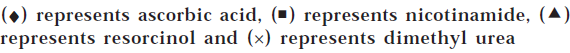- *Corresponding Author:
- N. K. Jain
Department of Pharmaceutical Sciences, Dr. H. S. Gour University, Sagar-470 003, India.
E-mail: jnarendr@yahoo.co.in
| Date of Submission | 02 May 2005 |
| Date of Revision | 18 February 2006 |
| Date of Acceptance | 06 September 2006 |
| Indian J Pharm Sci 2006, 68 (5): 608-614 |
Abstract
Acquired immunodeficiency syndrome (AIDS) pandemic is one of the biggest challenges of the 21st century. With the development of antiretroviral therapy, the count of human immunodeficiency virus (HIV)-infected people may decrease to a certain extent. Presently available formulations for this disease are found not to be very useful due to poor bioavailability, leading to poor efficacy, various side effects and high cost. In the present investigation, it was proposed to formulate the aqueous injection of saquinavir, which should definitely be more effective, economical, safe and with the least side effects as compared to its existing dosage forms, e.g., hard and soft gelatin capsule. The solubilization of saquinavir (antiHIV drug), practically insoluble in water, by means of physiologically active hydrotropes and cosolvents has been investigated. The results indicate that enhancement in solubility of saquinavir in the presence of hydrotrope at low concentration is due to weak ionic interaction. At higher concentrations (>0.4 M), complexation is found to be the probable mechanism for solubility enhancement by nicotinamide but nature of complex formed is not clear; whereas for ascorbic acid, self-association is the probable mechanism at these concentrations. Using these two approaches, various formulations of saquinavir were developed, and haemolysis study and dilution study of these formulations were carried out. Formulation containing nicotinamide as hydrotrope showed promising results.
Human immunodeficiency virus (HIV) infection and its clinical syndrome acquired immunodeficiency syndrome (AIDS) continue to be a major health problem worldwide. The development of highly effective antiretroviral chemotherapy for AIDS is found veryuseful in curbing the pandemic of this disease. Saquinavir (SQ, first protease inhibitor) is a transition state analogue peptide based inhibitor of the HIV protease [1]. It is a potent inhibitor of HIV replication in vitro and suppresses viral load and increases CD4+ cell counts in HIV patients in vivo [2]. Major drawback of protease inhibitors is their high cost; and for SQ, its low and variable oral bioavailability, which is approximately 4% in the fed and 1% in the fasted state [3]. Since the effect of SQ on viral replication is dose dependent and the therapy with SQ is expensive, there is an urgent need for methods to increase its bioavailability. In this present work, efforts were made to develop an economical and effective parenteral formulation of SQ with increased bioavailability and one that eliminated side effects related with oral administration of SQ. The main constraint in the development of aqueous formulation was poor solubility of drug (30 mg/l) [4]. Solubility enhancement was carried out using various cosolvents and hydrotropes.
Hydrotropy is a term originally coined by Neuberg [5] to describe the increase in the solubility of a solute by the addition of fairly high concentration of alkali metal salts of various organic acids. Saleh et al. [6] made an attempt to extend the definition of “hydrotropic agents” to include cationic and non-ionic organic compounds bearing the essential structural features of Neuberg’s hydrotropes. Hydrotropic solubilization of nifedipine [7], carbamazepine [8], ketoprofen [9] piroxicam [10], flurbiprofen [11] and nimesulide [12] has been reported by our team.
The solubility of weak electrolyte and non-polar molecules can be increased by the addition of water-miscible solvent in which drug has good solubility. This process is known as “cosolvency,” and solvents used in the combination to increase the solubility of solute are known as “cosolvents” [13]. It is proposed that the cosolvent systems work by reducing the interfacial tension between predominately aqueous solution and hydrophobic solute [14]. In the present work, aqueous injection of SQ was formulated, and haemolysis study and dilution study of these formulations were carried out. Similar efforts have been reported for carbamazipine [8], ketoporfen [15,16], piroxicxam [10] and flurbiprofen [11] by our team.
Materials and Methods
Saquinavir (Hoffman La Roche, Basel, Switzerland); ascorbic acid and nicotinamide (Loba Chemie Indoaustranol Co., Mumbai, India); resorcinol (Rankem, Ranbaxy Fine Chemical Limited, New Delhi, India); methanol (Qualigens Fine Chemical, Mumbai, India); sodium bisulfite [E. Merck (I) Ltd., Mumbai, India]; aluminium seals glass vials (10, 30 ml), rubber plugs (Modern Labs, Indore, India); membrane filters (0.2 μm) (Sartorius, Germany); membrane filters (0.45 μm) (W and R, Balgton Ltd., England) were used. Water used was double distilled in an all-glass apparatus. Hydrotrope concentrations used for present investigation were 0.1-1.2 M.
Determination of equilibrium solubility
Excess of SQ was added to screw-capped 10 ml amber-coloured glass bottles containing fixed volumes (2 ml) of the hydrotrope, cosolvent and various phosphate buffer saline (PBS) solutions (pH 2, 4, 6.4, 8 and 9), separately. These bottles were shaken mechanically (Toshniwal, Mumbai, India) at 25±2° in a constant temperature bath for 24 h. These mixtures were allowed to equilibrate for the next 12 h and then centrifuged for 3 min at 2000 rpm at 37±2°. The supernatant was filtered through 0.45 μm membrane (Millipore, Red Ford, MA, USA). An aliquot of the filtrate was diluted with water and the resulting solutions were analyzed spectrophotometrically at 239 nm. A similar procedure was followed for solubility determinations at 37±2°.
Studies for illustration of mechanism of solubilization
The UV spectra of SQ (λmax values) in fresh double-distilled water, hydrotrope solution and mixture of SQ and hydrotrope were recorded on a double-beam spectrophotometer (GBC, Cintra 10). The pH of different hydrotropes alone and in the presence of drug was measured at 25±2° using a digital pH meter (Elico Pvt. Ltd., Mumbai, India). The specific gravity of different hydrotrope solutions was determined at 25±2° using a 10 ml glass pyknometer [ASGI (I) Industry, Agra, India], considering water as a reference liquid. The viscosity of different solutions was determined relative to water at an ambient temperature of 25±2° using an Ostwald viscometer [ASGI (I) Industry, Agra, India]. The surface tension of different hydrotrope solutions was determined at 25±2° using stalagmometer [ASGI (I) Industry, Agra, India]. The refractive index of different hydrotrope solutions was determined at 25±2° using an Abbe refractometer (Carl Zeiss, Germany). The resistance of different hydrotrope solutions was determined using a conductivity bridge. Observations were recorded after the solutions had equilibrated in a water bath at 25±2° (Table 1). TLC study of SQ, different hydrotropes, solubilized products of drug and hydrotropes was done. Plates of silica gel GF254 were activated for 1 h at 110±2° and various solvent systems, detection reagents were used for visualization of spots [17]. Sample of SQ and different hydrotropes, physical mixtures (1:1) of hydrotropes with SQ and solubilized products, all previously vacuum-dried for 24 h, were subjected to IR spectroscopic studies. Five milligrams of each sample was mixed with about 100 milligrams of potassium bromide (vacuum-dried for 24 h) and compressed as pellets, and spectra were recorded on IR spectrophotometer.
| Solution property | Hydrotrope | |||
|---|---|---|---|---|
| A A | NIC | RL | DMU | |
| Break point concentration | 0.2 M | 0.4 M | 0.2 M | X |
| Specific gravity | - | - | - | - |
| Relative viscosity | + | + | + | + |
| Surface tension | ! | ! | ! | ! |
| Refractive index | - | - | - | - |
| Specific conductance | - | - | - | - |
| Equivalent conductance | - | - | - | - |
Table 1: Properties of Hydrotropes
Formulation of aqueous injection
Optimization of solubility-enhancement with intravenous dose of drug (12 mg) [18] was done. On the basis of results obtained from optimization studies (Table 2), aqueous injections of SQ using cosolvents and hydrotropes were formulated. In each case, except formulation containing ascorbic acid, 0.1% (w/v) sodium bisulfite was added to preclude the chances of oxidation. Methyl and propylparaben (0.018% and 0.02%) were added as preservative in each formulation.
| Solubilizing agent | Solubility enhancementa |
Optimized concentration |
|---|---|---|
| Ascorbic acid Nicotinamide DMA PEG 200 PEG 200, ethanol |
6.84 12.64 7.67 7.00 4.8 |
0.1 M 0.8 M 60% 40% PEG 200 40%, ethanol 20% |
Table 2: Optimization of Cosolvents and Hydrotropes
Stability testing
The sealed vials of the selected formulations were visually inspected daily for 45 d under fluorescent light against black and white background to detect any changes in physical appearance of the solutions, e.g., colour, turbidity, precipitation, etc. These studies were performed under room temperature in dark (RTD), freezing temperature in dark (FTD) and temperature cycling with shaking (TCS).
On the basis of the results of simultaneous physical stability testing, the promising formulations were further subjected to exhaustive chemical stability tests at 45±2°, 55±2° and 65±2° for a period of 4 w.
Haemolysis study
Haemolysis study (Table 3) on various formulations was performed by colorimetric methods [16] . Different formulations of SQ were incubated with 0.5 ml suspension of RBC at 25±2° for 45 min after making volume up to 10 ml with normal saline.
| Volume in ml of formulation (drug concentrationb) |
Haemolysis (%) in formulationa | |||
|---|---|---|---|---|
| SQAAc | SQNICc | QPEGETd | SQDMAd | |
| 1 | 1.7 | 2.65 | 3.10 | 2.47 |
| 2 | 1.78 | 2.76 | 3.9 | 4.3 |
| 3 | 1.92 | 3.42 | 4.7 | 4.8 |
Table 3: Haemolysis Study in Different Formulations
Dilution study
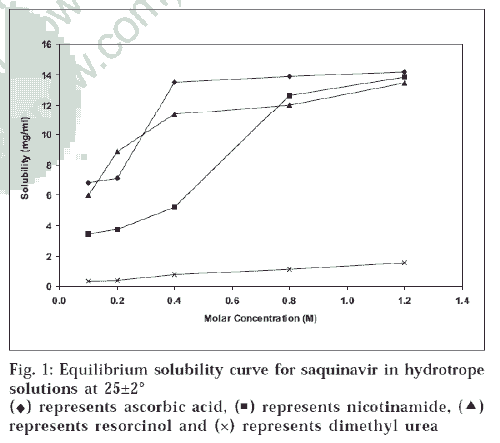
In the present study, all four formulations of SQ were studied for effect of dilution with infusion fluids such as dimethyl urea at 25±2°. The solubility also increased by increasing temperature from 25±2° to 37±2°. This shows that solubilization of SQ is endothermic. Solubilizing power of hydrotropes could be ranked as ascorbic acid>nicotinamide>resorcinol>dimethyl urea. In a similar fashion, solubilizing power of different cosolvents could be ranked as DMF>DMA>PEG200 (fig. 2). Equilibrium solubility diagram of nicotinamide depicts slight rise in the solubility, but after 0.4 M concentration there is PEG + ethanol, and DMA cosolvents respectively normal saline and 5% dextrose solution (Table 4). The serial dilutions of formulations were prepared in ratio of 1:5 to 1:100 and examined visually for the appearance of microcrystals.
| Dilution | Time (h) for first precipitation to occur after dilution with | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal saline | 5% w/v dextrose solution | ||||||||
| SQAAa | SQNICa | SQPEGETb | SQDMAb | SQAAa | SQNICa | SQPEGETb | SQDMAb | ||
| 1:5 | 6 | 3 | 2 | 1 | 3 | 6 | 1 | 1 | |
| 1:10 | 24 | 6 | 2 | 1 | 6 | 6 | 1 | 1 | |
| 1:30 | - | 24 | 2 | 2 | 6 | 24 | 2 | 1 | |
| 1:50 | - | - | 6 | 3 | 24 | 24 | 6 | 3 | |
| 1:70 | - | - | - | 6 | - | - | - | 4 | |
| 1:90 | - | - | - | - | - | - | - | - | |
| 1:100 | - | - | - | - | - | - | - | - | |
Table 4: Dilution Study in Different Formulations
Results and Discussion
Equilibrium solubility determinations in different hydrotropic agents in 0.1, 0.2, 0.4, 0.8 and 1.2 M were carried out. Equilibrium solubility diagram (fig. 1) of SQ in hydrotrope solutions showed 473-fold solubility enhancement with ascorbic acid, 462-fold with nicotinamide, 449-fold with resorcinol and 52-fold with dimethyl urea at 25±2°. The solubility also increased by increasing temperature from 25±2° to 37±2°. This shows that solubilization of SQ is endothermic. Solubilizing power of hydrotropes could be ranked as ascorbic acid>nicotinamide>resorcinol>dimethyl urea. In a similar fashion, solubilizing power of different cosolvents could be ranked as DMF>DMA>PEG200 (fig. 2).
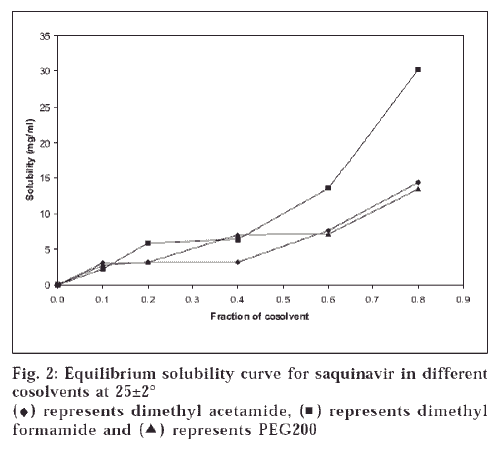
Equilibrium solubility diagram of nicotinamide depicts slight rise in the solubility, but after 0.4 M concentration there is fromsteep rise in solubility; while ascorbic acid shows steep rise in the solubility at 0.2 M (fig.1). Therefore, increase in solubility is not a linear function of hydrotrope concentration. To illustrate the current trends of solubility and understand the mechanism of solubilization, molecular structures of drug and these hydrotropes were studied (fig. 3). Centres with different electronegativities in SQ are (a) N in ring, (b) CONH, (c) OH; in nicotinamide are (a) pyridine N2, (b) CONH; and in ascorbic acid are (a) COOH, (b) hetroatomic ring with oxygen. These molecules may interact due to difference in electronegativity at these centres of drug and hydrotrope molecules. These interactions may be due to charge transfer phenomenon. Such weak interactions lead to planer stacking of ring system present in SQ, which causes bending of hydrophobic ring to the inside such that more hydrophilic groups are exposed to water molecules, which may account for initial linear increase in the solubility.
In order to understand the sharp increase in the solubility of SQ above certain concentration, which is defined as critical solute concentration (CSC), various solution properties like specific gravity, relative viscosity, surface tension, specific conductance, equivalent conductance of hydrotrope solution were studied (Table 1). At higher concentration positive deviation from linearity is characteristic of hydrotrope solubilization. This positive deviation in plots suggests that more than one molecule of hydrotrope can react with individual drug molecule19. Whether this aggregate formation is complexation or simple self-association can be determined from change in above-mentioned solution properties and also with the help of IR, TLC and UV spectral studies.
Negative deviation in specific gravity value was due to increase in partial molal volume upon aggregation [20], suggesting aggregate formation. Viscosity plots of hydrotropes showed positive deviation. This again indicates the increase in viscosity is due to aggregate formation [21]. Similarly, negative deviation of refractive index clearly indicates the possibility of some molecular aggregation. Apparent discontinuity (specific conductance) and decrease in conductance (equivalent conductance) are indicative of molecular aggregation [22]. Hydrotrope solutions showed decrease in surface tension in all cases but least with nicotinamide. The lowering of surface tension is due to self-association and thus supports the possibility of self-association at higher concentration in ascorbic acid vis-à-vis ruling out the possibility of same in case of nicotinamide. It was reported in literature that derivatives of ascorbic acid undergo self-association and form micelles, thus dissolving many hydrophobic drugs [23,24].
The increase in solubility of SQ in ascorbic acid can be partially attributed to pH effect as SQ solubility determined in various phosphate buffers saline ranging from pH 2-9 varied to a considerable extent, and higher solubility was observed in acidic pH. This may be due to basic nature of SQ.
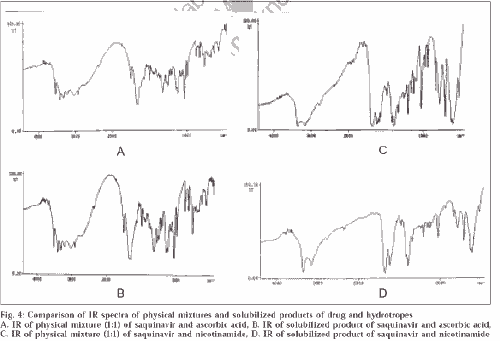
UV study of ascorbic acid and drug showed no shift in λmax, which rules out the chance of permanent interaction. There was no basis for assuming that there was any complex formed between the drug and hydrotrope molecules, because the complex formation is evident by formation of new chromophore (by appearance of new peak or merging of two peaks to generate a common peak). The TLC study showed two different spots of drug and ascorbic acid at these concentrations, confirming that no permanent interaction takes place and this aggregate formation could be only due to self-association. Moreover, unchanged IR spectra (fig. 4) of solubilized product of SQ and ascorbic acid to physical mixture (1:1, SQ and ascorbic acid) confirmed that no permanent chemical interaction takes place and aggregate formation is simple self-association.
Figure 4: Comparison of IR spectra of physical mixtures and solubilized products of drug and hydrotropes
A. IR of physical mixture (1:1) of saquinavir and ascorbic acid, B. IR of solubilized product of saquinavir and ascorbic acid, C. IR of physical mixture (1:1) of saquinavir and nicotinamide, D. IR of solubilized product of saquinavir and nicotinamide
To understand the aggregation phenomenon in nicotinamide, some specific studies like UV, TLC and IR study were carried out. In UV study of these solutions at different concentrations, a shift in λmax from 262 (0.1 M) to 289 (0.8 M and higher) was observed, suggesting that complexation might have occurred. IR and TLC studies confirmed that complexation had occurred. The result of TLC study revealed single spot of solubilized product at higher concentration, which confirmed that complex formation had occurred. If this were not the case, we would have got two separate spots corresponding to Rfvalue of each.
IR studies confirmed that complex formation had occurred. The changes in IR spectra of solubilized product of saquinavir and nicotinamide occurred mainly between wave numbers 3800-3600 and 1600-1400. IR spectra of solubilized product showed very few peaks of O-H stretch of saquinavir at 3836, 3787, 3745 and 3698, while equimolar physical mixture showed large number of peaks at 3867, 3856, 3851, 3845, 3835, 3824, 3799, 3742, 3737, 3714, 3673, 3651 and 3646. The other group affected was primary amide of both nicotinamide and saquinavir. Similar changes in number of peaks were observed for N-H stretch at 3300-3100; and C=C, C=N stretch at 1600-1400. The comparison with the IR of physical mixture confirmed that these differences were only due to complex formation and not because of simple overlapping in solubilized product (fig. 4).
Thus, complexation played a critical role in solubility enhancement of SQ by nicotinamide, which is consistent with the findings of many investigators [25-28]. But the type of complex formed, whether 1:1, 1:2 or higher, was not clear.
Four formulations were developed using ascorbic acid, nicotinamide, DMA ethanol and PEG200. Additives were added as per requirement of the formulation. Methyl paraben and propyl paraben (0.018% w/v and 0.02% w/v), generally accepted preservatives for injection formulation in their standard concentration [29], were added. Sodium bisulphate (0.1% w/v) is generally recommended for intermediate pH ranges and was therefore used in the present formulations. Formulation containing ascorbic acid as hydrotrope (SQAA) did not contain any antioxidant since ascorbic acid (solubilizing agent) also functions as antioxidant.
Tonicity adjustments were made in order to produce isotonic solution using molecular weight method [30]. Sodium chloride was used for tonicity adjustment. Formulations I and II did not contain any tonicity adjuster since they were hypertonic; thus they could be used without any problem. No buffering agents were used since drug showed good stability at all pH; moreover, it might affect the solubility of formulations. Solubility studies of SQ showed higher solubility in ascorbic acid and nicotinamide; therefore, these were selected for formulation development. Nicotinamide is a nontoxic vitamin (vitamin B5) that has been shown to enhance solubilities of many drugs [25,31,32] and hence was used for
Similarly, ascorbic acid (vitamin C) was used in injection formulation for solubility enhancement. Again this is a vitamin that may be beneficial as a vitamin supplement. Antioxidant nature could impart considerable stability to the formulation.
In case of cosolvent solubilization, SQ was found to have good solubility in DMF, DMA, PEG200 and ethanol. DMA, PEG200 and ethanol were used for formulation development. Although drug showed the highest solubility enhancement in DMF, yet the use of this cosolvent has not been justified in parenteral formulation. The vehicle should contain a minimum amount of these agents to reduce toxicity and difficulties with respect to blood compatibility and injection. The safe concentrations of ethanol, propylene glycol and PEG200 are 5-50, 32 and 50% respectively in isotonic saline solutions [33-35]. Therefore ternary blend of cosolvents [36] for optimizing these factors was used in injection formulation containing PEG200 and ethanol as cosolvents (SQPEGET).
All four formulations were subjected to physical and chemical testing. Physical stability testing under different stress conditions to study the effect of light, freezing temperature, freeze thaw cycling on the formulated injection was performed. The observations were made in respect of change in colour and occurrence of precipitate. It was observed that all formulations were stable to colour change.
Formulation SQDMA showed maximum precipitate formation. The earliest precipitate appeared in freeze thaw cycling with shaking after 7 d in SQDMA, while the same formulation showed considerable stability at room temperature and precipitate formation occurred after 19 d. Similarly, at low temperature (4°), first precipitate in the formulation was observed after 13 d. The possible reason for precipitate formation is the presence of nuclei in any solution, and these nuclei are expected to give rise to crystal growth. Simultaneously, these formulations were subjected to accelerated stability studies at temperatures 45°, 55° and 65°. The stability study testing was based on simplified technique of testing for liquid formulation at higher temperature for longer period [37] There is no significant change in potency of all four formulations SQNIC, SQPEGET, SQDMA, and SQAA after 145, 120, 105 and 104 d respectively.
All formulations were subjected to in vitro haemolytic study using rabbit blood. The degree of haemolysis was estimated by colorimetric method. The drug and additives required to design a dosage form may have haemolytic effect [34,35,38]. Hence haemolytic studies were carried out on the additives that were used for solubilization [16]. Haemolytic behaviour of whole formulations was studied and reported (Table 3). Haemolytic effects of all four formulations were found to be in the order SQAA<SQNIC<SQPEGET<SQDMA. The effects observed were insignificant because during administration injection is diluted to nearly 25 times.
For a drug to be therapeutically active, the concentration at the site of administration should exceed its aqueous solubility. Precipitation of drug upon injecting a solubilized formulation into body fluids often occurs [39-41]. This ultimately results in poor patient compliance. The amount of precipitation can be correlated with the rate at which the drug is injected. Any remedy which reduces or eliminates precipitation ensures more efficacious formulation. Method for determination of such effect is dilution [16]. Formulation containing cosolvent showed more precipitation in comparison to hydrotrope containing formulation on dilution (Table 4). The aqueous nature of hydrotropes may account for this type of behaviour; moreover, for higher dilution absence of precipitation is redissolution of these precipitates. Formulation SQDMA showed marked turbidity that did not disappear even at higher dilution, while formulation SQPEGET showed lesser degree of precipitate formation than formulation SQDMA. The decreasing order of precipitate formation was found as SQDMA>SQPEGET>SQAA>SQNIC.
Acknowledgements
Authors thank M/s Hoffman La Roche, Basel, Switzerland, for providing gift sample of SQ; and University Grants Commission, New Delhi, India, for financial support.
References
- Roberts, N.A., Martin IA_ Handa, B.K., Kay, J. and Krohn, A., Science, 1990, 248, 358.
- Mohan, P. and Baba, M., In; AntiAIDS drugs development: Challenges strategies and prospects, 1st Edn., Harwood Academic Publishers, USA, 1998, 93.
- Noble, S. and Faulds, D., Drugs, 1996, 52, 93.
- Budavari, S., Eds., In; The Merck Index, l2th Edn., Merck & Co.. Inc., Whitehouse Station, NJ,1996,1816.
- Neuberg, C., Biochem. Z, 1916, 76, 107.
- Saleh, A.M. and El-Khordagui, L.K., Int. J. Pharm., 1985, 20231.1
- Jain, N.K. and Jahagirdar, A., Pharmazie, 1989, 44. 727
- Jain, N.K., Agrawal, R.K. and Singhai, A.K., Die Pharmzie. 1990. 45, 223.
- Jain, N.K., Jain, S. and Singhai, A.K.. Die Pharmazie, 1996. 51. 151.
- Jain. N.K., Jain, S. and Singhai, A.K., Die Pharmazie. 1997. 52. 12.
- Gupta, G.D., Jain, S. and Jain, N.K., Die Pharmazie. 1997. 52. 621.
- Agrawal, S., Pancholi, S.S., Jain, /4,1Cald Agrawal. G.P., Ind J.. Pharm., 2004, 274, 149.
- Yalkowsky, S.H., Amidon, G Xografi, tittlirFly*L7, J. Pharm. Sci., 1975, 64, 48.
- Yalkowsky, S.H., and Rubino. J.T., J. Pharm. Sci., 1985. 74, 416.
- Singhai, A., Jain, S. andstain. N.K.. Die Pharmazie, 1997a. 52, 226.
- Singhai, A.K., Jain,,S;and Jain. N.K.. Die Pharmazie, 19976, 52, 149.
- Stall, E., In; Thin Layer Chromatography, 2nd Edn., Springer International Student Edition. Published by Toppers Company Limited, Japan, 1969,54, 299.
- Ha, H.R., Follath, F., Bloemhard, Y. and Krahenbuhl, S., J.Chromatogr. B, 1997, 694, 427.
- Poochikian, G.K. and Cradock, IC., J. Pharm. Sci., 1979, 68, 728.
- Badwan, A.A. and El-Khordagui, L.K., Int. J. Pharm., 1983 13, 67.
- Thomas, L.H., J. Chem. Soc., 1960, 4914.
- Mukerjee, A.T., Adv. Colloid. Interface Sci., 1967, 1, 242.
- LoNostro, P., Capuzzi, G., Mulinacci, N. and Romani, A., Langmuir, 2000, 16, 1744.
- Hagao, M., Manzo, B., Auman, D., Fratoni, E. and Nostro. P., J. Pharm. Sci., 2002, 91, 1810.
- Higuchi, T. and Bolton, S., J. Amer. Pharm. Assoc. Sci., 1959, 48, 557.
- Hata, S., Miztmo. J. and Tomioka, S., Chem. Pharm. Bull., 1970, 15, 1791.
- Truelove, J.E., Bawarshi-Nassar, R., Chen, N.R. and Hussain, A., Int. J. Pharm., 1984, 19, 17.
- Fawzi, M., Davison, E. and Tute, M., J. Pharm. Sci., 1980, 69, 104.
- Indian Pharmacopoeia, 4th Edn.. Ministry of Health and Family Welfare, Govt. of India, Th9 Controller of Publication, New Delhi, 1996, 199.
- Jain, N.K. and Shama. S.N. In: Textbook of Professional Pharmacy. 3rd Edn.. Vallabh Prakashan. Delhi. 1994. 330.
- Rassol, A.A.. Htisaip, and Diittert, L.W., J. Pharm. Sci., 1991. 80. 387.
- Khalafallah. N.. El mi, F., Daabis, N, and Saleh, A., J. Drug Rest 1973. 5. 175.
- United States Pharmacopoeia, XXIII, NF XVIII, The United States , Pharmacopoeia! Convention Inc., 1995, 572.
- Cadwallader, D. Brit. J. Anaesth., 1978, 50, 81.
- Cadwallader. D.E.. Wickcliffe. B.W. and Smith, B.L., J. Pharm.,Sci., 1964. 53. 927.
- Gould. P.L., Howard. J.P. and Oldershaw. G.A.. Int. J. Pharm., 1989, 51, 195.
- Lachnian.t., Liberman. H.A., and Kanig, J.L., In; Theory and Practice of Industrial Pharmacy, 3rd Edn., Varghese Publishing House, Mumbai,1991,780
- Smith, B.L. and Cadwallader, D.E., J. Pharm. Sci., 1967, 56, 351.
- Jusko, W.J., Uretch. M. and Gassett, R., J. Amer. Med. Assoc., 1973, 225, 176.
- Korttila, K., Sotlunan, A. and Andersson, P., Acta. Pharmacol. Toxicol., 1976,139, 104. 41. Yalkowsky, S.H. and Valvani, S.C., hit. Clin. Pharm., 1977, 11, 417.
- Yalkowslcy, S.H. and Valvani, S.C., Int. Clin. Pharm., 1977. 11, 417.