- *Corresponding Author:
- Sirisolla J
AU College of Pharmaceutical Sciences, Andhra University, Visakhapatnam-530003, India
E-mail: janaki.sirisolla@gmail.com
| Date of Received : | 15 July 2014 |
| Date of Revised : | 14 January 2015 |
| Date of Accepted : | 02 June 2015 |
| Indian J Pharm Sci 2015;77(3):321-327 |
This is an open access article distributed under the terms of the Creative Commons Attribution?NonCommercial?ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non?commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The objective of the present work is to design sustained release matrix tablets of cefixime trihydrate by incorporating drug in a matrix made up of release retardant polymers, which prolong drug release leading to minimization of the peak and valley effect in the plasma and provide patient convenience. The effect of combination of polymers on parameters like release pattern, release mechanism of the drug were studied. Total nine formulations each containing 200 mg of drug were prepared by direct compression method. The formulations F-1, F-2, F-3 were prepared with a 1:1 drug to polymer ratio using hydroxypropyl methylcellulose, carboxymethyl cellulose sodium and ethyl cellulose. F-4 was prepared with a 1:1 ratio of hydroxypropyl methylcellulose, carboxymethyl cellulose sodium, F-5 as prepared with a 1:1 ratio of hydroxypropyl methylcellulose and ethyl cellulose, F-6 was prepared with a 1:1 ratio of carboxymethyl cellulose sodium and ethyl cellulose, F-7, F-8, F-9 were prepared by using polymers hydroxypropyl methylcellulose, carboxymethyl cellulose sodium and ethyl cellulose in the ratios of 0.5:0.5:1, 0.5:1:0.5, and 1:0.5:0.5. Designed matrix tablets were evaluated for various pre-compression and post-compression parameters. Formulation F-5 showed 102.15 % release at the end of 12 h and it is selected as the best formulation. All Formulations followed zero order with non-Fickian diffusion method.
Keywords
Sustained release, patient compliance, HPMC, Sodium CMC, ethyl cellulose
Infectious diseases are most common in developing countries. The infectious bacterial classes are both Gram positive and Gram negative hence, the treatment is necessary with an agent, which have broad spectrum of activity. Cephalosporins possess a wide range of activity against Gram positive and Gram negative bacteria; these are act by inhibiting bacterial cell wall synthesis. Cefixime trihydrate is an orally active third generation cephalosporins [1,2]. Sustained release dosage forms have number of advantages over conventional dosage forms, improved patient convenience due to less frequent dosing, reduction in fluctuation in steady-state levels and therefore better control of disease, maximum utilization of drug enabling reduction in total amount of dose administered [3-5]. The objectives of the present work are to design, formulate and evaluate matrix tablets of cefixime trihydrate for sustained release dosage form. As the effect of sustained release dosage form is relatively more, incorporating the drug in the matrix tablet will prolong the drug release. These are prepared by direct compression method. The matrix tablets of cefixime trihydrate designed using polymers such as hydroxypropyl methylcellulose K5M (HPMC K5M), carboxymethyl cellulose sodium (sodium CMC) and ethyl cellulose (EC) and evaluated for various precompression and post-compression parameters [6,7]. The effect of combination of HPMC K5M and EC, HPMC and sodium CMC, EC and sodium CMC, HPMC, EC and sodium CMC on response parameters like release pattern, cumulative percent release of the drug, drug release mechanism were studied.
Materials and Methods
Cefixime trihydrate, microcrystalline cellulose were purchased from KAPL., Bengaluru, India, HPMC K5M, EC and sodium CMC were purchased from Colorcon Asia Pvt. Ltd., Mumbai. Magnesium stearate was purchased from Loba Chemie Pvt. Ltd. Sodium Hydroxide, hydrochloric acid were purchased from S. D. Fine Chem. Limited, Mumbai and potassium dihydrogenortho-phospahte from Ranbaxy Fine Chemicals.
Fourier Transform Infrared (FTIR) spectroscopy
The Fourier transform infrared (FTIR) spectra of samples were obtained using FTIR spectrophotometer (Perkin Elmer). Pure drug, individual polymers and optimized formulations were subjected to FTIR study. About 2-3 mg of sample was mixed with dried potassium bromide of equal weight and compressed to form a KBr disk. The samples were scanned from 500 to 4000 cm−1.
Formulation of sustained release matrix tablets
Sustained release matrix tablets were prepared by direct compression method as per the formula given in the Table 1. Initially the drug was passed through Sieve #30, all the polymers were passed through Sieve #40 and microcrystalline cellulose and magnesium stearate were passed through Sieve #60. Required quantity of the drug, polymers and diluents were mixed thoroughly. This powder mixture was blended and compressed using a single punch-tableting machine (Cadmach Machinery Co. Pvt. Ltd) with hardness of the tablets maintained between 8-11 kg/cm [8,9].
| Formulation code | Amount of drug | HPMC | Sodium CMC |
EC | MCC | Magnesium stearate |
Total |
|---|---|---|---|---|---|---|---|
| F‑1 | 200 | 200 | ‑ | ‑ | 95 | 5 | 500 |
| F‑2 | 200 | ‑ | 200 | ‑ | 95 | 5 | 500 |
| F‑3 | 200 | ‑ | ‑ | 200 | 95 | 5 | 500 |
| F‑4 | 200 | 100 | 100 | ‑ | 95 | 5 | 500 |
| F‑5 | 200 | 100 | ‑ | 100 | 95 | 5 | 500 |
| F‑6 | 200 | ‑ | 100 | 100 | 95 | 5 | 500 |
| F‑7 | 200 | 50 | 50 | 100 | 95 | 5 | 500 |
| F‑8 | 200 | 50 | 100 | 50 | 95 | 5 | 500 |
| F‑9 | 200 | 100 | 50 | 50 | 95 | 5 | 500 |
HPMC is hydroxypropyl methylcellulose, sod CMC is sodium carboxy methylcellulose, EC is ethyl cellulose, and MCC is microcrystalline cellulose. All quantities are in mg
Table 1: Composition of Tablet Formulations
Pre-compression evaluation parameters
Bulk density is the ratio of total mass of powder to the bulk volume of powder. It was measured by pouring a weighed quantity of powder (passed through standard Sieve #20) into a measuring cylinder and the initial volume (bulk volume) was noted. From this, the bulk density is calculated. Tapped density is the ratio of total mass of powder to the tapped volume of powder. It was measured by pouring the weighed powder into a measuring cylinder and then it was subjected to 500 tappings from a height of 2 inches. The volume was measured and tapped density was calculated. Three determinations were done for each parameter [10].
Hausner’s ratio is the ratio of tapped density to bulk density. It was calculated by noted tapped density and poured density values. Carr’s index was calculated as 100 times the ratio of the difference between tapped density and bulk density to the tapped density. It was measured by calculated tapped density and poured density values. These determinations were carried out in triplicate[10]. Angle of repose is defined as the maximum angle possible between the surface of a pile of powder and the horizontal plane; it was measured by pouring the weighed powder mixture into the funnel which was fixed to a stand at a definite height (h). The drug excipient blend was allowed to flow through the funnel freely on to the surface of a graph sheet. Then the height and diameter of the heap formed were noted and the angle of repose was calculated. Three determinations were performed [11]. The angle of repose can be calculated using the formula, Tan θ=h/r, where h is the height, r is the radius and θ is the angle of repose.
Post-compression evaluation for formulated matrix tablets
Hardness or tablet crushing strength is the force required to break a tablet in a diametric compression. Hardness of the tablet was determined using the Monsanto hardness tester (Shreeji Chemicals). The hardness was computed by deducting the initial pressure from the final pressure. Test was performed on six tablets and average was calculated [12]. Weight variation was carried out according to European pharmacopoeia. Twenty tablets were selected at random and average weight was determined. Then individual tablets were weighed and the individual weight was compared with the average weight [12]. Three tablets were selected randomly from each batch and thickness was measured using vernier calipers. The tablet was placed between two arms of the vernier calipers and thickness was measured [12]. The Roche friability test apparatus (Roche Rich Pharma, Mumbai) was used to determine the friability of the tablets. This device chamber revolves at 25 rpm. About 10 tablets were selected randomly, dedusted and weighed. Then they were placed in the drum and rotated 100 times. Then tablets were dedusted again to remove loose dust and were reweighed. The percent loss in weight was calculated, which gave a measure of friability [12].
Ten Tablets were selected randomly, weighed and average weight of the tablet calculated. Tablets were ground individually to a fine powder. Powder equivalent to 280 mg of cefixime trihydrate was transferred to a 100 ml volumetric flask, dissolved in 80 ml of pH 7.2 buffer. The volume was made up to 100 ml with the buffer and filtered through a Whatman filter paper. Absorbance of the sample solution was measured using UV/Vis spectrophotometer (Elico) and concentration of the drug in the sample was calculated [12].
In vitro dissolution of sustained release tablets of cefixime trihydrate was studied in USP XXIII dissolution apparatus (Electro lab) rotated at 100 rpm. Nine hundred millilitres of pH 1.2 buffer for the 2 h, followed by pH 7.2 buffer for the next 10 h were used as the dissolution media. The temperature of dissolution medium was maintained at 37±0.5° throughout the experiment. One tablet was used in each test. Samples of dissolution medium (5 ml) were withdrawn using a syringe fitted with a pre-filter at known intervals of time and analyzed for drug content by measuring the absorbance at 288 nm. The volume withdrawn at each time interval was replaced with fresh quantity of dissolution medium. The dissolution studies were carried out in triplicate. Cumulative percent drug released was calculated and plotted against time [12].
Dissolution data obtained from the above experiments with all formulations was applied to Zero order and First order kinetic Eqns. Zero order as cumulative amount of drug released vs. time and the Zero order Eqn. being C=K0 t, where K0 is the zero-order rate constant expressed in units of concentration/time and t is the time in h. A plot of concentration vs. time would yield a straight line with a slope equal to K0 and intercept the origin of the axes [13]. First order as log cumulative percent of drug remaining vs. time, and the Fisrt order Eqn. being LogC=LogC0− kt/2.303, where C0 is the initial concentration of drug, k is the first order constant, and t is the time [13].
Higuchi’s model, which is cumulative percent drug released vs. square root of time and the Higuchi Eqn. being, Q=K t1/2, where K is the constant reflecting the design variables of the system and t is the time in h. Hence, drug release rate is proportional to the reciprocal of the square root of time [14]. Korsmeyer Peppas Eqns were applied to evaluate the mechanism of drug release from the dosage form, data for the first 60% of drug release were plotted in Korsmeyer et al. proposed Eqn. log cumulative percent drug released vs. log time, and the exponent n was calculated through the slope of the straight line. Mt/M∞=Ktn, where Mt/M∞ is the fractional solute release, t is the release time, K is a kinetic constant characteristic of the drug/polymer system, and n is an exponent that characterizes the mechanism of release of tracers. For cylindrical matrix tablets, if the exponent n=0.45, then the drug release mechanism is Fickian diffusion, and if 0.45<n<0.89, then it is non- Fickian or anomalous diffusion. An exponent value of 0.89 is indicative of Case-II Transport or typical zero-order release [15].
Results and Discussion
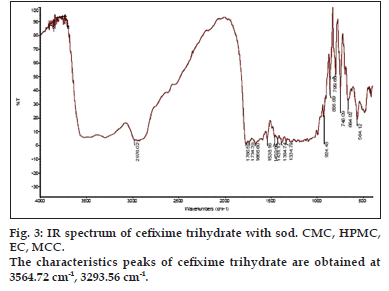
IR spectrum of cefixime trihydrate pure, physical mixtures of drug, excipients and the polymers were taken. The characteristics peaks of cefixime trihydrate are obtained at 3564.72 cm-1, 3293.56 cm-1, 1777.21 cm-1, 1677.30 cm-1, 1588.11 cm-1, 1581.17 cm-1, 1184.94 cm-1, 1224.18 cm-1, 803.17 cm-1, 863.83 cm-1 and 746.09 cm-1. The IR spectra obtained indicate good compatibility between drug and excipients. All the spectra are shown in the figs. 1 to 3.
Figure 1: Spectra of cefixime trihydrate alone and with excipients and polymers. IR spectra of (a). cefixime trihydrate alone and (b). cefixime trihydrate with HPMC and MCC. The characteristics peaks of cefixime trihydrate are obtained at 3564.72 cm-1, 3293.56 cm-1, 1777.21 cm-1, 1677.30 cm-1, 1588.11 cm-1, 1581.17 cm-1, 1184.94 cm-1, 1224.18 cm-1, 803.17 cm-1, 863.83 cm-1 and 746.09 cm-1.
Bulk densities of the powder blends of all the formulations ranged from 0.255 to 0.414 g/cc and tapped densities of the powder blends of all the formulations ranged from 0.274 to 0.453 g/cc as shown in Table 2. The Hausners ratio values ranged from 1.057 to 1.25. Evaluated values were less than 1.25 indicating good flow. It means that the powder flow properties were within the pharmacopoeias limits. The Carr’s index values ranged from 5.86 to 10%.
Carr’s index values between 5-15 indicate excellent flow. The results obtained indicates that the powder flow properties were within the pharmacopoeias limits (Table 2). Angle of repose is defined as maximum angle possible between the surface of the pile of powder and the horizontal plane. Angle of repose values obtained ranged from 28.17° to 34.36° which are <35, indicative of good flow properties of granules, and it was observed to be within the pharmacopoeias limits (Table 2).
| Formulation code | Bulk density (g/ml) | Tapped density (g/ml) | Compressibility index (%) | Hausner’s ratio | Angle of repose |
|---|---|---|---|---|---|
| F‑1 | 0.273 ± 0.001 | 0.302 ± 0.001 | 6.74 ± 0.04 | 1.25 ± 0.03 | 33.92° |
| F‑2 | 0.414 ± 0.002 | 0.453 ± 0.001 | 10.0 ± 0.04 | 1.057 ± 0.03 | 34.36° |
| F‑3 | 0.333 ± 0.001 | 0.345 ± 0.002 | 9.27 ± 0.01 | 1.22 ± 0.04 | 32.31° |
| F‑4 | 0.304 ± 0.002 | 0.340 ± 0.001 | 10.0 ± 0.02 | 1.24 ± 0.01 | 28.17° |
| F‑5 | 0.294 ± 0.002 | 0.318 ± 0.002 | 7.54 ± 0.02 | 1.195 ± 0.04 | 32.22° |
| F‑6 | 0.304 ± 0.001 | 0.327 ± 0.004 | 9.27 ± 0.03 | 1.23 ± 0.07 | 32.73° |
| F‑7 | 0.257 ± 0.001 | 0.276 ± 0.003 | 6.58 ± 0.01 | 1.113 ± 0.06 | 31.22° |
| F‑8 | 0.272 ± 0.001 | 0.290 ± 0.003 | 5.86 ± 0.04 | 1.192 ± 0.04 | 34.11° |
| F‑9 | 0.255 ± 0.001 | 0.274 ± 0.003 | 6.56 ± 0.01 | 1.25 ± 0.01 | 31.81° |
All values are mean ± standard deviation (SD) for n=3 determinations
Table 2: Precompression Parameters of the Powder Mixture.
The hardness of all the formulations ranged from 2.7 to 3.3 kg/cm2. The pharmacopoeial limit for hardness is 3-5 kg/cm2. Hence all the formulations passed the test for hardness (Table 3). The weights of the tablets were between 500 to 504 mg, as the weight of the tablet is 500 mg the weight variation limit is ±5%. The pharmacopoeial specification for weight variation limit is ±5% for uncoated tablets weighing more than 324 mg. Hence all the formulations passed the weight variation test (Table 3). The thickness of all the formulations was between 2.73 to 3.1 mm, which was according to the pharmacopoeial specifications. The tablet mean thickness was almost uniform in all the formulations (Table 3). Friability of all the formulations was determined, and the values were in the range from 0.39 to 0.56%. Friability of the formulated tablets was found to be below 1%, which indicated good mechanical resistance of the tablets. Hence all the formulations were within the pharmacopoeial limits (Table 3). Percent drug content of the drug in all the formulated tablets was found to be within limit. Percent drug content of cefixime trihydrate was within 98.21 to 102.5% for all the nine formulations. The results are within the range indicate uniformity of mixing. Table 3 shows data of drug content uniformity.
| Formulation code | Thickness (mm) | Weight variation (mg) | Hardness (kg/cm2) | Friability (%) | Drug content (%) |
|---|---|---|---|---|---|
| F‑1 | 2.92 ± 0.55 | 504.20 ± 3.57 | 2.7 ± 0.32 | 0.42 ± 0.02 | 99.61 ± 0.45 |
| F‑2 | 2.73 ± 0.24 | 503.10 ± 4.25 | 3.2 ± 0.33 | 0.49 ± 0.01 | 101.31 ± 0.25 |
| F‑3 | 2.83 ± 0.32 | 504.10 ± 4.94 | 3.3 ± 0.65 | 0.51 ± 0.04 | 99.54 ± 0.55 |
| F‑4 | 2.9 ± 0.33 | 503.20 ± 5.50 | 3.28 ± 0.69 | 0.40 ± 0.05 | 99.79 ± 0.58 |
| F‑5 | 2.96 ± 0.85 | 502.90 ± 4.59 | 3.3 ± 0.58 | 0.39 ± 0.03 | 99.82 ± 0.54 |
| F‑6 | 2.83 ± 0.55 | 505.33 ± 3.33 | 2.9 ± 0.22 | 0.48 ± 0.02 | 99.44 ± 0.06 |
| F‑7 | 2.94 ± 0.24 | 503.10 ± 4.74 | 2.8 ± 0.55 | 0.56 ± 0.03 | 99.31 ± 0.41 |
| F‑8 | 2.98 ± 0.63 | 503.20 ± 4.04 | 2.8 ± 0.51 | 0.47 ± 0.02 | 99.48 ± 0.52 |
| F‑9 | 3.1 ± 0.66 | 503.10 ± 3.93 | 3.1 ± 0.69 | 0.46 ± 0.01 | 100.11 ± 0.44 |
All values are mean ± standard deviation (SD) for n=3 determinations
Table 3: Evaluation Parameters of the Compressed Tablets
Results obtained in the in vitro drug release study of different formulations are shown in Table 4. The data indicated that formulations F-1 released 99% of cefixime trihydrate at the 9th h, F-2 released 101.44% at the 6th hour, F-3 released 99.8% at the 11th hour, F-4 released 101.75% at the 9th hour, F-5 releases 102.15% at the 12th hour, F-6 released 100.99% at the 11th hour, F-7 released 99.89% at the 11th hour, F-8 released 99.78% at the 9th hour and Formulation F-9 releases100.97% of drug at the 9th hour. From the data obtained it was concluded that the formulation F-5 HPMC:EC in 1:1 ratio released 102.15 % of cefixime trihydrate in 12 h could be regarded as the best formulations.
| Time | Cumulative percent drug release | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (h) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
| 1 | 29.5 | 38.92 | 17.61 | 33.7 | 23.6 | 25.19 | 24.9 | 32 | 30.05 |
| 2 | 42.07 | 52.29 | 23.92 | 42.5 | 30.98 | 32.16 | 31.72 | 41.07 | 43.01 |
| 3 | 55.04 | 69.84 | 32.83 | 50.45 | 37.52 | 40.98 | 38.78 | 49.19 | 56.02 |
| 4 | 70.42 | 80.74 | 41.27 | 61.17 | 45.89 | 47.65 | 46.92 | 58.98 | 71.62 |
| 5 | 76.05 | 92.45 | 50.45 | 70.92 | 53.12 | 55.21 | 64.68 | 69.01 | 77.18 |
| 6 | 80.24 | 101.44 | 58.90 | 78.27 | 60.98 | 62.93 | 68.95 | 76.98 | 81.24 |
| 7 | 89.24 | ‑ | 67.62 | 88.19 | 69.72 | 72.12 | 70.08 | 87.03 | 90.18 |
| 8 | 94.5 | ‑ | 75.95 | 96.91 | 79.92 | 84.83 | 81.98 | 94.89 | 95.98 |
| 9 | 99.0 | ‑ | 83.62 | 101.75 | 85.09 | 88.14 | 86.38 | 99.78 | 100.97 |
| 10 | ‑ | ‑ | 91.9 | ‑ | 91.15 | 96.09 | 92.67 | ‑ | ‑ |
| 11 | ‑ | ‑ | 99.8 | ‑ | 96.95 | 100.99 99.89 | ‑ | ‑ | |
| 12 | ‑ | ‑ | ‑ | ‑ | 102.15 | ‑ | ‑ | ‑ | ‑ |
Formulation F5 showed drug release up to 12 h making it the optimal formulation.
Table 4: In Vitro Dissolution Profile For Formulations F1-F9.
Data of in vitro release were fitted to different Eqns and kinetic models to explain the release kinetics of cefixime trihydrate from the sustained release matrix tablet. The data were processed for regression analysis using MS-Excel statistical functions. To know the order of reaction from these formulations, the data were treated according to first-order (log cumulative percent drug remaining vs. time), Higuchi’s (cumulative percent drug released vs. square root of time), and Korsmeyer Pappas’s (log cumulative percent drug released vs. log time) Eqns along with zero order (cumulative amount of drug released vs. time) Eqn.
When the data were plotted according to the zero-order Eqn, the formulations showed fair linearity with regression values between 0.956 and 0.999, and when the data were plotted according to the first-order Eqn., the formulations showed regression values between 0.819 and 0.961. By studying the release kinetics of cefixime matrix tablets, the formulations did not follow a first-order release pattern but a zeroorder release pattern (Table 5).
| Formulation code | Correlation coefficient | |
|---|---|---|
| Zero order | First order | |
| F1 | 0.956 | 0.864 |
| F2 | 0.989 | 0.961 |
| F3 | 0.999 | 0.909 |
| F4 | 0.996 | 0.844 |
| F5 | 0.995 | 0.860 |
| F6 | 0.994 | 0.819 |
| F7 | 0.980 | 0.936 |
| F8 | 0.996 | 0.889 |
| F9 | 0.958 | 0.937 |
Correlation coefficient values of zero order, first order release
Table 5: Release Kinetics of Formulated Matrix Tablets
The in vitro release profiles of drug from all the formulations could be best expressed by Higuchi’s Eqn., as the plots showed high linearity with R2 values between 0.973 and 0.991. It indicating that diffusion mechanism involved in the release of the drug from the tablets. To confirm the diffusion mechanism, the data were fit into Korsmeyer Peppas Eqn. From the slope n values ranging from 0.527 to 0.630, the diffusion mechanism involved in formulations F1 to F9 was considered to be non-Fickian (Table 6).
| Formulation code | Kinetic models | ||
|---|---|---|---|
| Higuchi | Peppas Model | ||
| R2 | R2 | N | |
| F1 | 0.993 | 0.991 | 0.565 |
| F2 | 0.996 | 0.991 | 0.545 |
| F3 | 0.949 | 0.987 | 0.630 |
| F4 | 0.989 | 0.978 | 0.527 |
| F5 | 0.973 | 0.979 | 0.622 |
| F6 | 0.969 | 0.973 | 0.604 |
| F7 | 0.975 | 0.973 | 0.615 |
| F8 | 0.988 | 0.981 | 0.542 |
| F9 | 0.994 | 0.991 | 0.561 |
Correlation coefficient value of Higuchi and Peppas models
Table 6: Diffusion Characteristics of Formulated Matrix Tablets
Sustained release matrix tablets of cefixime trihydrate prepared by direct compression, using different polymers like HPMC, EC and sodium CMC in different ratios. Formulations F1, F2, F3 were prepared using polymers HPMC, sodium CMC and ethyl cellulose respectively on the basis of 1:1 drug to polymer ratio. F-4 was prepared using 1:1 ratio of HPMC and sodium CMC, F-5 was prepared with 1:1 ratio of HPMC and EC, F-6 was prepared by 1:1 ratio of sodium CMC and EC, Further, three formulations (F7, F8, F9) were prepared using polymers HPMC, sodium CMC and EC in the ratios of 0.5:0.5:1, 0.5:1:0.5 and 1:0.5:0.5, respectively. FT-IR spectra were compared to find that there is no interaction between drug and polymer. Tablets were evaluated for weight variation and thickness, drug content, in vitro dissolution. When compared to all the nine formulations, formulation F-5 HPMC:EC in 1:1 ratio released 102.15% of cefixime trihydrate in 12 h was selected as the optimized formulations. All the formulations followed zero order release kinetics with diffusive mechanism by non-Fickian.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Tripathi KD. Essentials of Medical Pharmacology. 5th ed. New Delhi: Jaypee Brothers Medical Publishers; 2003.
- Satoskar RS, Bhandarkar SD, Ainapure SS. Pharmacology and Pharmacotherapeutics. 16th ed. Mumbai: Popular Prakashan; 1999.
- Asija R, Rathi H, Asija S. Sustained released drug technology: A review. Int J Res Pharm Sci 2012;2:1-13.
- Chugh I, Seth N, Rana AC, Gupta S. Oral Sustained release drug delivery systems: An over view. Int Res J Pharm 2012;3:57-62.
- Gennaro AR. Remington: The Science and Practice of Pharmacy. 20th ed. Easton PA, USA: Mac Publishing Company; 2001.
- Chien YW. Novel Drug Delivery Systems. 2nd ed. New York: Marcel Dekker Inc; 1992.
- Bhalla HL, Raj PC. Release controlling polymers. Indian Drugs 1991;28:519-22.
- Patel MP, Patel MM, Patel KN. Formulation and optimization of controlled released floating matrix tablets of cefixime. J Pharm Res 2009;2:1110-2.
- Hayashi T, Kanbe H, Okada M, Suzuki M, Ikeda M, Onuki M, et al.Formulation study and drug release mechanism of a new Theophylline sustained-release preparation. Int J Pharm 2005;304:91-101.
- Lachman L, Lieberman HA, Kanig JL. The Theory and Practice of Industrial Pharmacy. 3rd ed. Mumbai: Varghese Pub House; 2003, P.67-72.
- Cooper J, Gunn C. Powder flow and compaction. In: Carter SJ. editors. Tutorial Pharmacy. New Delhi: CBS Publishers and Distributors; 1986. P 211-33.
- Indian Pharmacopoeia, Controller of Publication, Govt. of India. New Delhi, India: Ministry of Health and Family Welfare; 2010. p. 185.
- Wagner JG. Interpretation of percent dissolved?time plots derived from in vitrotesting of conventional tablets and capsules. J Pharm Sci1969;58:1253-7.
- Higuchi T. Mechanisms of sustained action medication: Theoretical analysis of rate release of solid drugs dispersed in solid matrices .J Pharm Sci 1963;52:1145-9.
- Korsmeyer R, Gwrny R, Peppas N. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 1983;15:25-35.