- *Corresponding Author:
- A. A. Kempwade
Department of Pharmaceutics, KLE College of Pharmacy, Belagavi, Karnataka 590010, India
E-mail: kempwadeamol@rediffmail.com
| Date of Submission | 08 December 2020 |
| Date of Revision | 20 September 2021 |
| Date of Acceptance | 11 July 2022 |
| Indian J Pharm Sci 2022;84(4):863-873 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study deals with formulation and evaluation of flexible liposome embedded thermoreversible in situ intranasal gel of rizatriptan benzoate. The flexible liposomes were formulated (F1-F9) and optimized with respect to percentage of ethanol and phospholipid to get optimum vesicle size and entrapment efficiency. The vesicles were characterized for their size, shape and zeta potential. The optimized stable vesicles were incorporated into in situ thermoreversible gel. The ex vivo permeation of drug through goat nasal mucosa was studied. The nasal mucosa used for drug permeation experiment was subjected to histopathological study to check for any damages. The in vivo pharmacokinetic study was carried out in Wistar rats. Vesicles size and entrapment efficiency of stable formulations (F2-F6) was varied from 71.2±14.16 to 199.3±26.03 nm and 72.98 % to 77.44 % respectively. The liposomes were nearly spherical in shape with zeta potential varied from -14.2±4.48 to -11.7±5.26 mV. The improved drug permeation and higher flux across nasal mucosa with enhancement ratio of 2.75 was observed for flexible liposomal thermoreversible gel F2 compared to normal thermoreversible hydrogel of drug. The histopathological study of nasal mucosa revealed that there was no damage to epithelial layer and was found to be intact. The area under the curve of optimized formulation (F2) was 3.98 and 1.53 times more than oral drug solution and intranasal gel respectively, confirming improvement in bioavailability. Thus present study concludes that flexible liposome enriched thermoreversible, mucoadhesive in situ intranasal gel of rizatriptan benzoate is a safe and effective drug delivery system for treatment of migraine.
Keywords
Flexible liposomes, intranasal, rizatriptan benzoate, thermoreversible gel
Rizatriptan benzoate is a selective 5-Hydroxytryptamine (5-HT) 1B/1D receptor agonist which is commonly prescribed for treatment of migraine. Various studies to determine the etiology of migraine concluded that symptoms are due to local cranial vasodilatation and/or due to the release of vasoactive and pro-inflammatory peptides from sensory nerve ending in an activated trigeminal system. In 70 % to 80 % of patients the symptoms have found to be relieved within 2 h by rizatriptan benzoate.
Rizatriptan benzoate is having oral bioavailability of 40 %-45 %. It undergoes extensive First Pass Metabolism (FPM) by Monoamine Oxidase A (MAO-A) isoenzyme to an inactive indole acetic acid metabolite. The nausea and vomiting which are observed during migraine attack, also limits its bioavailability. Various approaches have been tried to develop an effective formulation for rizatriptan benzoate. These studies have utilized various routes for drug administration viz. buccal, transdermal and nasal[1-3]. The buccal and transdermal routes are capable of avoiding FPM, but they lack drug targeting potential. In such case, intranasal route may be a viable alternative as it has advantage of targeting the drug to the brain. Earlier studies have proved that olfactory lobe of nasal cavity has a potential to deliver the drug directly to brain bypassing Blood Brain Barrier (BBB). However, the problem associated with nasal drug delivery is lower penetrability of drug through nasal mucosa and drainage of formulation into oral cavity. Thus rizatriptan nasal spray provided faster onset of action than the oral dosage form but the bioavailability was found to be 43 %±7 %[4]. In this context, a formulation which will increase absorption of the drug by bypassing FPM, BBB; having more residence time in nasal cavity and improving permeability through nasal mucosa would be highly beneficial.
Earlier, liposomes were considered as effective drug carriers for transdermal drug delivery but some recent studies have confirmed that the conventional liposomes are less penetrated through stratum corneum and remain confined to the upper layers of the skin. Further few studies consisting of intranasal utilization of conventional liposomes. The flexible liposomes like ethosomes have shown very promising results when administered as gel formulation via transdermal route[5-10]. Flexible liposomes (ethosomes) are varying from the conventional liposomes. The conventional liposomes are rigid due to presence of cholesterol while flexible liposomes have more bilayer flexibility due to absence of cholesterol and higher percentage of ethanol (20 %-40 %). Flexibility of liposomes improves the penetration through the intercellular pores which are smaller than that of liposomes. At intermediate concentrations, ethanol increases inter vesicle repulsion by promoting the fluctuation forces of the liposomes which avoids aggregation. Thus these liposomes are more stable than that of conventional liposomes. The flexible liposome (ethosomes) gets penetrated through stratum corneum and release the drug at deeper layers in the skin. These ethosomes are also achieving therapeutic drug level in the plasma when administered transdermally. Though ethosomes are showing very promising results by transdermal route, the study with respect to intranasal route of administration is not so much entertained till date. Two studies where ultraflexible liposomes have been used for intranasal drug delivery are available[11,12]. These studies concluded that flexible liposomes are better carriers than conventional liposomes with having improved pharmacokinetic profiles of galanthamine hydrobromide and salmon calcitonin respectively. A United States (US) Patent (US 2009/0047234 A1) explains various drug formulations containing phospholipids and alcohol for intranasal drug delivery. However, these studies have not addressed the draining off problem of nasal formulation.
The residence time of formulation in nasal cavity can be increased by increasing the viscosity and mucoadhesive strength of the formulation by formulating it as mucoadhesive thermoreversible gel[13,14]. In this context, in the proposed study flexible liposome enriched in situ thermoreversible mucoadhesive intranasal gel of rizatriptan benzoate was prepared and it’s in vivo pharmacokinetic performance has been checked.
Materials and Methods
Rizatriptan benzoate was received as a gift sample from Cipla Pvt. Ltd., Mumbai. Soya lecithin and Carbopol 934P were procured from Hi-Media Lab Pvt. Ltd. Mumbai. Poloxamer 407 was procured from Sigma (St Louis, Missouri (MO)). All other chemicals were procured from local market and were of Analytical Reagent (AR) grade.
Formulation and characterization of flexible liposomes:
Flexible liposomes were prepared by ethanol injectionsonication method as described by Troitou et al.[15]. Briefly, the drug and soya lecithin were dissolved in ethanol by mixing it with magnetic stirrer. Double distilled water was added slowly in the fine stream (500 μl/min) by using syringe to make volume up to 30 ml. The dispersion was stirred for 30 min at 750 rpm using magnetic stirrer (IKA India Pvt. Ltd.). The dispersion was covered with parafilm to avoid the loss of ethanol. The temperature of the system was kept at 30° during whole process. The formulated liposomes were sonicated by probe sonicator (Rivotek Ultrasonic sonicator, Mumbai, India) at 80 % amplitude for 3 cycles of 5 min with 5 min of rest period between each cycle. The sonication was carried out in ice cold environment to avoid excessive rise in temperature of the dispersion during process. Nine different formulations (F1-F9) were formulated based on the various concentrations of soya lecithin and ethanol.
Experimental design:
The flexible liposomes were optimized with respect to the percentage of the soya lecithin and percentage of ethanol to get desired particle size and entrapment efficiency. The study was carried out by using 32 full factorial design. The concentrations used for formulations were based on preliminary studies. The percentages of ethanol (X1) and soya lecithin (X2) in final formulation were taken as independent variables while vesicle size (Y1) and entrapment efficiency (Y2) were taken as dependent variables. Three different levels were coded as -1, 0 and +1 for minimum, moderate and maximum concentrations. The actual values of these levels and different formulations prepared were as shown in Table 1. The formulations were prepared and their responses were measured.
| Formulation code | Percentage (%) of soya lecithin (w/v) | Percentage (%) of ethanol (v/v) |
|---|---|---|
| F1 | -1 | -1 |
| F2 | 0 | -1 |
| F3 | +1 | -1 |
| F4 | -1 | 0 |
| F5 | 0 | 0 |
| F6 | +1 | 0 |
| F7 | -1 | +1 |
| F8 | 0 | +1 |
| F9 | +1 | +1 |
Note: % of soya lecithin in w/v (X2): -1=2 %, 0=2.5 %, +1=3 %, % of ethanol in v/v (X1): -1=15 %, 0=20 %, +1=25 %
Table 1: Compositions of F1-F9 with their Coded Values and Actual Concentrations
The obtained data was fed to the software and were simultaneously analyzed for their best fit model viz. linear, Two-Factor Interaction (2FI) and quadratic models. The level of statistical significance (p<0.05) and lack of fit of model were obtained for determination of appropriate polynomial model. These polynomial equations were used to determine the effect of independent variables on responses.
Characterization of formulated flexible liposomes:
Particle size and zeta potential: Particle size of the formulated flexible liposomes was determined by dynamic light scattering technique using particle size analyzer (Nanotrac R-150 Ultra, Microtrac Inc.). The Polydispersity Index (PDI) was calculated to check the particle size distribution. The zeta potential of all the formulations was determined by Malvern Zetasizer.
Vesicular shape and surface morphology of flexible liposomes: The Transmission Electron Microscopy (TEM) and Atomic Force Microscopy (AFM) techniques were used to visualize the formulated liposomes. Samples were negatively stained with phosphotungstic acid solution on carbon coated grid and viewed under microscope at 10 000 to 60 000 fold magnification at an accelerating voltage of 80 kV for TEM analysis. The AFM analysis was carried out on NanosurfAG AFM at room temperature using a scanning probe microscope in non-contact mode with silicon nitride cantilevers.
Entrapment efficiency: Entrapment efficiency of formulations was determined by the centrifugation method[16]. The formulations were centrifuged at 15 000 rpm at 4° for 2 h to separate the unentrapped drug from entrapped drug. The clear supernatant which consists of unentrapped drug was separated. The settled pellets of liposomes were washed with water to remove any unentrapped drug. The settled pellets were treated with 30 % v/v Triton X-100 to break the liposomes. The liberated drug is analyzed by spectroscopically at 224 nm by using previously constructed calibration curve (linearity, range=0.5-5.0 μg/ml, R2=0.997). The percent drug entrapped was calculated by formula.
Percent entrapment efficiency=Ws/Wa×100.
Here, Wa is the amount of drug initially added and Ws is the amount of drug liberated.
Incorporation of flexible liposomes in thermoreversible gel:
The thermoreversible gel formulations of stable liposomal dispersions (F2-F6) were prepared by cold method. The concentrations of poloxamer 407 and carbopol 934 were used based on preliminary studies to convert sol into gel at intranasal conditions[17]. The carbopol 934 (0.3 % w/v) was dispersed slowly into the distilled water using stirrer. The flexible liposomes dispersion was mixed into above solution by stirrer at 1000 rpm for 30 min to get final dispersion with drug concentration of 5 mg/ml. Poloxamer 407 was added into above mixture to get its concentration 18 % w/v. The mixture was placed at 4° overnight to get clear solution. The pH of the gel was adjusted to 6.2±0.2. The physicochemical characteristics like gelation time; mucoadhesive strength and viscosity of the gel (Brookfield DV-III Ultra Brookfield engineering laboratories, Middleboro, Massachusetts (MA)) were studied. The normal thermoreversible hydrogels were prepared by same method having same polymer concentrations but instead of the vesicles, plain drug was used.
Ex vivo drug permeation through goat nasal mucosa:
Nasal tissue of sheep was obtained immediately after its sacrifice from local slaughter house. It was safely transported to laboratory by keeping it in saline phosphate buffer pH 6.4. Nasal septum was separated from underlying bony cartilage without its damage. Tissue samples were fixed on Franz diffusion cell apparatus (PermeGear Inc., United States of America (USA)) having 6 cells with effective permeation area of 1.76 cm2 and 12 ml capacity. After initial 30 min of incubation time the stable flexible liposome enriched thermoreversible gel formulations (F2-F6) and normal thermoreversible hydrogels were placed in the donor compartment (gel equivalent to 5 mg of rizatriptan). The temperature of the chambers was maintained at 34°±1° using circulating hot water bath system (Thermo Fischer scientific, Haake, S5P Newington, USA). Saline phosphate buffer pH 6.4 was used as receptor medium. 0.5 ml of sample was withdrawn at predetermined time intervals with replacement of equal volume with phosphate buffer for 8 h and amount of drug permeated was analyzed by Ultraviolet (UV) spectrophotometer at 224 nm after suitable dilution. The amount of drug permeated was determined from previously constructed calibration curve (linearity, range=0.5-5.0 μg/ml, R2=0.997).
Histopathological study of nasal mucosa:
Histopathological evaluation of the nasal tissue which was used for the ex vivo drug permeation study was done and compared with the tissue which was incubated in Phosphate Buffered Saline (PBS) (pH 6.4). Tissue was fixed on glass slide by using 10 % buffered formalin. Paraffin sections were cut on glass slides and stained with haematoxylin and eosin. These sections were examined under light microscope by a pathologist who was unaware of the study, to detect any damage to tissue during ex vivo drug permeation.
Pharmacokinetic study:
The 3-4 mo old healthy Wistar rats of either sex, weighing 180-220 g were used for the study. The animals were housed in a clean environment at a temperature of 25°±1° and relative humidity of 45 % to 55 %, under 12/12 h light/dark cycle. The animals had free access to palletized food and water. The research protocol was approved by Institutional Animal Ethics Committee (IAEC) of KLEU’s College of Pharmacy, Belgaum, India (Resolution no-KLECOP/IAEC/Res. 16-20/04/2013). The animals were divided into three groups. Each group comprised of 6 animals. Each animal in all groups received 3 mg/kg dose. The animals in first group received oral drug solution. The second group was administered with normal thermoreversible gel while third group received optimized flexible liposome enriched thermoreversible gel (F2) via intranasal route (~40-50 μl/ nostril). The animals were kept in supine position for 2 min after administration of nasal dose. The blood samples were collected after predetermined time intervals from retro-orbital sac (0.25, 0.5, 1, 1.5, 2, 4, 8 and 12 h) in eppendroff tubes containing anticoagulant. The samples were centrifuged for 15 min at 13 500 rpm (Remi centrifuge, Mumbai, India). The supernatant plasma was separated by pipette and stored in -80° up to further analysis. Proteins in the plasma samples were removed by Acetonitrile (ACN), wherein 200 μl of ACN and plasma were taken in eppendorf tubes and mixed at 200 rpm for 15 min using mixmate eppendorf (Eppendorf AG, Hamburg, Germany). The supernatant was filtered by using Nylon syringe filter (pore size 0.45 μm) and 10 μl of supernatant was injected in High Performance Liquid Chromatography (HPLC) for analysis. The obtained data was analysed by Pharmacokinetics (PK) Solver software using one compartment model.
HPLC assay:
The developed and validated method was used for analysis. The HPLC Shimadzu system consisting of CBM-20A prominence communication bus module, SPD-M20A prominence diode array detector, SIL- 20AC HT Prominence auto sampler, LC solution software version 1.25 was used for data acquisition. The analytical conditions were optimized on Phenomenex Luna C18 column (150×4.6 mm, 5 μm) at room temperature. The mobile phase was consisted of 0.01 M potassium dihydrogen phosphate buffer pH 3.4:ACN (80:20 % v/v) at a flow rate of 1.0 ml/min. The analysis was carried out at wavelength of 225 nm. The already developed and validated calibration curve with R2 value of 0.984 was used for analysis (linearity, range 0.1-0.7 μg/ml, Limit of Quantitation (LOQ)-9.27 ng/ml).
Stability studies:
The formulated flexible liposomes were kept for stability studies at temperature of 4°. The stability of the samples was checked at predetermined time intervals (initial and 3 mo). The particle size and entrapment efficiency of the prepared formulations were checked. The formulations which found unstable were eliminated from study.
Results and Discussion
Flexible liposomes were prepared by ethanol injection method. Further size reduction was achieved by probe sonication. The size reduction achieved in probe sonication is due to cavitation effect wherein sonic waves are transferred into dispersion which produces millions of small bubbles. These small bubbles collide among themselves as well as with colloidal particles and increase the internal pressure of the system. This results in breakage of colloidal particles to nanosize. The flexible liposomes were successfully prepared and stored at cold temperature till further analysis. The particle size, PDI, zeta potential and entrapment efficiency of the vesicles obtained are shown in Table 2. The zeta potential of the vesicles was ranged from -14.2±4.48 mV to -11.7±5.26 mV. The higher zeta potential (+ or -) results in more repulsion between individuals particles and less chances of aggregation. PDI value of 0 indicates mono dispersion population. The PDI value in between 0 to 0.2 has narrow particle size distribution while above 0.2 the particles are heterogeneously dispersed. The PDI was found in between 0.411 to 0.659, which indicates highly polydispersity vesicle populations in dispersion.
| Formulation code | Particle size (nm) | PDI | Zeta potential (mV) | Entrapment efficiency (%) |
|---|---|---|---|---|
| F1 | 92.10±18.32 | 0.416 | -14.2±4.48 | 78.02 |
| F2 | 149.1±21.18 | 0.448 | -13.4±4.74 | 77.44 |
| F3 | 85.5±15.22 | 0.411 | -14.2±4.40 | 74.00 |
| F4 | 146.9±21.54 | 0.413 | -13.0±3.87 | 77.06 |
| F5 | 199.3±26.03 | 0.446 | -13.0±2.99 | 74.88 |
| F6 | 71.2±14.16 | 0.422 | -11.7±5.26 | 72.98 |
| F7 | 193.9±26.97 | 0.586 | -12.9±3.54 | 70.24 |
| F8 | 129.2±19.07 | 0.458 | -12.7±3.80 | 69.20 |
| F9 | 149.6±18.64 | 0.659 | -12.6±4.00 | 67.12 |
Note: (n=3, mean±Standard Deviation (SD))
Table 2: Characterization of flexible liposome formulations
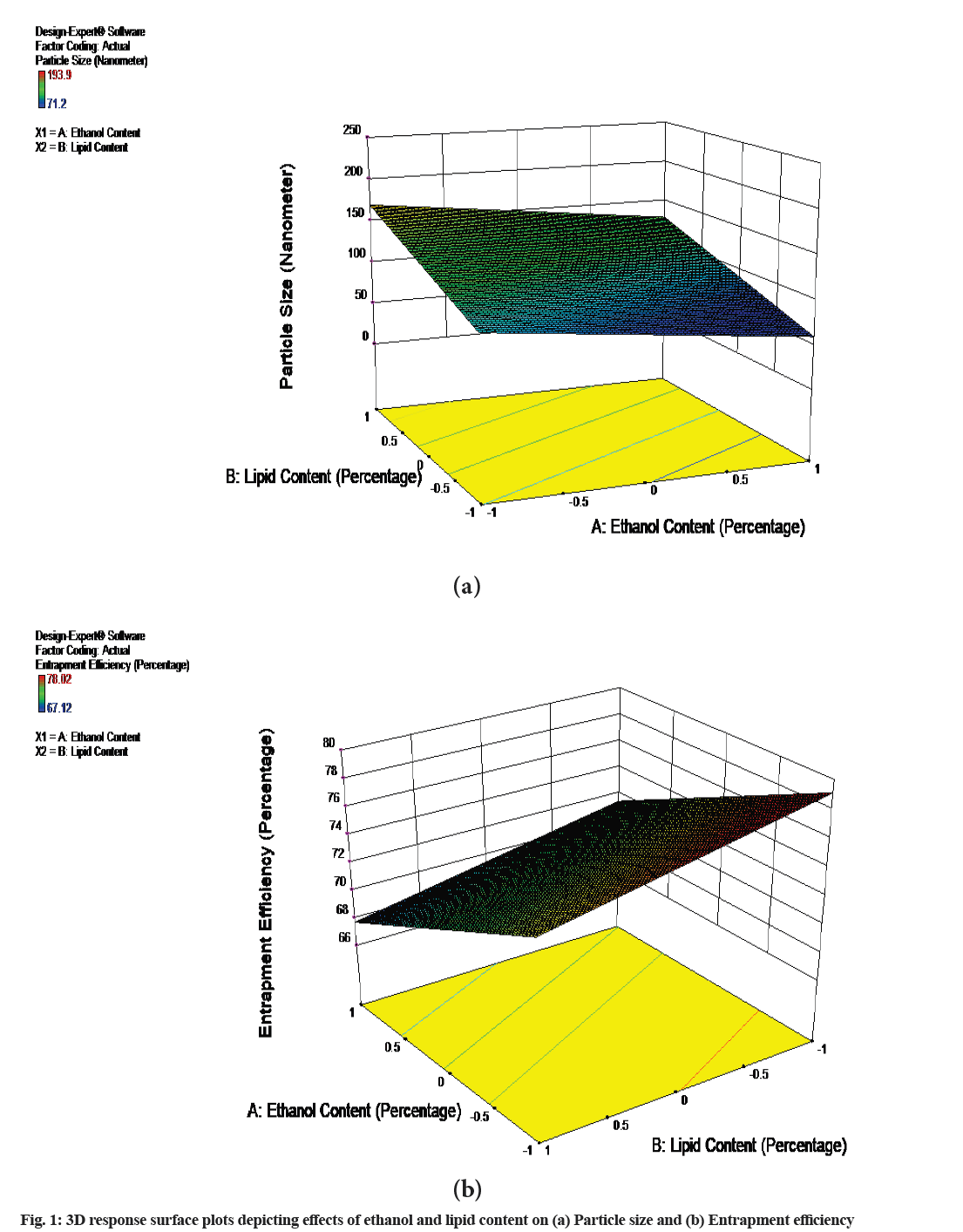
Optimization is a critical step in the formulation of any drug delivery system where in the processing parameters and materials composition is adjusted in such a way that obtained product will be of desired attributes. Now a day’s various softwares are utilized as efficient tool for optimizing such parameters. In the present study 32 factorial design was utilized to select the proper concentrations of ethanol and phospholipid to get the maximum entrapment efficiency and minimum particle size. According to the design, 9 formulations were prepared as shown in Table 1. The obtained results for particle size, PDI, zeta potential and entrapment efficiency are as shown in Table 2. Based on results, the quadratic model was found to be best fit model for the given data and polynomial equations depicting main effect were obtained from Analysis of Variance (ANOVA) provision in the software. The positive integers in the equation are depicting direct relationship between the independent variable allied to it. The quadratic equations for particle size and entrapment efficiency are as follows.
Particle size=114.90+29.4 X1-11.7 X2-27.13 Y1-15.53 Y2-25.07 X1Y1+4.20 X2Y1+18.13 X1Y2-7.80 X2Y2.
Entrapment efficiency=73.44+1.67 X1+0.40 X2+3.05 Y1+1.54 Y2-0.14 X1Y1+0.55 X2Y1+0.42 X1Y2-0.50 X2Y2.
The results obtained reveals that the concentration of ethanol plays significant role in particle size, PDI, zeta potential and entrapment efficiency of vesicles and not lipid concentration. As the concentration of ethanol increased, the particle size of vesicles was found to be increased which is in contrast to earlier findings[5,8].
This may be attributed to high ethanol concentration, which causes bilayer solubilization, suffices for changing vesicle morphology. Another reason may be that though intermediate ethanol concentration increases inter-vesicle repulsion by promoting fluctuation force; at high concentration, it causes vesicle fusion (owing to too strong fluctuations/ bilayer partial or local, solubilization). The entrapment efficiency of the vesicles was found to be decreased as the concentration of ethanol was increased. The likely reasons for the changes in entrapment efficiency are changed partition coefficient, possibly changed apparent dissociation constant and acid dissociation constant (pKa) of rizatriptan which together change distribution coefficient of the drug that is more soluble below pKa (in the bulk 6.40 but lower in bilayers), where it is positively charged. It was found that the formulations having ethanol concentration between 15 %-20 % v/v were yellowish colloidal appearance without drug precipitation, while the formulations with 25 % v/v ethanol shown the phase separation after a week with drug precipitation. This is in confirmation with earlier studies[16,18]. The higher ethanol concentrations made vesicles to leak the drug from membrane. As the formulations having ethanol concentration below 20 % v/v (F2-F6) provided best physical appearance, particle size and entrapment efficiency these were selected for further studies. Though the formulation F1 was having low ethanol concentration the vesicles were found to be aggregated with considerable increase in vesicle size as shown in Table 3, so these were not considered for further studies. Three Dimensional (3D) response surface plots for the effects are shown in fig. 1. The effect observed was found to be more pronounced for particle size than entrapment efficiency. When the data was analysed for ANOVA, it is observed that it follows linear model with r2 value of 0.6926 for particle size and 0.8969 for entrapment efficiency. These values are found to be higher than predicted values for particle size and entrapment efficiency.
| Formulation | Time in months | |||
|---|---|---|---|---|
| Initial | 3 mo | |||
| Particle size | % Entrapment efficiency | Particle size | % Entrapment efficiency | |
| F1 | 92.10±18.32 | 78.02 | 205.5±24.80 | 70.45 |
| F2 | 149.1±21.18 | 77.44 | 151.5±23.74 | 76.23 |
| F3 | 85.5±15.22 | 74 | 90.8±15.05 | 71.78 |
| F4 | 146.9±21.54 | 77.06 | 145.1±19.94 | 73.49 |
| F5 | 199.3±26.03 | 74.88 | 228.2±33.50 | 71.45 |
| F6 | 71.2±14.16 | 72.98 | 83.5±9.53 | 69.32 |
Note: (Mean±SD)
Table 3: 3 Mo Stability Study of formulations F1-F6
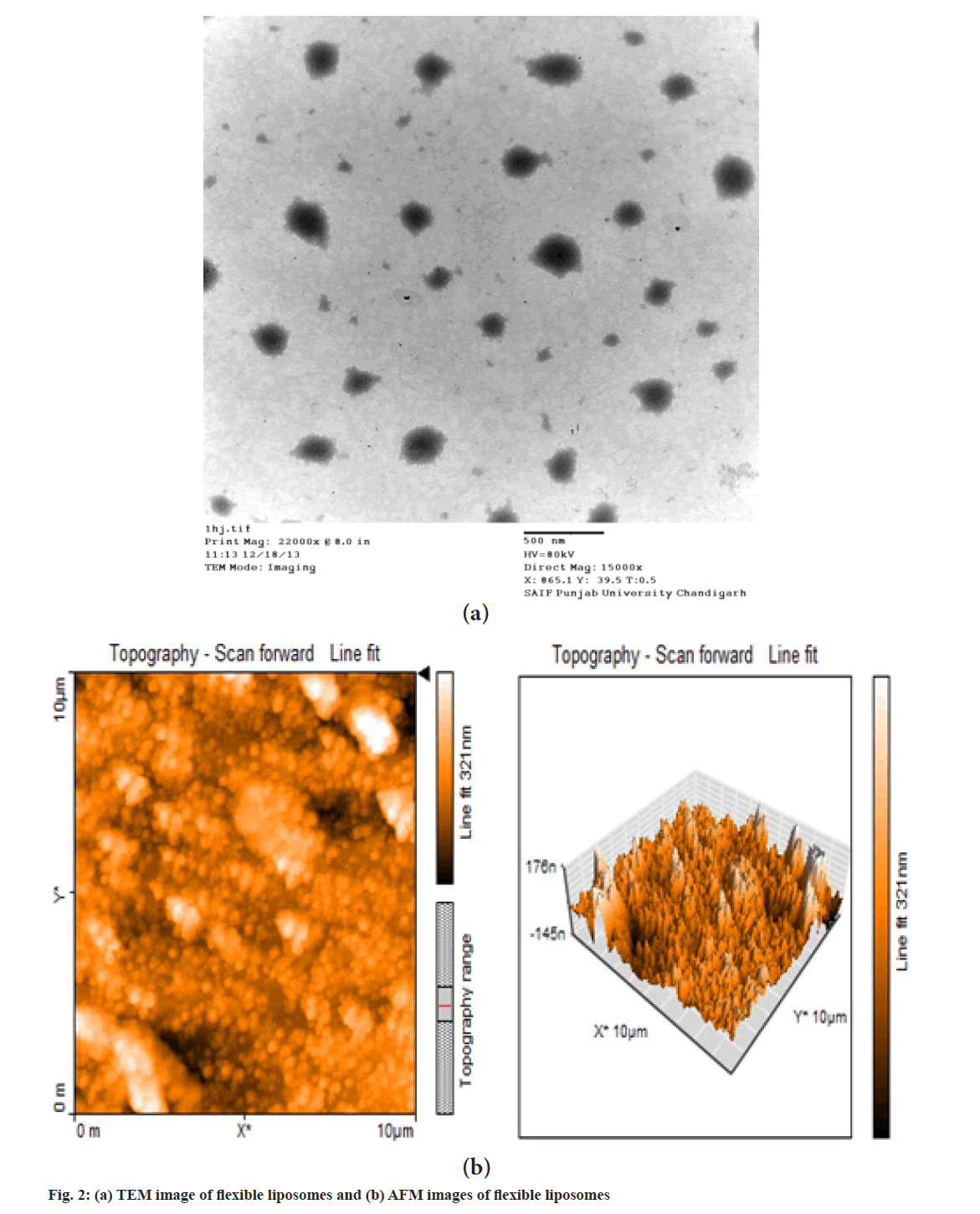
It is observed from TEM photomicrograph that flexible liposomes are unilamellar in nature and nearly spherical shape. Normally conventional liposomes are spherical in shape but the flexible liposomes are deviating from spherical shape, this may be due to absence of cholesterol. As cholesterol gives rigidity to the liposomal bilayer, the absence of cholesterol and higher concentration of alcohol gives flexibility to bilayer and makes them deviate from normal spherical shape. This observation supports the earlier findings by Touitou et al.[19]. The TEM image of flexible liposomes is shown in fig. 2a.
AFM images of flexible liposomes are as shown in fig. 2b. The AFM analysis was carried out to determine the surface topography and dimensions of flexible liposomes. The AFM images are showing uniformly distributed nearly spherical shaped liposomes.
The prepared gels were characterized for their physicochemical characteristics. The gelation time was found to be less than 15 s. As the temperature reaches 32°-34°, the gel was formed instantly. The mucoadhesive strength was found to be 4527±35 dynes/ cm2. The vicosity of the formulations were found to be 187±11 cps when measured at sol state at 10°.
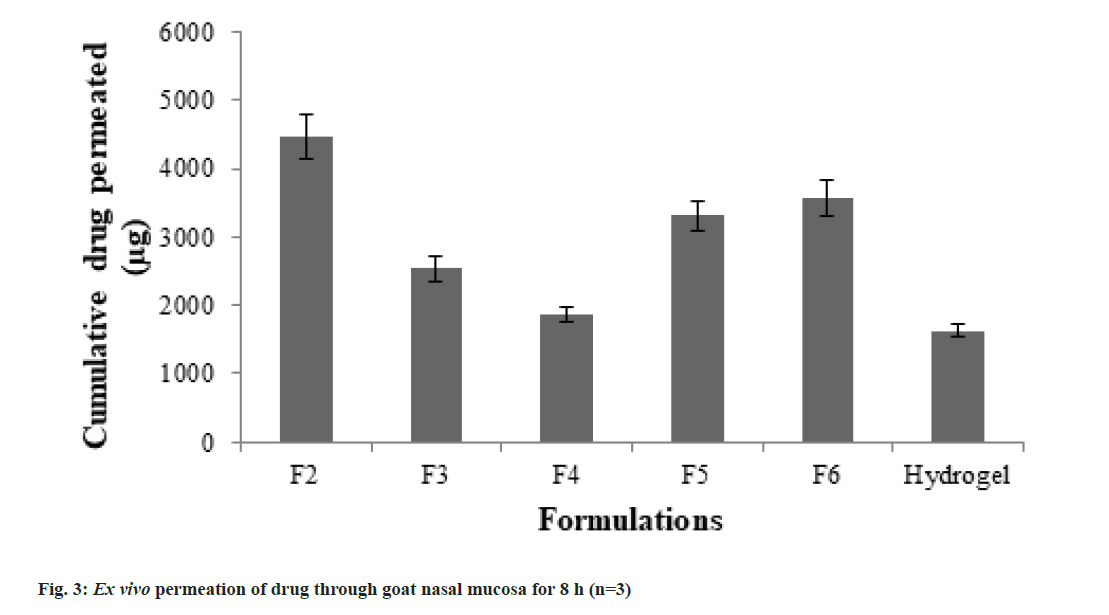
Ex vivo drug release study was carried out through freshly excised goat nasal mucosa. The cumulative amount of drug permeated in 8 h was calculated. The total drug permeation across goat nasal mucosa was found to be highest for F2 while it was lowest for normal hydrogel. The cumulative drug permeated in 8 h is shown in fig. 3. The flux values for F2 and normal hydrogel were found to be 3.53±0.23 and 1.28±0.11 μg/ cm2/min respectively. When the enhancement in drug permeation with respect to normal thermoreversible hydrogel was calculated from data, the highest enhancement ratio (2.75 times) was observed for F2. The enhancement ratios of different formulations are shown in Table 4.
| Formulation code | Flux (µg/cm)2/min | Enhancement ratio |
|---|---|---|
| F2 | 3.53±0.23 | 2.75 |
| F3 | 2.0±0.18 | 1.56 |
| F4 | 1.47±0.12 | 1.15 |
| F5 | 2.61±0.16 | 2.04 |
| F6 | 2.80±0.20 | 2.19 |
| Intranasal gel | 1.28±0.11 | - |
Note: (n=3, mean±SD)
Table 4: Drug Permeation Parameters of different formulations Across Goat Nasal Mucosa
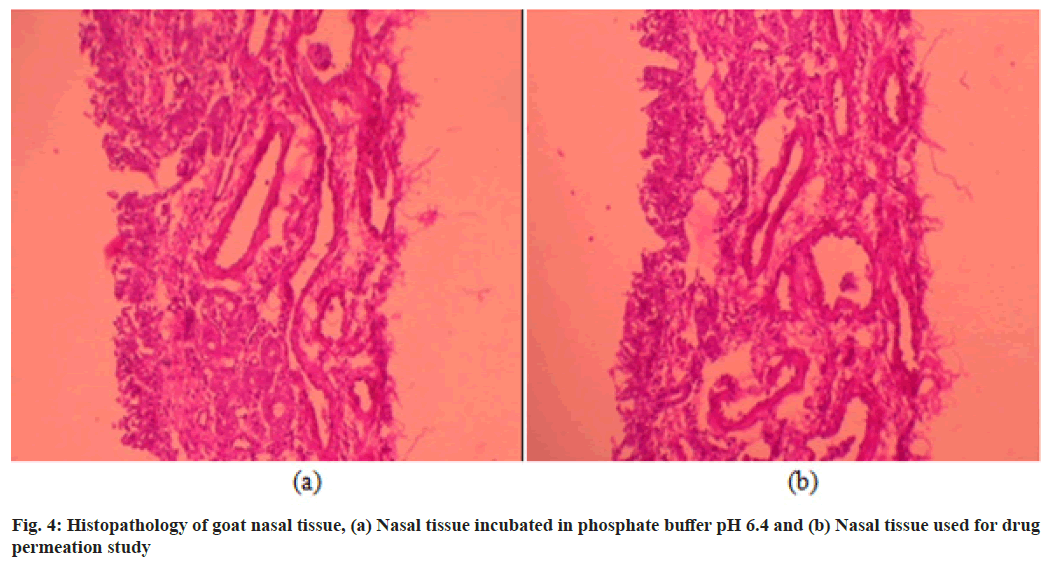
The histopathological study was carried out in order to detect if there is any toxic effect like cellular damage due to formulation on nasal mucosa. The histopathological images of nasal mucosa obtained are shown in fig. 4. Multiple sections were studied which shown normal pseudostratified columnar ciliated epithelium with normal lamina propria and mucus acini. The epithelium layer of tissue which was used for permeation study found to be intact and no any evidence of cellular damage was observed when compared with tissue which was placed in phosphate buffer pH 6.4. Thus flexible liposome based thermoreversible gel seems to be safe for the nasal mucosa and can be administered by intranasal route.
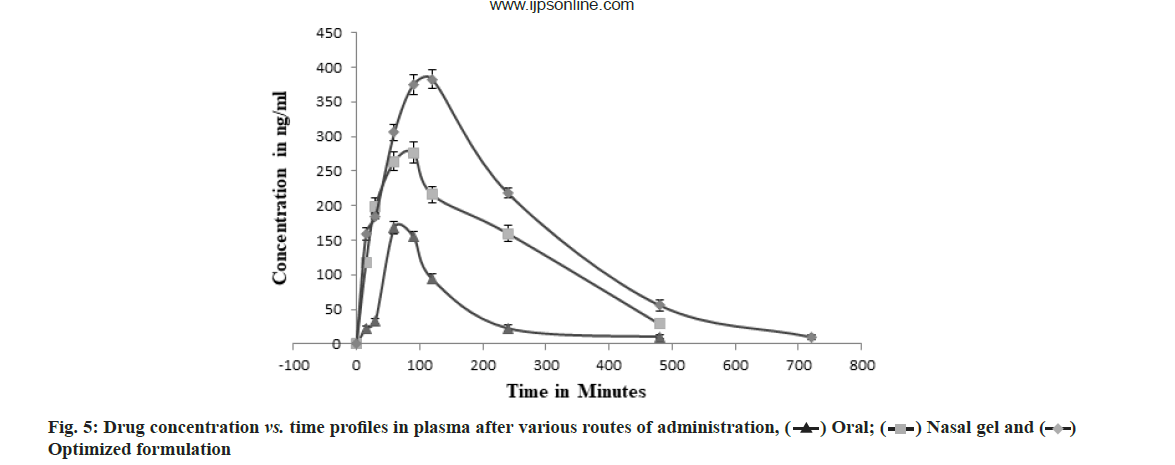
The concentration of rizatriptan in plasma after oral and intranasal route of administration was compared by validated HPLC method. The formulation F2 which was showing highest flux values per unit time during ex vivo study was administered during pharmacokinetic study. The various parameters like maximum Concentration (Cmax), Time to reach Cmax (Tmax), Area Under the Curve (AUC)0→t and AUC0→∞ were obtained and are shown in Table 5. The AUC0→t of three groups were compared, the group treated with optimized formulation had higher AUC0→∞ (1.762±0.078 μg×h/ml) when compared to oral drug solution (0.442±0.024 μg×h/ml) and intranasal hydrogel (1.276±0.094 μg×h/ml).
| Route of administration | Cmax (µg/ml) | Tmax (min) | AUC0→t (µg´h/ml) | AUC0→∞ (µg´h/ml) |
|---|---|---|---|---|
| Oral | 0.168±0.004 | 78.6±3.99 | 0.435±0.021 | 0.442±0.024 |
| Intranasal gel | 0.276±0.014 | 76.67±0.62 | 1.141±0.074 | 1.276±0.094 |
| Intranasal optimized formulation | 0.382±0.011 | 105.435±1.27 | 1.746±0.075 | 1.762±0.078 |
Note: (n=6, mean±SD)
Table 5: Pharmacokinetic parameters of rizatriptan in plasma
The AUC0→∞ of optimized formulation (F2) was 3.98 and 1.53 times more than oral drug solution and intranasal gel respectively, confirming improvement in bioavailability. The Tmax and Cmax values were also found to be increased for optimized formulation. The increase in Tmax is mainly due to the time taken by drug to diffuse from lipid layers of liposomes and gel. The plasma concentration vs. time profile graph is as shown in fig. 5.
The particle size and entrapment efficiency were determined at experimental conditions for 3 mo (4°). The formulations F7-F9 has shown phase separation after 1 w which were discarded from further study. No any significant change was observed for F2–F6 within specified time. The various parameters studied with their observations are as shown in Table 3.
In the current study rizatriptan benzoate embedded flexible liposome based in situ thermoreversible intranasal gel was formulated and optimized. The optimized gel formulation was found to be safe to the nasal mucosa with 4 and 1.53 times more bioavailability than oral drug solution and intranasal gel respectively. This approach has added advantage of bypassing the FPM and targeting the drug at the site of action in brain as compared to conventional tablet formulation. Thus the present study concludes that flexible liposome based thermoreversible gel could be an effective and safe drug delivery system via intranasal route for the drugs which are having low oral bioavailability and having their area of action in brain.
Acknowledgements:
Authors are thankful to the KLE University, Belgaum for providing grant to perform this research work. Authors extend their regards to Dr. Prabhakar Kore Basic Science Research Centre, KLE University Belgaum for providing their amenities to carry out this work. Authors are also thankful to Cipla Pvt. Ltd., Mumbai for providing rizatriptan benzoate as gift sample.
Conflict of interests:
The authors declared no conflict of interest
References
- Avachat AM, Gujar KN, Wagh KV. Development and evaluation of tamarind seed xyloglucan-based mucoadhesive buccal films of rizatriptan benzoate. Carbohydr Polym 2013;91(2):537-42.
[Crossref] [Google scholar] [PubMed]
- Garg T, Jain S, Singh HP, Sharma A, Tiwary AK. Elastic liposomal formulation for sustained delivery of antimigraine drug: In vitro characterization and biological evaluation. Drug Dev Ind Pharm 2008;34(10):1100-10.
[Crossref] [Google scholar] [PubMed]
- Sharma N, Kulkarni GT, Sharma A, Bhatnagar A, Kumar N. Natural mucoadhesive microspheres of Abelmoschus esculentus polysaccharide as a new carrier for nasal drug delivery. J Microencapsul 2013;30(6):589-98.
[Crossref] [Google scholar] [PubMed]
- Chen J, Jiang XG, Jiang WM, Gao XL, Mei N. Intranasal absorption of rizatriptan-in vivo pharmacokinetics and bioavailability study in humans. Pharmazie 2005;60(1):39-41.
[Google scholar] [PubMed]
- Chourasia MK, Kang L, Chan SY. Nanosized ethosomes bearing ketoprofen for improved transdermal delivery. Results Pharma Sci 2011;1(1):60-7.
[Crossref] [Google scholar] [PubMed]
- Li G, Fan Y, Fan C, Li X, Wang X, Li M, et al. Tacrolimus-loaded ethosomes: Physicochemical characterization and in vivo evaluation. Eur J Pharm Biopharm 2012;82(1):49-57.
[Crossref] [Google scholar] [PubMed]
- Maheshwari RG, Tekade RK, Sharma PA, Darwhekar G, Tyagi A, Patel RP, et al. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: A comparative assessment. Saudi Pharm J 2012;20(2):161-70.
[Crossref] [Google scholar] [PubMed]
- Verma P, Pathak K. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomedicine 2012;8(4):489-96.
[Crossref] [Google scholar] [PubMed]
- Arumugam K, Subramanian GS, Mallayasamy SR, Averineni RK, Reddy MS, Udupa N. A study of rivastigmine liposomes for delivery into the brain through the intranasal route. Acta Pharm 2008;58(3):287-97.
[Crossref] [Google scholar] [PubMed]
- Vyas SP, Goswami SK, Singh R. Liposomes based nasal delivery system of nifedipine: Development and characterization. Int J Pharm 1995;118(1):23-30.
- Li W, Zhou Y, Zhao N, Hao B, Wang X, Kong P. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ Toxicol Pharmacol 2012;34(2):272-9.
[Crossref] [Google scholar] [PubMed]
- Chen M, Li XR, Zhou YX, Yang KW, Chen XW, Deng Q, et al. Improved absorption of salmon calcitonin by ultraflexible liposomes through intranasal delivery. Peptides 2009;30(7):1288-95.
[Crossref] [Google scholar] [PubMed]
- Majithiya RJ, Ghosh PK, Umrethia ML, Murthy RS. Thermoreversible-mucoadhesive gel for nasal delivery of sumatriptan. AAPS PharmSciTech 2006;7(3):E80-6.
[Crossref] [Google scholar] [PubMed]
- Agrawal A, Maheshwari RK. Formulation development and evaluation of in situ nasal gel of poorly water soluble drug using mixed solvency concept. Asian J Pharm 2011;5(3):131-40.
- Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes-novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J Control Release 2000;65(3):403-18.
[Crossref] [Google scholar] [PubMed]
- Limsuwan T, Amnuaikit T. Development of ethosomes containing mycophenolic acid. Procedia Chem 2012;4:328-35.
- Kempwade A, Taranalli A. Formulation and evaluation of thermoreversible, mucoadhesive in situ intranasal gel of rizatriptan benzoate. J Solgel Sci Technol 2014;72(1):43-8.
- Barupal AK, Gupta V, Ramteke S. Preparation and characterization of ethosomes for topical delivery of aceclofenac. Indian J Pharm Sci 2010;72(5):582-6.
[Crossref] [Google scholar] [PubMed]
- Lodzki M, Godin B, Rakou L, Mechoulam R, Gallily R, Touitou E. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J Control Release 2003;93(3):377-87.
[Crossref] [Google scholar] [PubMed]
 Optimized formulation
Optimized formulation



