- *Corresponding Author:
- S. Saggar
Department of Pharmaceutical Sciences, MJRP College of Health Care & Allied Sciences, Mahatma Jyoti Rao Phoole University, Jaipur-302 019, India
E-mail: sachinsaggar@yahoo.com
| Date of Submission | 03 November 2018 |
| Date of Revision | 27 February 2019 |
| Date of Acceptance | 01 June 2019 |
| Indian J Pharm Sci 2019;81(4):661-672 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The solid self-emulsifying drug delivery system of bambuterol hydrochloride was designed, prepared and evaluated to overcome poor bioavailability. The designing process included selection of oil phase, surfactant and co-solvent/co-surfactant based on saturated solubility studies. Psuedoternary phase diagram was constructed using dilution method to identify the self-emulsifying region. Liquid self-emulsifying drug delivery formulations were prepared from components obtained from these studies and were converted to solid self-emulsifying drug delivery systems by adsorption technique using microcrystalline cellulose:aerosil mixture as the adsorbent. The prepared solid self-emulsifying drug delivery system-based formulations were evaluated for drug content, morphology, globule size, micromeritic properties, ex vivo permeability and stability. Formulation F1 containing 10 mg bambuterol hydrochloride, triacetin (12.50 % w/w), Tween 80 (43.75 % w/w) and ethanol (43.75 % w/w) was concluded to be optimal. These results suggested that bambuterol hydrochloride can be formulated as a solid self-emulsifying drug delivery system, which could be used to improve oral bioavailability of bambuterol hydrochloride.
Keywords
Solid self-emulsifying drug delivery system, bambuterol hydrochloride, SEM, phase diagram, bioavailability
Self-emulsifying drug delivery system (SEDDS) refers to a formulation comprising an isotropic mixture of natural and synthetic oils with hydrophilic or lipophilic surfactants and co solvents, which spontaneously emulsifies when exposed to gastrointestinal fluid to form oil-in-water emulsion[1-4]. The emulsion so produced is a clear dispersion in which the particle size of the dispersed phase ranges from nanometers to several microns. According to Đekić and Primorac, microemulsions are isotropic, transparent systems, which contain spherical droplets of water phase or oil phase, with diameter of average size from 10 to 100 nm, dispersed in a continuous oil or water phase, respectively whereas nanoemulsions are oil-in-water or water-in-oil emulsions with droplet size in the range of 50-1000 nm (preferably from 100 to 500 nm)[5]. The main difference between microemulsions and nanoemulsions is regarding their physical stability, appearance, and microstructure[5]. Pouton et al. reported that SEDDS can be dispensed in either soft gelatin or hard gelatin capsules. Upon oral administration, these systems form fine emulsions (or microemulsions) in the gastro-intestinal tract (GIT) with mild agitation provided by gastric mobility[6,7]. The difference between a SEDDS and self-micro emulsifying drug delivery system is that the former when diluted results in a droplet size between 100 and 300 nm and the later results in a droplet size of less than 50 nm[8].
SEDDS can overcome the problems associated with various drugs falling in various BCS classes, in case of BCS class III drugs, SEDDS can overcome the problem of enzymatic degradation, gut wall efflux and bioavailability[9].
On ingestion of SEDDS, it is initially acted upon by the gastric lipase in the stomach, which digests the lipid part of the formulation and further the gastric emptying enables the emulsification process before the formulation enters the duodenum. Presence of the lipids in the formulation leads to the delay in the gastric emptying resulting in the increase in the gastric residence time. Therefore, the drug remains for prolonged time in the GIT, which results in better dissolution of the drug at the absorption site and therefore increases the absorption[10].
A number of lipids have shown ability to alter the permeability of the gut wall and increase the permeability of the intestine[11]. Lipids may enhance the extent of lymphatic transport and increase the bioavailability directly or indirectly by reduction in first pass metabolism. Certain lipids and surfactants have shown the tendency to reduce the activity of the efflux transporters in the GI wall, thereby increasing the amount of drug absorbed. This mechanism may also lead to reduced intra-enterocyte metabolism since there is interplay between P-gp and CYP3A4. Cremophor EL, Labrasol, Polysorbate 80, Polysorbate 20 have shown P-gp inhibitory activity[12].
Bambuterol hydrochloride is 5-[(1RS)-2-[(1,1- dimethylethyl)amino]-1-hydroxyethyl]-1,3-phenyl enebis (dimethylcarbamate) hydrochloride[13]. Bambuterol is a long acting β-adrenoceptor agonist used in the treatment of asthma. It is a prodrug of terbutaline. Bambuterol causes smooth muscle relaxation, resulting in dilation of bronchial passages. It is freely soluble in water[13]. On average, 20 % of an oral dose is absorbed. Protein binding of bambuterol is low, 40-50 % at therapeutic concentrations. The terminal half-life of bambuterol after an oral dose is 9-17 h. Bambuterol is metabolized in the liver and terbutaline is formed by both hydrolysis and oxidation. After absorption from the gut, about 2/3 of the absorbed bambuterol survives first-pass metabolism, rest 1/3 is metabolized to intermediary metabolites, which are still prodrugs of terbutaline. Terbutaline has a bioavailability of about 10 % of administered dose[14,15]. Renal clearance after oral administration is around 1250 ml/min. Bambuterol is available as 10 and 20 mg tablet for oral administration. The dosage of bambuterol is 10 mg per day but it may also be administered upto 20 mg per day. Bambuterol is also available in liquid dosage form in strength of 1 and 2 mg/ml.
The absorption of a β-agonist after oral administration is dependent on its lipophilicity, the aim of the present study was to develop the solid SEDDS of bambuterol to enhance its bioavailability, which may lead to reduction in dose and side effects of the drug like tremors, muscle cramps, tachycardia, palpitation and anxiety.
Materials and Methods
Bambuterol hydrochloride was purchased from Yarrow Chem Products, Mumbai. Tween 80, micro crystalline cellulose was gifted by Eden drugs Pvt. Ltd. Amritsar, Aerosil was gifted by Medimax, Amritsar. Triacetin was gifted by Alkon Lab, Amritsar. Distilled water, hydrochloric acid (Ramkem LR grade), potassium chloride (Qualigens AR grade), potassium dihydrogen phosphate (CDH LR), disodium hydrogen phosphate, sodium chloride (S. D. Fine Chem, extra pure), ethanol (Changshu Hongsheng Fine Chemical, AR), were used in the study.
Solubility of bambuterol in oil, surfactant and co solvent:
The solubility studies of the drug in various oils (isopropyl myristate, triacetin, oleic acid, ethyl oleate, Labrafil 1944), surfactant (Tween 80, Labrasol and Cremophor EL) and co-solvent/co-surfactant (propylene glycol, polyethylene glycol 400, ethanol, Transcutol) was determined by dissolving excess amount of drug in 3 ml of each of selected oil, surfactant and co-solvent/co-surfactant in a 5 ml stoppered vial. The excess amount of drug was mixed using a vortex mixer. The vials were then kept at 37±1° in an isothermal shaker for 72 h to get to equilibrium. The equilibrated samples were then centrifuged at 3000 rpm for 15 min. The supernatant of the centrifuged samples was carefully separated/removed with help of syringe and needle without disturbing the precipitated portion of the samples. The concentration of the drug in each of the samples was estimated by diluting the samples with isopropyl alcohol and measuring the absorbance at 265 nm using UV spectrophotometer (UV 1800 Shimadzu, Japan)[16-20].
Construction of pseudoternary phase diagram:
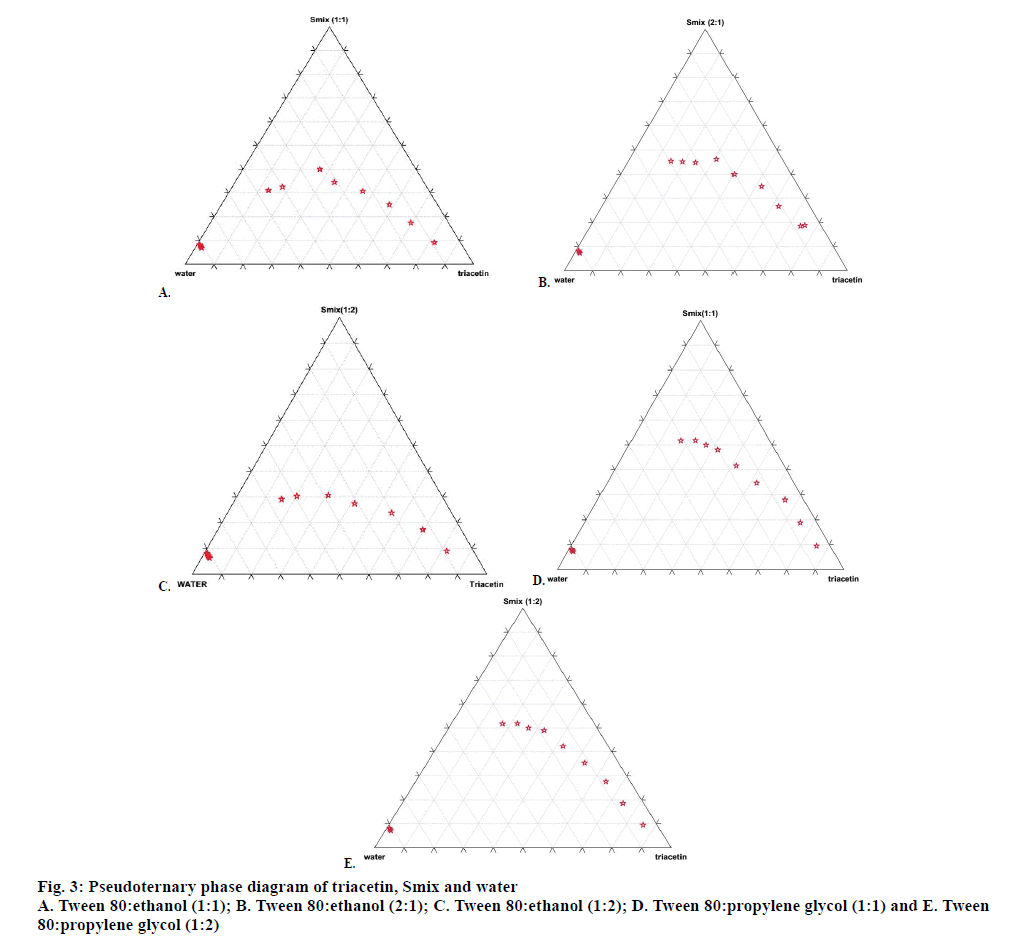
Pseudoternary phase diagrams were constructed to identify the regions like o/w microemulsion or nanoemulsion, coarse emulsion and gel/viscous region by diluting specific oil/surfactant/co-solvent mixture with water. The phase diagram is helpful to determine the appropriate concentration range and ratios of the components that can result in micro or nanoemulsion. The aqueous titration method or spontaneous emulsification method was used to construct the phase diagram. First of all, various ratios of surfactant and co-solvent/co-surfactant were prepared by mixing selected surfactant and selected co-solvent/cosurfactant in various proportions like 1:1, 1:2, 1:3 w/w. This mixture of surfactant and co-solvent/co-surfactant is termed as Smix. Thereafter different types of Smix were mixed with selected oil in ratios of 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, and 1:9 w/w in a glass vial at room temperature by vortexing for at least 5 min. Each ratio of Smix dissolved in oil, was then titrated with distilled water in increments of 25 μl of water using a micropipette and the samples were vigorously mixed by vortexing for at least 2 min and then kept at room temperature for 10 min to reach equilibrium before next addition of water. The process was repeated either till the samples turned turbid or until 90.90 % w/w addition of water. The phase behaviour of each ternary phase system was minutely observed during the titration. The percent composition of each component in the ternary system was determined and the results were plotted on the triangular coordinates to construct the phase diagram[21-23]. The same process was repeated with all the other ratios of surfactant and co-surfactant. Pseudo-ternary phase diagram was constructed using Triplot software.
Preparation of liquid self-emulsifying drug delivery system:
The amount of the oil, surfactant and co-solvent/ co-surfactant to be taken was decided on the basis of microemulsification region in the ternary phase diagram. Bambuterol hydrochloride was accurately weighed and dissolved in required quantity of oil and Smix (surfactant and co-solvent/co-surfactant) taken in a screw-capped glass vial, by sonicating at 37° for maximum up to 30 min. Thirteen formulations each containing different concentrations of oil, surfactant and co-surfactant were prepared. Each formulation contained 10 mg of bambuterol hydrochloride.
Preparation of solid SEDDS:
Solid SEDDS were formulated by adsorption technique. 10 mg (standard oral dose) of bambuterol hydrochloride was dissolved in each pre-concentrate by ultrasonication of the drug and pre-concentrate mixture at 37° for up to 30 min as mentioned above. A mixture of microcrystalline cellulose and aerosil in ratio of 1:1 was chosen as adsorbent. Drug pre-concentrate mixture was added drop-wise on the adsorbent material and homogenized, using a stainless steel spatula in a glass mortar and pestle, to ensure uniform distribution of formulation. The proportion of drug pre-concentrate mixture to adsorbent was 1:0.5. The resultant material was dried at ambient temperature[17,18]. Adsorbent used was a 1:1 mixture of microcrystalline cellulose:aerosil. Solid SEDDS is a mixture of bambuterol and preconcentrate: adsorbent in the ratio of 1:0.5.
Physiochemical evaluation of solid SEDDS, morphological studies:
Scanning electron microscopy (SEM) for solid SEDDS and bambuterol hydrochloride was performed using scanning electron microscope (Jeol, Japan) at accelerated voltage at 10 KV for samples and 2 KV for drug using InLens detector and magnification of 10 000-100 000X to study the topography[17,18].
Differential scanning calorimetry and infrared studies:
Physical state of bambuterol hydrochloride in solid SEDDS was characterized using a differential scanning calorimeter. Thermograms of drug and solid SEDDS were obtained using DSC Q 20 (TA Instrument) differential scanning calorimeter to study their thermal behaviour. The samples were scanned at the speed of 15°/min at a heat flow from 0° to 250°[17,18]. Infrared studies of the bambuterol and the solid SEDDS were carried out to compare the spectra of bambuterol and solid SEDDS[17].
Micromeritic properties[18,24-26]:
Micromeritic properties such as angle of repose, bulk density, tap density, Hausner ratio and compressibility index of the prepared solid SEDDS were evaluated. These studies were done in triplicate. A funnel was kept on a vertical stand in such a manner that the difference in height of the tip of the funnel and paper placed on horizontal surface is constant and specified. The bottom of the funnel was closed with the finger and sample was loaded in the funnel. The funnel was opened to release the powder on to the paper so as to form a conical heap. The borders of the conical heap were marked circularly and the diameter of the conical heap was measured at four points. The height of the conical heap was also measured. The angle of repose was measured by using the following Eqn., angle of repose = tan-1 2h/diameter or tan-1 h/r, where, h = height; r = radius, which is half the diameter.
Solid SEDDS were weighed and carefully transferred to a 100 ml Borosil glass measuring cylinder. The measuring cylinder was dropped from a height of 1 inch on to a hard surface until no further volume change was observed. The tap density is obtained by dividing the weight of the solid SEDDS formulation (g) by final volume of the formulation (cm3).
Solid SEDDS were weighted and then carefully transferred to a 100 ml Borosil glass measuring cylinder. The final volume of the formulation was noted. The pour density or bulk density is obtained by dividing the weight of the solid SEDDS formulation (g) by final volume of the formulation (cm3). Compressibility index is described as (tap density-bulk density/tap density)×100 and Hausner ratio is described as H = tap density/bulk density.
Globule size determination:
One gram of each solid SEDDS containing bambuterol hydrochloride equivalent to 10 mg was diluted to 100 ml of distilled water in a volumetric flask and was sonicated at 37° for 10 min. The sample was allowed to equilibrate for 15 min. The size of the droplets of the ensuing emulsion was determined by photon correlation spectroscopy that analyses the fluctuation in the light scattering due to Brownian motion of droplets as function of time using Zetasizer Nano series (Beckman Coulter-Delsa Nano). The same process was repeated in 0.1 N HCl[17,18]. The magnitude of the surface charge is directly associated/linked to the stability of the emulsion. The zeta potential of the each solid SEDDS formulation diluted with distilled water was measured using a Zeta analyser (Beckman Coulter-Delsa Nano)[17,18].
Drug content:
Bambuterol hydrochloride was extracted from the solid SEDDS in pH 6.8 buffer using ultrasonication technique. The dispersions were sonicated for 10 min at 37° and then allowed to equilibrate for another 10 min. Thereafter the contents were filtered through Whatman filter paper. The filtrates were analysed for content of bambuterol hydrochloride using UV spectrophotometric technique at 264 nm. Drug content estimation studies were done in triplicate[17,18].
Disintegration test:
Solid SEDDS formulations containing bambuterol hydrochloride equivalent to 10 mg were filled in hard gelatin capsules. The test was carried out as per USP. One capsule was placed in each tube and the basket rack is positioned in distilled water at 37±2° such that capsule remains 2.5 cm below the surface of the water on their upward movement and descend not closer than 2.5 cm from the bottom of the beaker. The apparatus moves a distance of 5 to 6 cm at a frequency of 28 to 32 cycles per minute. Perforated plastic discs were placed on the top of capsules. The apparatus was operated till the capsules disintegrate and the time taken for disintegration was noted. The capsule complies with the test, if the capsules disintegrate, and all particles pass through the 10-mesh screen in the time specified. If any residue remains, it must have a soft mass with no palpably firm core. The tests were conducted in triplicate.
As per ICH harmonized tripartite guideline for new drug products[27], for rapidly dissolving (dissolution >80 % in 15 min at pH 1.2, 4.0 and 6.8) products containing drugs, which are highly soluble throughout the physiological range (dose/solubility volume <250 ml from pH 1.2 to 6.8), disintegration may be substituted for dissolution. In such cases dissolution testing may not be necessary.
In vitro dissolution test:
The in vitro dissolution study was carried out using USP type II apparatus at 75 rpm and at 37±0.5°. The dissolution medium consisted of phosphate buffer (pH 6.8). The method was slightly modified as per the International Pharmacopoeia[28]. Since bambuterol hydrochloride has good solubility in water, instead of 900 ml of dissolution media 500 ml of dissolution media was used. The solid SEDDS formulations containing bambuterol hydrochloride equivalent to 10 mg were placed in the dissolution media and the dissolution test carried out. Samples were withdrawn after 15 min and were analysed by measuring the absorbance at 264 nm on a UV spectrophotometer. The studies were done in triplicate.
Comparative ex vivo intestinal permeability studies:
The everted gut technique was employed for ex vivo intestinal permeability studies. Fresh intestine of chicken was taken and from the intestine, 5-6 cm long portion of ileum was removed and washed thoroughly and then everted immediately. The removed portion of ileum was checked for leaks by filling it with 0.9 % w/v normal saline solution. The ileum was then suspended-hung in a beaker filled with Krebs-Ringer phosphate (KRP) buffer (pH 7.2-7.4) in “U shaped” manner in such a way that both the ends of the ileum were available for withdrawing and replenishing the sample. Thereafter solid SEDDS F1 loaded with bambuterol hydrochloride was introduced in the beaker with stirring. The system was maintained at 37±1° and stirred at 50 rpm with help of magnetic stirrer. The ileum was filled with 5 ml of KRP buffer (pH 7.2- 7.4). Two milliliters of sample was withdrawn from the ileum using a syringe at predetermined intervals and the amount withdrawn from the ileum was immediately replenished with KRP Buffer to maintain sink condition. Samples were withdrawn up to 2 h. The drug content in the samples was determined by UV spectrophotometric method. The studies were done in triplicate. The whole process was repeated by replacing the solid SEDDS F1 with bambuterol hydrochloride dissolved in distilled water[29]. The amount of the drug permeating across the membrane/intestine for the drug in solution and the solid SEDD system was compared. The intestinal permeability is one of the major factors that govern the rate and extent of absorption[30].
Stability studies:
Stabilities studies for the solid SEDDS were performed at 25°±2°/60±5 % relative humidity. The samples were tested for drug content after the time intervals of 90 d. The drug content in the samples was estimated spectrophotometrically[18].
Statistical analysis:
Origin 9.0 software was used for statistical analysis. Where the studies have been done in triplicate, the data represent the mean±standard deviation. The statistical analysis was performed using student’s t-test. A difference below the probability level was considered as statistical significant (p<0.05).
Results and Discussion
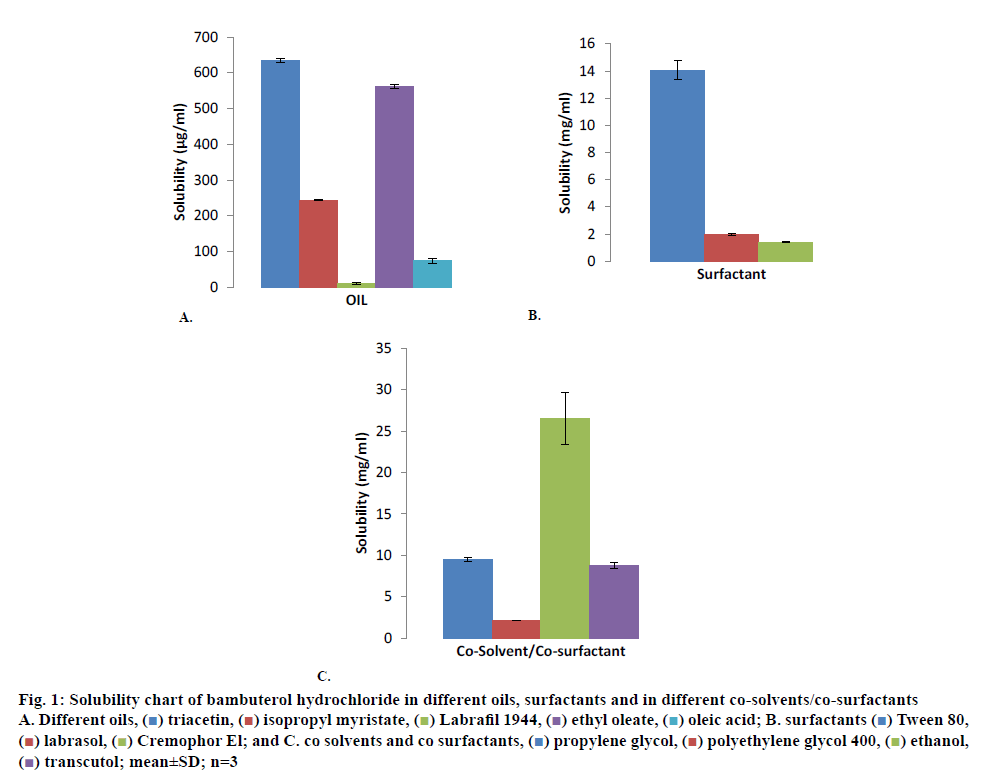
Bambuterol hydrochloride showed maximum solubility in triacetin (635.12±4.88 μg/ml; fig. 1A) compared to other oils evaluated for solubility, similarly bambuterol showed maximum solubility in Tween 80 (14.05±0. 697 mg/ml; fig. 1B) compared to other surfactant and in ethanol (26.538±3.135 mg/ml; fig.1C; p<0.05, t-test) when compared to other co-solvents/co-surfactant. Bambuterol hydrochloride also showed sufficient solubility in propylene glycol (9.464±0.260 mg/ml), therefore two systems, one constituting triacetin, Tween 80 and ethanol and the other constituting triacetin, Tween 80 and propylene glycol were selected as oil, surfactant and co-solvent/co-surfactant, respectively for further studies. The standard curve for UV spectrophotometric method for estimation of drug content using isopropyl alcohol at 265 nm is depicted in fig. 2 and the regression Eqn. for the method is Y = 0.00123x+0.01196 (R-square: 0.99933).
Figure 1: Solubility chart of bambuterol hydrochloride in different oils, surfactants and in different co-solvents/co-surfactants
A. Different oils, ( ) triacetin, (
) triacetin, ( ) isopropyl myristate, (
) isopropyl myristate, ( ) Labrafil 1944, (
) Labrafil 1944, ( ) ethyl oleate, (
) ethyl oleate, ( ) oleic acid; B. surfactants (
) oleic acid; B. surfactants ( ) Tween 80, (
) Tween 80, ( ) labrasol, (
) labrasol, ( ) Cremophor El; and C. co solvents and co surfactants, (
) Cremophor El; and C. co solvents and co surfactants, ( ) propylene glycol, (
) propylene glycol, ( ) polyethylene glycol 400, (
) polyethylene glycol 400, ( ) ethanol, (
) ethanol, ( ) transcutol; mean±SD; n=3
) transcutol; mean±SD; n=3
From the phase diagram, the percent composition w/w of triacetin, Tween 80, ethanol and water that will form self-micro-emulsifying drug delivery system was determined. Smix prepared by mixing Tween 80 and ethanol in ratio 1:1, 2:1, 1:2 was selected for further studies and Smix ratio 3:1 was rejected because the micro emulsion region decreased with increase in proportion of Tween 80 and was therefore not conducive for further studies (figs. 3A, B and C). The same process was repeated with triacetin, Tween 80 and propylene glycol. Smix was prepared by mixing Tween 80 and propylene glycol in following proportions 1:1, 2:1 and 1:2, w/w, respectively. Smix prepared by mixing Tween 80 and propylene glycol in ratio 1:1 and 1:2 was selected for further studies and Smix ratio 2:1 was rejected because the micro emulsion region decreased with the increase of Tween 80 (fig. 3D and E).
Various formulations were prepared by mixing triacetin, Tween 80 and ethanol in various percent w/w proportions. These formulations were further evaluated for phase separation on storage and globule size. The same procedure was also adopted for preparation and evaluation of formulations containing triacetin, Tween 80 and propylene glycol. On the basis of the phase diagram studies, thirteen formulations/composition (Table 1) were selected for further studies.
Composition Code |
Smix | Smix Ratio | Pre-concentrate | Oil:Smix | ||
|---|---|---|---|---|---|---|
| Oil (parts) |
Tween 80 (parts) | Ethanol (parts) |
||||
| Formulation F 1 Formulation F 2 Formulation F 3 Formulation F 4 Formulation F 5 Formulation F 6 Formulation F 7 Formulation F 8 Formulation F 9 |
Smix1 Smix1 Smix1 |
1:1 1:1 1:1 |
12.50 13.80 15.38 12.50 13.80 15.38 12.50 13.80 15.38 |
43.75 43.10 42.31 58.33 57.47 56.41 29.17 28.73 28.20 |
43.75 43.10 42.31 29.17 28.73 28.20 58.33 57.47 56.41 |
1:7 1:6.25 1:5.5 1:7 1:6.25 1:5.5 1:7 1:6.25 1:5.5 |
| Smix2 | 2:1 2:1 2:1 |
|||||
| Smix2 | ||||||
| Smix2 | ||||||
| Smix3 | 1:2 1:2 1:2 |
|||||
| Smix3 Smix3 |
||||||
| Composition Code | Smix | Smix Ratio | Pre-concentrate | Oil:Smix | ||
| Oil (parts) | Tween 80 (parts) | P.G. (parts) | ||||
| Formulation F 10 Formulation F 11 Formulation F 12 Formulation F 13 |
Smix4 | 1:2 1:2 1:1 1:1 |
9.09 14.30 9.09 14.30 |
30.30 28.57 45.45 42.85 |
60.60 57.13 45.45 42.85 |
1:10 1:6 1:10 1:6 |
| Smix4 | ||||||
| Smix5 | ||||||
| Smix5 | ||||||
PG is propylene glycol; Smix is a mixture of surfactant and co-surfactant
Table 1: Selected compositions of oil, surfactant and co-surfactant
Thirteen liquid SEDDS formulations (F1-F13, Table 1) with different concentration of oil, surfactant and co-surfactant each containing 10 mg of bambuterol hydrochloride were prepared by ultrasonication method. The liquid SEDDS were transformed to solid SEDDS by adsorption technique. The formulations of the solid SEDDS were evaluated.
According to Jang et al. and Gershanik et al., the droplet size of the solid SEDDS is the most crucial factor in SEDDS performance as it determines the rate and extent of drug release and absorption. Smaller droplet size will present a large area for drug absorption and hence increase bioavailability[3,31]. The globule size of the solid SEDDS in distilled water ranged from 176.8 to 665 nm. The lowest globule size was observed in the formulation F1 (Table 2). Since the dilution of solid SEDDS resulted in a droplet size between 100 and 300 nm[8], it was observed that solid SEDDS formulation of bambuterol can produce droplet size, which falls in SEDDS range. The presence of surfactants in the solid SEDDS cause interfacial film to stabilize and condense, while co-surfactant causes the film to expand; thus relative proportion of surfactant and co-surfactant have varied effects on the globule size[18]. Further the globule size of solid SEDDS in 0.1 N HCl was also determined and it was found to be in the range of 287.5 to 1029.3 nm. This result revealed that some of the solid SEDDS would produce droplets in self-emulsifying range in the gastric pH as well (Table 2).
Formulation |
Globule size (nm) | Zeta Potential | PDI | |
|---|---|---|---|---|
| Distilled water | ||||
| F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 F13 |
176.8 498.1 425.6 510.6 665 581.3 446.2 533.7 526.7 356.9 441.7 224.2 342.8 |
-2.74 0.77 1.88 3.98 -8.27 -1.28 25.99 -6.96 2.46 -4.74 -0.37 11.71 10.94 |
0.226 0.326 0.362 0.297 0.292 0.301 0.191 0.319 0.284 0.279 0.310 0.238 0.280 |
|
| 0.1 N HCl | ||||
| Formulation | Globule size (nm) | PDI | ||
| F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 F13 |
287.5 476.3 555.6 779.5 1350 499.2 704.1 1029.3 354.5 948.2 434.2 965.8 500.3 |
0.262 0.227 0.251 0.335 0.434 0.228 0.305 0.407 0.248 0.382 0.221 0.366 0.265 |
||
PDI is polydispersity index; the measurement was done with 1:100 dilutions of solid SEDDS in distilled water
Table 2: Globule size, zeta potential and polydispersity index of bambuterol solid SEDDS in distilled water and 0.1 N HCl
The polydispersity index (PDI) of all the solid SEDDS ranged from 0.191 to 0.362, the lowest PDI was reported for formulation F7 (Table 2). The PDI below 0.3 indicates good uniformity in the globule size distribution after dilution with water[32,33]. PDI of solid SEDDS F1 was found to be 0.226, which indicates uniform globule distribution (Table 2).
Zeta potential of the solid SEDDS in distilled water was found to be in the range of +25.99 to –8.27 mV. Generally formulations having zeta potential value of ±30 mV are considered to be stable. Solid SEDDS comply with zeta potential requirement for stability (Table 2). Formulation F1, F2, F6, F9, F11, F13 were chosen for further studies based on the globule size of these formulation in distilled water and 0.1 N HCl.
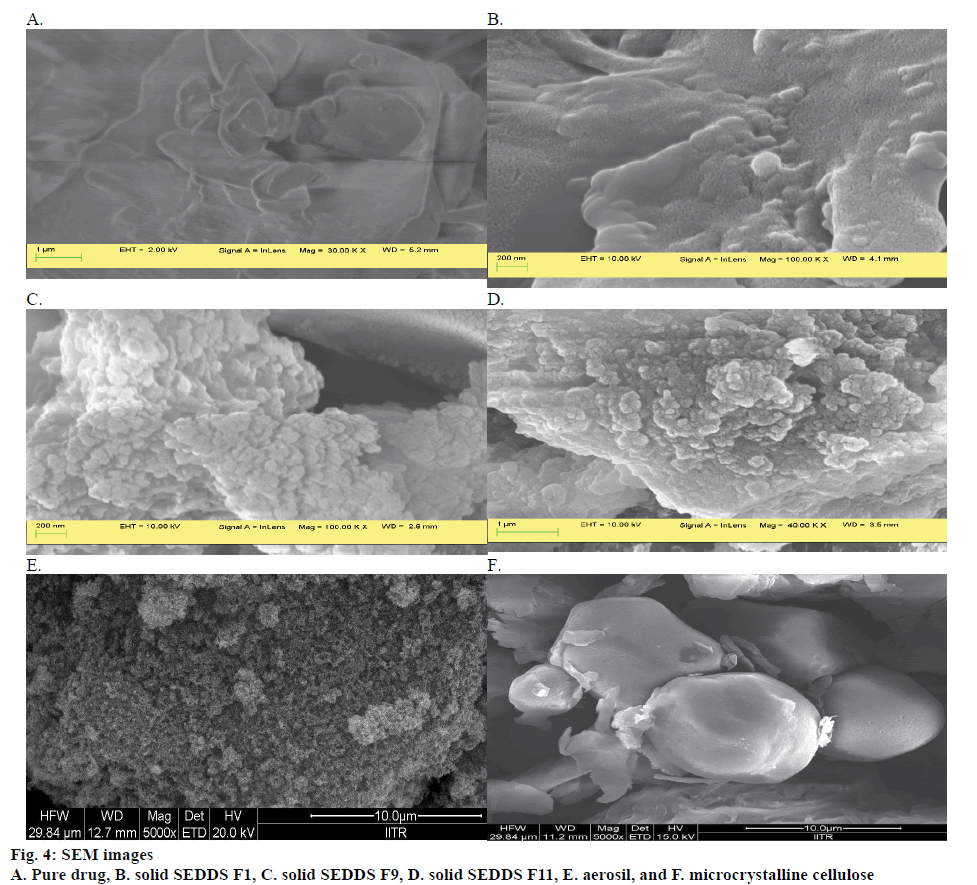
SEM studies were carried out to compare the surface morphology of the pure bambuterol and bambuterol adsorbed on the solid surface of the adsorbent. Pure bambuterol appears as smooth surface (fig. 4A). The micrograph of the solid SEDDS F1 (fig. 4B), F2, F6, F9 (fig. 4C), F11 (fig. 4D) and F13 also indicates smooth surface. This suggests that the entire drug was uniformly adsorbed on to the adsorbent surface without much agglomeration or aggregation. The micrograph of the aerosil and microcrystalline cellulose is depicted in fig. 4E and F, respectively.
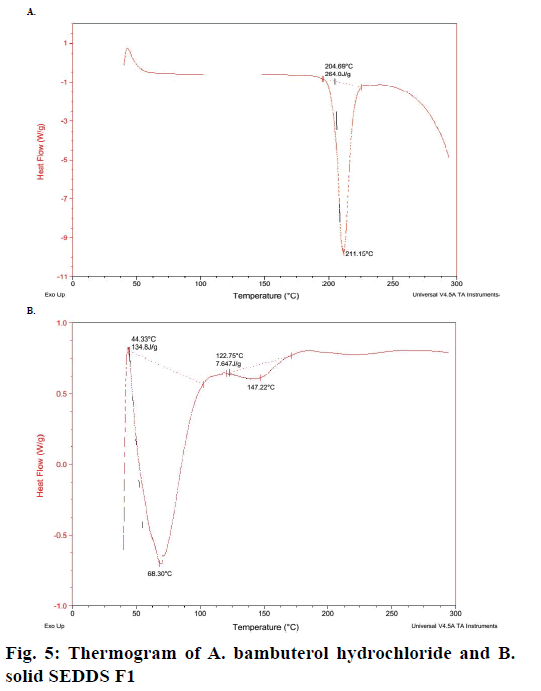
The differential scanning calorigraph of the pure bambuterol represent sharp endothermic peak at 211.15° (fig. 5A), which corresponds to its melting point. In the thermogram of the solid SEDDS F1 (fig. 5B), the endothermic peak is absent, which indicates the change in melting behaviour of the drug and inhibition of crystallization following solubilisation using lipid surfactant and physical mixing with solid carrier.
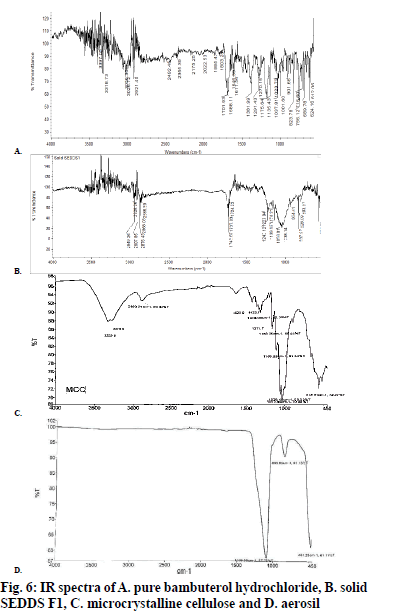
Pure bambuterol hydrochloride (fig. 6A) shows major peak at 823.78, 1291.43, 1701.93 and 2900 (cm-1). The IR spectra of the solid SEDDS F1 (fig. 6B) reveals that there is no considerable change in the major peaks when compared with spectra of the pure bambuterol hydrochloride. This shows that there was no interaction between the drug and the excipients. The IR spectra of microcrystalline cellulose and aerosil are represented by fig. 6C and D, respectively.
Formulations F7-F9 indicate angle of repose <30, formulations F1-F3 indicate angle of repose around 30 whereas formulations F4-F6 indicates angle of repose >31. It was observed that with increase in concentration of surfactant Tween 80, the angle of repose increases, which shows that high concentration of surfactant could cause loss of flow in solid SEDDS. Further formulations F9-F13 indicate angle of repose around 35, which shows that formulations containing Tween 80 and propylene glycol as Smix have poor flow properties as compared to formulations containing Tween 80 and ethanol as Smix. Formulations F7-F9 showed excellent flow properties whereas F10, F11 showed fair flow properties. Overall the flow properties of the formulations were within the acceptable limits (Table 3).
Formulation |
Angle of repose (o) |
Bulk density (g/cm3) |
Tap density (g/cm3) |
Compressibility Index (%) | Hausner ratio |
|---|---|---|---|---|---|
| F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 F13 |
30.14±0.27 30.03±0.92 29.88±0.24 32.78±0.30 33.85±0.61 31.55±0.94 25.57±0.47 26.86±0.25 26.83±0.98 36.11±0.31 37.10±0.21 35.78±0.89 32.23±0.65 |
0.475±0.012 0.438±0.015 0.441±0.015 0.514±0.036 0.523±0.019 0.584±0.017 0.483±0.023 0.503±0.023 0.515±0.023 0.523±0.032 0.532±0.017 0.513±0.020 0.493±0.053 |
0.562±0.017 0.499±0.019 0.503±0.019 0.589±0.047 0.601±0.024 0.551±0.021 0.525±0.026 0.546±0.028 0.529±0.027 0.590±0.041 0.601±0.21 0.594±0.028 0.582±0.050 |
13.83±0.40 12.28±0.35 12.36±0.44 12.87±0.90 13.02±0.45 12.27±0.32 7.98±0.24 7.84±0.53 8.12±0.32 11.31±0.70 11.51±0.35 13.59±0.65 15.40±2.02 |
1.161±0.005 1.138±0.002 1.140±0.005 1.146±0.011 1.148±0.004 1.139±0.002 1.086±0.002 1.084±0.006 1.088±0.003 1.127±0.008 1.129±0.004 1.157±0.008 1.181±0.02 |
Mean±SD; n=3
Table 3: Angle of repose, bulk density, tap density, Carr’s index and Hausner ratio of solid SEDDS formulations
The compressibility index of the formulations F7-F9 was found to be lowest whereas that of F13 was found to be highest. Formulations F7-F9 showed excellent flow properties whereas F13 showed good flow properties as per compressibility index. This further reaffirms the results obtained by angle of repose studies. Similarly the Hausner ratio values obtained in these studies indicated that formulations F7-F9 have excellent flow character whereas rest of the formulations possessed good flow character. These studies were conducted in triplicate.
Irrespective of the concentration of oil, surfactant and co-surfactant the drug content in the solid SEDDS formulations was found to be in the range of 95.79 to 101.01 % (p<0.05, t-test) indicating uniform dispersion of the drug in the solid SEDDS (Table 4). The drug content of the samples was determined in triplicate. The disintegration test carried out on the formulations showed that all the formulations disintegrated in less than 5 min. Therefore the solid SEDDS formulation passed the disintegration test. The test was carried out in triplicate. (Table 4)
Formulation |
Percent drug content | Disintegration time | Percent drug release |
|---|---|---|---|
| F1 F2 F6 F9 F11 F13 |
98.17±1.80 96.46±1.06 95.79±0.87 99.43±1.39 101.01±0.94 98.35±0.93 |
Less than 5 min Less than 5 min Less than 5 min Less than 5 min Less than 5 min Less than 5 min |
99.64±0.96 98.10±1.66 96.40±1.87 100.56±1.75 98.71±1.62 99.33±1.16 |
Mean±SD; n=3
Table 4: Drug content, disintegration and in vitro dissolution of various solid SEDDS formulations
Though as per the ICH guidelines for new products, which are rapidly dissolving (dissolution >80 % in 15 min at pH 1.2, 4.0 and 6.8) products and contain drugs, which are highly soluble throughout the physiological range, in vitro dissolution test is not mandatory but then also the test was carried out to determine and assess the dissolution of the drug. Single-point measurements are normally considered to be suitable for immediate-release dosage. It was observed that all the formulations i.e. F1, F2, F6, F9, F11 and F 13 released the drug within 15 min (p<0.05, t-test, Table 4). The test was carried out in triplicate. From the results of the dissolution and disintegration studies it is evident that the solid SEDDS formulation and its excipients do not have any negative effect on the solubility of the drug.
Intestinal permeability is one of the major factors that govern bioavailability, therefore permeability studies are a strong indicator of bioavailability. Solid SEDDS formulation F1 was chosen for permeability studies since its globule size was the smallest. Diffusion profile of the solid SEDDS F1 was compared with diffusion profile of bambuterol hydrochloride dissolved in distilled water. After 2 h, 10.87±0.43 % of the drug diffused from the solid SEDDS F1 whereas from the drug solution the diffusion was found to be 10.02± 0.35 %. (p<0.05, t-test; Table 5). After 2 h, the amount of drug diffused out of solid SEDDS F1 is 1.08 times more than that from bambuterol solution.
Time (min) |
Percent CDP Drug solution |
Percent CDP Solid SEDDS F1 |
|---|---|---|
| 0 30 60 90 120 |
0 4.02±0.13 6.87±0.28 8.99±0.21 10.02±0.35 |
0 3.92±0.11 6.98±0.18 9.23±0.39 10.87±0.43 |
CDP is cumulative drug permeability; Mean±SD; n=3
Table 5: Comparative cumulative drug permeability from drug solution and solid SEDDS F1
Compared with other oral and peroral drug formulations, a drug dissolved in an aqueous solution is the most bioavailable and consistent form. Peroral drug solutions are often used as the reference preparation for solid peroral formulations[34]. The ex vivo studies of the solid SEDDS indicated that formulation F1 has slightly better permeability than bambuterol dissolved in distilled water. Therefore it can be concluded from these studies that solid SEDDS formulation F1 containing bambuterol would have greater bioavailability than ordinary solid oral formulation of bambuterol. Stability studies of solid SEDDS were conducted at 25°±2°/60± 5 % relative humidity. The solid SEDDS were found stable up to 90 d as there was very negligible change in drug content over 90 d.
In this study, solid SEDDS of bambuterol hydrochloride, which has poor bioavailability was formulated successfully by adsorption method for oral administration. Components of the solid SEDDS formulation and their ratios were obtained by solubility studies and pseudoternary phase diagram. Morphology, globule size, zeta potential, micromeritic properties, disintegration test, dissolution test, drug content and loading, stability and ex vivo permeability of the solid SEDDS formulations were evaluated. The solid SEDDS formulation F1 containing 10 mg bambuterol hydrochloride, triacetin (12.50 % w/w), Tween 80 (43.75 % w/w) and ethanol (43.75 % w/w) was concluded to be the best. The optimized solid SEDDS not only showed optimum globule size, zeta potential, and drug content but also had good flow character, and was found to be stable. These studies indicated that there was no chemical interaction between the adsorbent and the drug and further the drug was properly adsorbed on the surface of the adsorbent. The optimized solid SEDDS showed slightly increased permeability of the drug across the intestinal membrane when compared to drug solution, in ex vivo studies. These results suggested that bambuterol hydrochloride can be formulated as solid SEDDS, which could be used to improve the oral bioavailability of bambuterol hydrochloride.
Acknowledgments:
The authors thank the Chandigarh University, Mohali, Punjab and Gyani Inder Singh Institute of Professional Studies, Dehradun for providing the necessary facilities for carrying out these studies and experiments. The authors are grateful to Dr. Jitender Singh, UIPS, Chandigarh University, Mohali, Punjab for DSC and Dr. Anupinder Singh, Dept. of Physics, GNDU, Amritsar for SEM studies.
References
- Pouton CW. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci 2006;29(3-4):278-87.
- Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res 1995;12(11):1562-72.
- Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm 2000;50(1):179-88.
- Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother 2004;58:173-82.
- Đekić L, Primorac M. Microemulsion and Nanoemulsions as carrier for delivery of NSAIDS. In: Čalija B, editor. Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs. Formulation Challenges and Potential Benefits. Cambridge, MA: Academic Press; 2017. p. 69-94.
- Pouton CW. SEDDS: Assessment of the efficiency of emulsification. Int J Pharm 1985;27;335-48.
- Shah NH, Carvajal MT, Patel CI, Infeld MH, Malick AW. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzedglycerides for improving in vitro dissolution and oral absorption of lipophillic drugs. Int J Pharm 1994;106:15-23.
- Chitneni M, Peh KK, Darwis D, Abdulkarim M, Abdullah GZ, Qureshi MJ. Intestinal Permeability Studies of Sulpiride incorporated into Self-Micro emulsifying Drug Delivery System (SMEDDS). Pak J Pharm Sci 2011;24(2):113-21.
- Wasan KM. Formulation and physiological and biopharmaceutical issues in the development of oral lipid-based drug delivery systems. Drug Dev Ind Pharm 2001;27:267-76.
- Serajuddin AT, Sheen PC, Mufson D, Bernstein DF, Augustine MA. Effect of vehicle amphiphilicity on the dissolution and bioavailability of a poorly water-soluble drug from solid dispersions. J Pharm Sci 1988;77(5):414-7.
- Porter CJ, Charman WN. In vitro assessment of oral lipid based formulations. Adv Drug Deliv Rev 2001;50(suppl 1):127-47.
- Chen ML. Lipid excipients and delivery systems for pharmaceutical development: A regulatory perspective. Adv Drug Deliv Rev 2008;60:768-77.
- British Pharmacopoeia. Vol. 1. London, United Kingdom: The British Pharmacopoeia Commission; 2001.
- Rosenberg J, Larsson P, Nyberg L. Pharmacokinetics of bambuterol during oral administration of plain tablets and solution to healthy adults. Br J Clin Pharmacol 2000;49(3):199-206.
- Nyberg L, Rosenborg J, Weibull E, Nilsson M, Jönsson S, Kennedy BM. Pharmacokinetics of bambuterol in healthy subjects. Br J Clin Pharmacol 1998;45:471-8.
- Sabale V, Vora S. Formulation and evaluation of microemulsion-based hydrogel for topical delivery. Int J Pharm Investig 2012;2:140-9.
- Gaikwad S, Godbole M, Potnis V, Daud A. Formulation and Evaluation of Self-Emulsifying Drug Delivery System of Orlistat. Am J PharmTech Res 2012;2:297-311.
- Jaiswal P, Aggarwal G, Harikumar SL, Singh K. Development of self-microemulsifying drug delivery system and solid-self-microemulsifying drug delivery system of telmisartan. Int J Pharm Investig 2014;4:195-206.
- Deshmukh A, Kulkarni S. Novel Self Micro- emulsifying drug delivery System (SMEDDS) of Efavirez. J Chem Pharm Res 2012;4:3914-9.
- Modi J. Formulation and evaluation of Nanoemulsion based drug delivery of NSAIDS [dissertation]. Jodhpur, Rajasthan: Jodhpur National University; 2012.
- Ahmad J, Amin S, Kohli K, Mir SR. Construction of Pseudoternary phase diagram and its evaluation: Development of dispersible oral formulation. Int J Drug Dev Res 2013;5:84-90.
- Syed HK, Peh KK. Identification of Phases of Various Oil, Surfactant/Co-Surfactants and Water System by Ternary Phase Diagram. Acta Pol Pharm 2014;71(suppl 2):301-9.
- Prajapati ST, Joshi HA, Patel CN. Preparation and Characterization of Self micro-emulsifying drug delivery system of Olmesartan Medoxomil for Bioavailability Improvement. J Pharm 2013;2013:728425.
- United States Pharmacopeia and National Formulary (USP 28-NF 23). Rockville, MD: United States Pharmacopeial Convention; 2005.
- Marshal K. Compression and Consolidation of powdered solids. In: Leon L, Liberman H, Joseph K, editors. The theory and practice of Industrial Pharmacy. 3rd ed. Mumbai: Varghese Publication House; 1987. p. 67.
- Martin A, Swarbick J, Cammarata A. Physical Chemical Principles in the Pharmaceutical Sciences. 3rd ed. Mumbai: K.M. Varghese Company; 1991.
- ICH harmonised tripartite guideline. Specifications: test procedures and acceptance criteria for new drug substances and new drug products: chemical substances Q6A. October 1999. Available from: https://www.ich.org/products/guidelines/quality/article/quality-guidelines.html.
- World Health Organization. The International Pharmacopoeia: Dissolution testing of tablets and capsules. World Health Organization, Dept. of Essential Medicines and Pharmaceutical Policies; 2016. Available from: http://apps.who.int/phint/pdf/b/10.3.1.Dissolution-testing-of-tablets-and-capsules.pdf.
- Liu W, Pan H, Zhang C, Zhao L, Zhao R, Zhu Y, et al. Developments in Methods for Measuring the Intestinal Absorption of Nanoparticle-Bound Drugs. Int J Mol Sci 2016;17:1171.
- USFDA. Waiver of in vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System: Guidance for Industry. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER); 2017. Available from: https://www.fda.gov/media/70963/download.
- Jang DJ, Jeong EJ, Lee HM, Kim BC, Lim SJ, Kim CK, et al. Improvement of bioavailability and photostability of amlodipine using redispersible dry emulsion. Eur J Pharm Sci 2006;28:405-11.
- Raval M, Patel J, Patel A, Sheth N. Formulation and development of a self- nanoemulsifying drug delivery system of irbesartan. J Adv Pharm Technol Res 2011;2(1):9-16.
- Charman SA, Charman WN, Rogge MC, Wilson TD, Dutko FJ, Pouton CW. Self-emulsifying drug delivery systems: Formulation and biopharmaceutic evaluation of an investigational lipophilic compound. Pharm Res 1992;9(1):87-93.
- Block LH. Biopharmaceutics and drug delivery systems. In: Shargel L, Mutnick AH, Souney PF, Swanson LN, editors. Comprehensive Pharmacy Review. 7th ed. Philadelphia: Lippincott William and Wilkins; 2010. p. 89-90.
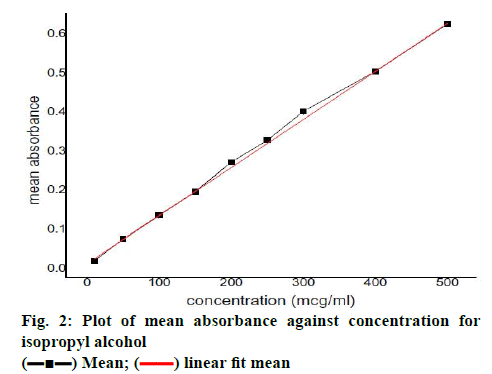
 ) Mean; (
) Mean; ( ) linear fit mean
) linear fit mean