MJ, Perez-Marcos B, Gomez-Amoza JL, Martinez-Pacheco R, Souto C, Concheiro A. Influence of technological variables on release of drugs from hydrophilic matrices. Drug Dev Ind Pharm 1992;18:1355-75.
- Velasco M, Ford JL, Rowe P, Rajabi-Siahboomi AR. Inßuence of drug: Hydroxypropylmethylcellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from HPMC tablet. J Control Release 1999;57:75.
- Desai SJ, Singh P, Simonelli AP, Higuchi WI. Investigation of factors influencing release of solid drug dispersed in inert matrices, II: Quantitation of procedures. J Pharm Sci 1966;55:1224.
- Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. J Pharm Pharmacol 2005;57:533.
- Narasimhan B, Peppas NA. Molecular analysis of drug delivery systems controlled by dissolution of the polymer carrier. J Pharm Sci 1997;86:297.
- Hara PO, Hickey AJ. Respirable PL. A micropsheres containing Rifampicin for the treatment of tuberculosis: Manufacture and characterization. Pharm Res 2000;17:955.
- Dutt M, Khuller GK. Sustained release of isoniazid from a single injectable dose of poly (DL-lactide-co-glycolide) microparticles as a therapeutic approach towards tuberculosis. Int J Antimicrob Agents 2001;17:115.
- Labana S, Pandey R, Sharma S, Khuller GK. Chemotherapeutic activity against murinetuberculosis of once weekly administered drugs (isoniazid and rifampicin) encapsulated in liposomes. Int J Antimicrob Agents 2002;20:301.
- Huiyu Z, Yanling Z, Danielle LB, Mark CM, Theodore W. Microparticle based lung delivery of isoniazid decreases isoniazid metablolism and targets alveolar macrophages. J Control Release 2005;107:288.
- Jain NK, Kulkarni K, Talwar N. Controlled-release tablet formulation of isoniazid. Pharmazie 1992;47:277-8.
- Bulutoner F, Capan Y, Kas S, Oner L, Hincal AA. Sustained release isoniazid tablets: I- formulation and in vitro evaluation. Farmaco 1989;44:739-52.
- Lucinda-Silva RM, Evangelist RC. Microspheres of alginate chitosan containing isoniazid. J Microencapsulation 2003;20:145-52.
- Sharma A, Sharma S, Khuller GK. Lectin-functionalized poly (lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. J Antimicrob Chemother 2004;54:761-6.
- Bamba M, Puisievx F, Marty JP, Carstensen JT. Release mechanism in gel forming sustained release formulations. Int J Pharm 1979;2:307.
- USP 29-NF 24. United States Pharmacopeial Convention Inc: Rockville, MD; 2006. p. 3017.
- Vendruscolo CW, Andreazza IF, Ganter JL, Ferrero C, Bresolin TM. Xanthan and galactomannan (from M. Scabrella) matrix tablets for oral controlled delivery of theophylline. Int J Pharm 2005;296:1.
- Skoug JW, Mikelsons MV, Vigneron CN, Stemm NL. Qualitative evaluation of the mechanism of release of matrix sustained release dosage forms by measurement of polymer release. J Control Release 1993;27:227.
- Lee PI, Peppas NA. Prediction of polymer dissolution in swellable controlled release systems. J Control Release 1987;6:207.
- Gao P, Nixon PR, Skoug JW. Diffusion in HPMC gels, II: Prediction of drug release rates from hydrophilic matrix extended release dosage forms. Pharm Res 1995;12:965.
- Available from: http://www.dow.com. [Last assessed on 2006 Mar 20].
- Kirilmaz L. Data mining for the controlled dosage form. Pharm Tech (Europe) 2001;1.
- Langenbucher F. Linearization of dissolution rate curve by the weibull distribution. J Pharm Pharmacol 1972;24:979.
- Dokoumetzidis A, Papadopoulou V, Macheras P. Analysis of dissolution data using modified versions of Noyes-Whitney equation and the Weibull function. Pharm Res 2006;23:256.