- *Corresponding Author:
- D. M. Patel
Department of pharmaceutics and pharmaceutical technology, Shri sarvajanik pharmacy college, Mehsana - 384 001, India
E-mail: justdmpatel@rediffmail.com
| Date of Submission | 12 July 2006 |
| Date of Revision | 18 August 2007 |
| Date of Acceptance | 17 November 2007 |
| Indian J Pharm Sci, 2007, 69 (6): 763-767 |
Abstract
Floating tablets of carbamazepine were developed using melt granulation technique. Bees wax was used as a hydrophobic meltable material. Hydroxypropylmethylcellulose, sodium bicarbonate and ethyl cellulose were used as matrixing agent, gas-generating agent and floating enhancer, respectively. The tablets were evaluated for in vitro buoyancy and dissolution studies. A simplex lattice design was applied to investigate the combined effect of 3 formulation variables i.e. amount of hydroxypropyl methylcellulose ( X 1 ), ethyl cellulose ( X 2 ) and sodium bicarbonate ( X 3 ). The floating lag time (F lag ), time required for 50% (t 50 ) and 80% drug dissolution (t 80 ) were taken as responses. Results of multiple regression analysis indicated that, low level of X 1 and X 2 , and high level of X 3 should be used to manufacture the tablet formulation with desired in vitro floating time and dissolution. Formulations developed using simplex lattice design were fitted to various kinetic models for drug release. Formulation S3 was selected as a promising formulation and was found stable at 40 o and 75% relative humidity for 3 months. Present study demonstrates the use of simplex lattice design in the development of floating tablets with minimum experimentation.
Keywords
Floating tablet, Carbamazepine, Simplex lattice design, Melt-granulation
The high cost involved in the development of a new drug molecule has diverted the pharmaceutical companies to investigate various strategies in the development of new drug delivery systems [1]. Drug release from the delivery devices can be sustained up to 24 h for many drugs using current release technologies. However, the real issue in the development of oral controlled release dosage forms is to prolong the residence time of the dosage form in the stomach or upper gastrointestinal (GI) tract until the drug is completely released [2].
Several approaches are currently used to retain the dosage form in the stomach. These include bioadhesive systems [3], swelling and expanding systems [4,5], floating systems [6,7] and other delayed gastric emptying devices [8,9]. The principle of floating preparation offers a simple and practical approach to achieve increased gastric residence time for the dosage form and sustained drug release.
Carbamazepine (CBZ) is used for anticonvulsant and antineuralgic effects. The popularity of this drug is related to several beneficial properties, including, proven efficacy in controlling different types of seizures. It is poorly soluble in water with erratic oral absorption and bioavailability less than 70%. Moreover CBZ has a narrow therapeutic range and shows bioavailability differences [10]. In efforts to reduce the frequency of dosing required for chronic CBZ therapy and to decrease variability in plasma concentration, various extended release formulations have been developed by many researchers [11-13]. Preparing the drug in a floating dosage form can control the extent of bioavailability for such poorly water-soluble drug. The objective of the present investigation is to develop floating tablets containing carbamazepine using simplex lattice design as an optimization technique.
Materials and Methods
Carbamazepine USP was a gift from Hindustan Chemicals Ltd., Chennai, India. Bees wax was purchased from Ases Chemical Works, Jodhpur, India. Hydroxypropyl methylcellulose K4 M (HPMC K4 M), ethyl cellulose (EC) and sodium bicarbonate were purchased from Laser Chemicals, Ahmedabad, India. Magnesium stearate and talc were purchased from Apex Chemicals, Ahmedabad, India. All other ingredients used were of analytical grade and used as received.
Preparation of carbamazepine floating tablets
Bees wax was melted in a large petridish and the required quantity of CBZ was added to the molten mass. Previously prepared geometric mixture of HPMC K4 M and/or EC and sodium bicarbonate was added to the molten CBZ-bees wax mixture and stirred well to mix. The mass was removed from the hot plate and subjected to scrapping until it attained room temperature. The coherent mass was passed through 60 mesh and the resulting granules were resifted using100 mesh to separate the fines. The granules (50 g) of 60/100 mesh were collected and mixed with talc (2%) and magnesium stearate (1%). This lubricated blend was compressed into tablets using 12 mm flat face round tooling on a Rimek-I rotary tablet machine (Karnavati Eng. Pvt. Ltd, Ahmedabad). Compression force was adjusted to obtain tablets with hardness in range of 5-6 kg/cm2. Tablets were weighing 515±4 mg round flat face with average diameter of 12±0.1 mm and thickness of 4.6±0.2 mm. Formulations of the preliminary trial batches (P1 to P7) and the simplex lattice design batches (S1 to S7) are shown in Tables 1 and 2, respectively.
| Formulation ingredients | P1 | P2 | P3 | P4 | P5 | P6 | P7 |
|---|---|---|---|---|---|---|---|
| Bees wax (%) | 10 | 10 | 10 | 10 | 10 | 15 | 20 |
| HPMC K4 M (%) | 45 | 40 | 35 | 30 | 20 | 30 | 25 |
| Sodium bicarbonate (%) | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Ethyl cellulose (%) | 0 | 5 | 10 | 15 | 25 | 10 | 10 |
| Floating lag time (seconds) | 300 | 280 | 265 | 261 | 257 | 250 | 325 |
| Floating time without rupture of tablets (min) | <180 | <180 | <180 | <180 | <180 | >720 | >720 |
All batches contained 40% w/w carbamazepine, 2% w/w talc and 1% w/w magnesium stearate. Average weight of each tablet is 515 mg.
Table 1: Tablet formulation and evaluation results of preliminary trials
| Batch code | Transformed fractions of variables? | Flag ±SD (s) | t50 ±SD (min) | t80 ±SD (min) | ||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | ||||||
| S1 | 1 | 0 | 0 | 255±3.1 | 479±2.3 | 767±4.4 | ||
| S2 | 0 | 1 | 0 | 175±1.2 | 388±1.9 | 620±1.2 | ||
| S3 | 0 | 0 | 1 | 158±0.9 | 366±3.2 | 586±2.9 | ||
| S4 | 0.5 | 0.5 | 0 | 186±1.4 | 549±2.7 | 878±5.4 | ||
| S5 | 0 | 0.5 | 0.5 | 167±0.8 | 411±3.1 | 657±3.6 | ||
| S6 | 0.5 | 0 | 0.5 | 185±1.7 | 398±1.8 | 633±2.9 | ||
| S7 | 0.33 | 0.33 | 0.33 | 153±0.6 | 479±2.5 | 767±5.6 | ||
All batches contained 200 mg carbamazepine, 75 mg bees wax, 2% w/w talc and 1% w/w magnesium stearate. Coded value 0 represents actual values? 125 for X1, 50 for X2 and 0 for X3, and coded value 1 represents actual values? 175 for X1, 100 for X2 and 50 for X3, respectively. ?X1 is amount HPMC K4 M (mg); X2 is amount of sodium bicarbonate (mg); X3 is amount of ethyl cellulose (mg). Flag indicates, floating lag time; and SD, standard deviation. Average weight of each tablet is 515 mg.
Table 2: Formulation and evaluation of batches in simplex lattice design
In vitro buoyancy studies
In vitro buoyancy was determined by floating lag time as per the method described by Rosa et al [14]. The tablets were placed in a 100 ml glass beaker containing simulated gastric fluid (SGF), pH 1.2 as per USP. The time required for the tablet to rise to the surface and float was determined as floating lag time.
In vitro dissolution studies
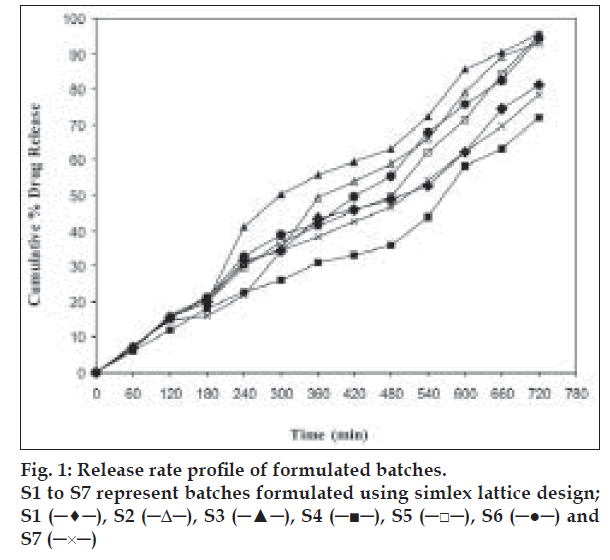
The in vitro dissolution study of CBZ tablets was performed using USP apparatus (model TDT-06T, Electrolab, India) fitted with paddles (75 rpm) at 37±0.5° using simulated gastric fluid (pH 1.2; 900 ml) as a dissolution media. At the predetermined time intervals, 10 ml samples were withdrawn, filtered through 0.45 μ membrane filter, diluted, and assayed at 285 nm using a Shimadzu UV/Vis double beam spectrophotometer (Kyoto, Japan). Cumulative percentage drug release was calculated using an equation obtained from the calibration curve. The drug release profile is shown in fig. 1. The time required for 50% and 80% drug release was calculated.
Simplex lattice design
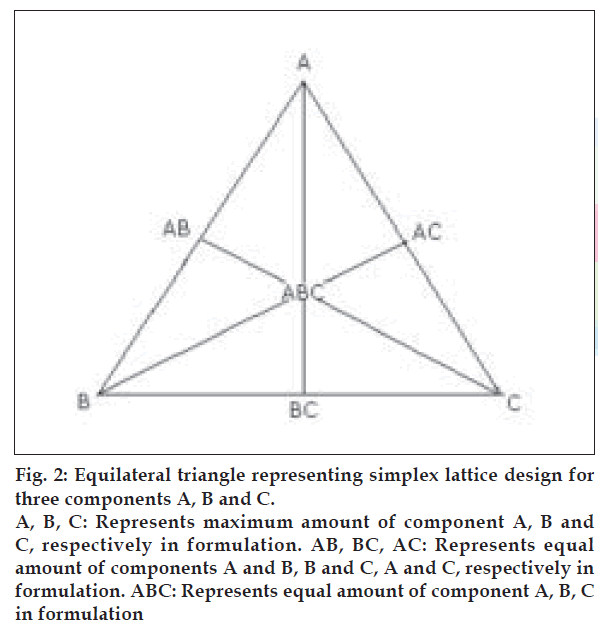
A simplex lattice design [15] was adopted to optimize the formulation variables. In this design, three factors were evaluated by changing their concentrations simultaneously and keeping their total concentration constant. The simplex lattice design for three- component system is represented by an equilateral triangle (fig. 2) in two-dimensional space. Seven batches (S1 to S7) were prepared, one at each vertex (A, B, C), one at half way between vertices (AB, BC, AC) and one at the center point (ABC). Each vertex represents, a formulation containing the maximum amount of one component, with the other two components at a minimum level. The half way between the two vertices represents, a formulation containing the average of the minimum and maximum amount of the two ingredients represented by two vertices. The center point represents, a formulation containing one third of each ingredient. The amounts of matrixing agent, HPMC K4 M (X1), gas-generating agent, sodium bicarbonate (X2) and floating enhancer, EC (X3), were selected as independent variables. The floating lag time (Flag), time required for 50% (t50) and 80% drug dissolution (t80) were taken as responses.
Figure 2: Equilateral triangle representing simplex lattice design for three components A, B and C. A, B, C: Represents maximum amount of component A, B and C, respectively in formulation. AB, BC, AC: Represents equal amount of components A and B, B and C, A and C, respectively in formulation. ABC: Represents equal amount of component A, B, C in formulation
Kinetic modeling of drug release
The dissolution profile of all the batches was fitted to various models like zero-order, first-order [16], Higuchi [17], Hixon-Crowell [18], Korsmeyer and Peppas [19-21] and Weibull models [22-24] to ascertain the kinetic modeling of drug release. The method of Bamba et al, was adopted for deciding the most appropriate model [25].
Results and Discussion
Bees wax was selected as a hydrophobic meltable material to impart sufficient integrity to the tablets. HPMC K4 M was selected as a matrixing agent considering its widespread applicability and excellent gelling activity in sustained release formulations. Sodium bicarbonate generates CO2 gas in the presence of hydrochloric acid present in dissolution medium. The gas generated is trapped and protected within the gel formed by hydration of HPMC K4 M, thus decreasing the density of the tablet. As the density of the tablet falls below 1 (density of water), the tablet becomes buoyant. EC was used as floating enhancer. It also works as a dissolution retardant being insoluble in gastric pH. Five batches (P1 to P5) were prepared using same amounts of sodium bicarbonate and bees wax while different amounts of HPMC K4 M and EC. The amount of HPMC K4 M was decreased while EC was increased from batch P1 to P5. From the evaluation results (Table 1), it was observed that as the amount of EC was increased from 0% to 25%, Flag was decreased and this effect was significant on reducing Flag up to 10% of EC. Hence it was decided to optimize the amount of EC in between 0% and 10%. As the amount of HPMC K4 M was increased from 20% to 45%, the Flag was increased indicating that high amount of HPMC K4 M is undesirable to achieve low Flag. Below 25%, HPMC K4 M might not give sufficient strength to the matrix to prolong drug release up to 12 h. Hence, it was decided to optimize HPMC K4 M in between 25% and 35%. Formulations P1 to P5 were subjected to in vitro dissolution study. All the tablets ruptured within 3 h with more than 80% drug release. This might be due to poor strength of tablets or due to insufficient binding provided by bees wax that failed to keep the matrix intact. Formulations P6 and P7 were prepared using 15% and 20% of bees wax, respectively. These were found to remain intact for more than 12 h under stirring at 75 rpm during dissolution studies. Formulation P7 exhibited floating lag time of 325 s. This might be due to poor penetration of SGF in a tablet core due to high amount of bees wax. Hence it was decided to keep the bees wax at 15%. It is well known that higher percentage of sodium bicarbonate decreases the Flag. So it was decided to optimize sodium bicarbonate between 10% and 20% to keep the Flag below 3 min. In order to optimize the formulation for acceptance criteria of Flag, less than 3 min; t50, in between 300 and 420 min; and t80, in between 540 and 600 min; a simplex lattice design was employed in the present investigation.
The amounts of matrixing agent (HPMC K4 M, X1), gas-generating agent (sodium bicarbonate, X2) and floating enhancer (EC, X3) were selected as independent variables in a simplex lattice design. The floating lag time (Flag) and times required for 50% (t50) and 80% drug dissolution (t80) were taken as responses. A statistical model incorporating seven interactive terms was used to evaluate the responses.
Y=b0+b1X1+b2X2+b3X3+b12X1X2+b23X2X3+b13X1X3 +b123X1X2X3 (1), where Y is the dependent variable, b0 is the arithmetic mean response of seven runs and bi is the estimated coefficient for factor Xi. The main effects (X1, X2 and X3) represent the average result of changing 1 factor at a time from its low to high value. The interaction terms (X1X2, X2X3, X1X3 and X1X2X3) show how the response changes when two or more factors are simultaneously changed. The statistical analysis of simplex lattice design batches was performed by multiple linear regression analysis using Microsoft Excel. The values (Table 2) for Flag, t50 and t80 for all the 7 batches (S1 to S7) showed a wide variation (i.e., 153 to 255 seconds, 366 to 549 minutes, and 586 to 878 minutes, respectively). The data clearly indicate that the values of Flag, t50 and t80 are strongly dependent on the selected independent variables. The fitted equations relating the responses Flag, t50 and t80 to the transformed factor are shown in Equation 2, Equation 3, and Equation 4, respectively.Flag=–1928.7+2183.7X+2103.78X+2086.28X3-118.17X1X2-85.67X1X3, (R2=0.999) (2), t50= 2093.17–1624.93X1–1691.9X2–1724.67X3 +455.81X1X2 (R2=0.945) (3) t80=3380.34–2631.38X1–2738.65X2–2791.4X3 +729.58X1X2 (R2=0.940) (4) The high values of correlation coefficients for Flag, t50 and t80 indicate a good fit i.e., good agreement between the dependent and independent variables. The polynomial equations can be used to draw conclusions after considering the magnitude of coefficient and the mathematical sign it carries (i.e., positive or negative). The equation for Flag suggests that the factor X1 has more significant effect on floating lag time followed by factor X2 and X3. Therefore, high level of factor X1 should not be selected for lower floating lag time. From the equation 3 and equation 4, it can be concluded that, factor X1 has more important role in prolonging both, t50 and t80. The magnitude of coefficients indicates that factor X2 has more favorable effect on both the dependent variables than factor X3. The high value of X1X2 coefficient also suggests that the interaction between X1 and X2 has a significant effect on t50 and t80. From the results of multiple linear regression analysis, it can be concluded that the drug release pattern can be changed by appropriate selection of the X1, X2 and X3 levels.
The promising formulation was selected on the basis of the acceptance criteria for Flag,t50 and t80 as mentioned earlier. Formulations S2, S3, S5 and S7 passed the criteria for Flag. Formulations S2, S3, S5 and S6 passed the criteria for t50. The criterion for t80 was fulfilled only by formulation S3. Hence formulation S3 was selected as promising formulation from the simplex lattice design batches.
All the simplex lattice design batches showed good in vitro buoyancy with maximum floating lag time of 255 s. All the tablet formulations remained buoyant for more than 12 h in SGF, pH 1.2. In vitro buoyancy study was also conducted at an elevated pH condition (pH 4.5). The floating tendency of all the formulations remained unaltered at higher pH.
The dissolution data of batches S1 to S7 was fitted to zero-order, first-order, Higuchi, Hixon-Crowell, Korsmeyer and Peppas, and Weibull models. The method of Bamba et al was adopted for deciding the most appropriate model. The results of F-statistics were used to select the most appropriate model. The release profile of promising batch, S3, fitted best to zero-order model (F= 23.64). This superiority is statistically insignificant with the Korsmeyer and Peppas model (F= 41.81) as well as Weibull model (F= 26.54) as shown by the goodness-of-fit test (F ratio test). But priority should be given to the model with the lowest F value. Thus, it may be concluded that drug release from floating CBZ tablets is best explained by zero order model. The other simplex lattice design batches also followed zero order model with either significant or insignificant difference with the other models.
The factorial batches were subjected to short-term stability studies at 40o and 75% RH for three mo. Samples withdrawn after three mo showed no significant change in appearance of the tablets, floating lag time and in vitro drug release.
Based on the results it was concluded that the addition of matrixing polymer, HPMC K4 M, and gas generating agent, sodium bicarbonate was essential to achieve in vitro buoyancy. A systematic study using a simplex lattice design revealed that the amount of HPMC K4 M, sodium bicarbonate and EC had a significant effect on Flag, t50 and t80. Thus, by selecting a proper optimization technique, proper balance of formulation variables can be achieved rapidly with minimum efforts to produce required in vitro buoyancy and drug dissolution profile.
Acknowledgements
Authors thank Hindustan Chemicals Ltd., Chennai (India) for providing gift sample of carbamazepine USP. Authors also wish to thank Shri M. L. Gandhi Higher Education Society, Modasa (India), for extending required laboratory facilities for the present work.
References
- Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: Gel layer behavior, mechanism and optimal performance. Pharm SciTechnol Today 2000;3:1-8.
- Baumgartner S, Kristi J, Vrecer F, Vodopivec P, Zorko B. Optimization of floating matrix tablets and evaluation of their gastric residence time. Int J Pharm 2000;195:125-35.
- Santus G, Lazzarini G, Bottoni G. An in vitro-in vivo investigation of oral bioadhesive controlled release furosemide formulations. Eur J Pharm Biopharm 1997;44:39-52.
- Deshpande AA, Rhodes CT, Shah NH, Malick AW. Controlled-release drug delivery systems for prolonged gastric residence: An overview. Drug Develop Ind Pharm 1996;22:531-9.
- Deshpande AA, Shah NH, Rhodes CT, Malick W. Development of novel controlled-release system for gastric retention. Pharm Res 1997;14:815-9.
- Menon A, Ritschel WA, Sakr A. Development and evaluation of monolithic floating dosage form for furosemide. J Pharm Sci 1994;83:239-45.
- Whitehead L, Fell JT, Collett JH, Sharma HL, Smith AM. Floating dosage forms: An in vivo study demonstrating prolonged gastric retention. J Control Release 1998;55:3-12.
- Singh B, Kim K. Floating drug delivery systems: An approach to oral controlled drug delivery via gastric retention. J Control Release 2000;63:235-59.
- Chawla G, Bansal A. A means to address regional variability in intestinal drug absorption. Pharm Tech 2003;27:50-68.
- Larkin JG, Mecllelan A, Munday A, Sutherland M, Bulter E, Brodie MJ. A double blind comparison of conventional and controlled release formulations of carbamazepine in healthy subjects. Br J Clin Pharm 1989;27:313-22.
- Giunchedi P, Conte U, La Manna A. Carbamazepine modified release dosage forms. Drug Develop Ind Pharm 1991;17:1753-64.
- Giunchedi P, Maggi L, Conte U, La Manna A. Linear extended release of a water insoluble drug, carbamazepine, from erodible matrix. Int J Pharm 1993;94:15-22.
- Stevens RE, Limsakun T, Evans G, Mason DH. Controlled multidose pharmacokinetic evaluation of two extended release carbamazepine formulations (Carbatrol and Tegretol XR). J Pharm Sci 1998;87:1531-4.
- Rosa M, Zia H, Rhodes T. Dosing and testing in vitro of a bioadhesive and floating drug delivery system for oral application. Int J Pharm 1994;105:65-70.
- Banker GS, Anderson NR. Tablets. In: Lachman L, Lieberman HA, Kanig JL, editors. The Theory and Practice of Industrial Pharmacy. 3rd ed. Mumbai: Varghese Publishing House; 1987. p. 283.
- Wagner JG. Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J Pharm Sci 1969;58:1253-7.
- Higuchi T. Mechanism of sustained-action medication: Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 1963;52:1145-9.
- Hixon AW, Crowell JH. Dependence of reaction velocity upon surface and agitation. IndEnggChem 1931;23:923-31.
- Korsmeyer R, Gurny R, Peppas N. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 1983;15:25-35.
- Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm ActaHelv 1985;60:110-1.
- Harland RS, Gazzaniga A, Sangalli ME, Colombo P, Peppas NA. Drug/ polymer matrix: Swelling and dissolution. Pharm Res 1988;5:488-94.
- Langenbucher F. Linearization of dissolution rate curves by the Weibull distribution. J Pharm Pharmcol 1988;24:979-81.
- Goldsmith JA, Randall N, Ross SD. Methods of expressing dissolution rate data. J Pharm Pharmacol 1978;30:347-9.
- Romero P, Costa JB, Chulia D. Statistical optimization of a controlled release formulation obtained by a double compression process: Application of a Handmade matrix and factorial design. In: Wells JI, Rubistein MH, Horwood E, editors. Pharmaceutical Technology, Contorlled Drug Release. Vol 2. New York, NY: Ellis Harwood; 1991. p. 44-58.
- Bamba M, Puisieux F. Release mechanisms in gel forming sustained release preparation. Int J Pharm 1979;2:307-15.