- Corresponding Author:
- J. Ali
Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard, Hamdard Nagar, New Delhi-110 062, India.
E-mail: jali@jamiahamdard.ac.in
| Date of Submission | 29 January 2005 |
| Date of Revision | 05 October 2005 |
| Date of Acceptance | 15 July 2006 |
| Indian J Pharm Sci, 2006, 68 (4): 442-447 |
Abstract
In the present study, an attempt was made to develop a low-dose controlled-release delivery system for the treatment of periodontal infections. Nylon fibres were taken as core material. The coating solution contained polyvinyl acetate and amoxycillin trihydrate. The fibres were coated five times to maximize drug loading. The coating composition was optimized and fibres were subjected to in vitro release studies. For the study, a continuous-flow-through apparatus for in situ drug release, simulating the in vivo conditions of periodontal pocket, was designed in a manner that the drug released was well above the minimum inhibitory concentration of amoxycillin trihydrate. In situ samples were further subjected to microbiological evaluation against the microorganisms which are implicated in periodontal infections. Optimized fibre was further subjected to permeation rate study using modified Franz diffusion cell. The drug-coated fibres provided sustained effect up to a period of 11 d (264 h) and followed first-order release. The drug release followed Fickian diffusion mechanism. In situ samples revealed that the drug level at different time intervals remained above its minimum inhibitory concentration (1.5 µg/ml) for a period of 11 d. In situ release samples when subjected to microbiological evaluation against microorganisms inhibited the growth of S. aureus, S. mutans and B. cereus . Permeation rate studies through bovine cheek pouch membrane revealed that only a low level of drug permeated through the membrane and it followed zero-order permeation rate. The retentive fibres were shown to provide controlled delivery of amoxycillin trihydrate.
The oral cavity provides a diverse environment for colonization by a wide variety of microorganisms. The early oro-dental infections are due to facultative or strict aerobes and at later stages most bacteria in oral infections are anaerobic species. Facultative indigenous oral streptococci and anaerobic species, especially the Bacteroides, Fusobacterium, Anaerobic cocci and Actinomyces species, are the most common agents of pyogenic submucosal oro-facial infections [1]. Treatment of infectious diseases frequently involves the use of antimicrobial agents as a primary therapy. Other delivery systems include oral rinses, irrigation, local delivery and systemic administration [2].
There are many antibiotics which are commonly prescribed by the physicians for periodontal diseases. Each of them has certain limitations. Tetracycline is a broad-spectrum antibiotic that inhibits the anaerobic and facultative aerobes [3]. Films and gels of metronidazole have been developed which are effective only against anaerobes to deliver the drug into periodontal pocket. Their replacement at regular intervals was required to produce a sustained antimicrobial effect [4,5]. Systemic administration of antibiotic is not advisable due to a number of side effects, including gastrointestinal disturbances and hypersensitivity reactions. Moreover, the drug reaches the periodontal pocket in a much diluted state and the subject gets exposed to high dose. A site-specific drug delivery is a better alternative in such cases.
Polyhydroxybutyric acid strip containing tetracycline hydrochloride and metronidazole 25% showed sustained release in simulated gingival fluid for about 5 d [6]. Strips loaded with amoxycillin-clavulanic acid were developed, with the highest level of drug being released during 24 h followed by sustained effect up to 5 d [7].
The use of fibres was first introduced by Goodson et al. [8] to deliver tetracycline hydrochloride to periodontal pocket. The hollow cellulose acetate fibres of 0.25 mm diameter containing 300 μg tetracycline per cm were developed. Rapid release of drug took place within 24 h due to release from both sides [8]. Goodson et al. [9] developed monolithic tetracycline fibres for controlled delivery to periodontal pocket by melt extrusion technique [9].
Amoxycillin trihydrate is the most widely used and prescribed medication for oro-dental infections. Amoxycillin trihydrate is a broad spectrum antibiotic and is active against most periodontal pathogens, particularly facultative and aerobic bacteria. It is also active against bacteria that are responsible for periodontal diseases, namely, Bacteroides gingivilis, B. intermedius and anaerobic bacilli. It is bactericidal in nature and has a low minimum inhibitory concentration against most oral pathogens.
In order to achieve sustained action of drug, polyvinyl acetate was used to provide prolonged action of amoxycillin at the targeted site. PVA was selected on the basis of its nontoxicity, non-irritant behaviour and compatibility with the drug and other excipients. It is highly hydrophobic in nature, considered to be a good film-forming agent and is a component of many controlled-release matrices and films.
The main objective of the present study was to develop a low-dose delivery system in the form of a fibre for local delivery of amoxycillin for treating infections and to maintain the concentration of the drug above its minimum inhibitory concentration for a prolonged period of time at the site of infection. Our objective was also to test the efficacy of the formulation against the microorganisms causing periodontitis.
Materials and Methods
Polyvinyl acetate (Vamipol-30) was obtained from Vam Organic Chemicals Ltd., Muradabad. Nylon fibre was obtained from Modipon Fiber Ltd., Modinagar, Merrut. Microbiological culture was obtained from B.V. Patel Chest Institute, University of Delhi. Mueller Hinton agar was obtained from Hi Media, Mumbai. Amoxycillin trihydrate was obtained from Ranbaxy Research Laboratories Ltd., Gurgaon, Haryana.
Preparation of drug-loaded fibres
The drug-loaded fibres were prepared by coating technique. In order to coat maximum amount of drug on fibres, polymer and drug solutions were prepared in ethanol and were mixed in different ratios (3:2, 1:1, 2:3 and 4:1). The fibres were coated five times. After each coating, the fibre was weighed to determine the drug load. Amount of drug loaded was further confirmed by shaking the fibre in ethanol and the resulting solution was filtered and analysed spectrophotometrically by measuring the absorbance at 229 nm and actual concentration of drug was extrapolated from calibration curve of amoxycillin prepared at 229 nm. Fibres were coated by the two following methods.
A. Using dilute solution of drug
Using ethanol as solvent, a 20% w/v solution of polymer was prepared. Drug solution was prepared to give a concentration of 3 mg/ml. Polymer solution and drug solutions were mixed properly on weight basis. Fibres (10 cm long) were weighed and dipped in coating solution for about 5.0 min and then removed and dried at 45° for half an hour. The weight of the coated fibre was taken and the amount of drug coated on it was estimated on a weight basis. The drug loading was also determined spectrophotometrically at 229 nm. Coating was done five times and each time, the content of drug present in the coating was estimated. The amount of drug in coated fibres with different number of coatings is presented in Table 1.
| Set | Coating | Average content of drug in fibre (mg) n=3(±S.D) | ||||
|---|---|---|---|---|---|---|
| No. | composition (g) | |||||
| A | P=6 | A-I | A-II | A-III | A-IV | A-V |
| D=4 | 0.34 (0.012) | 0.58 (0.012) | 0.68 (0.044) | 0.917 (0.020) | 1.54 (0.065) | |
| B | P=5 | B-I | B-II | B-III | B-IV | B-V |
| D=5 | 0.19 (0.016) | 0.345 (0.004) | 0.622 (0.009) | 0.7 (0.024) | 1.04 (0.065) | |
| C | P=4 | C-I | C-II | C-III | C-IV | C-V |
| D=6 | 0.09 (0.001) | 0.105 (0.004) | 0.288 (0.001) | 0.534 (0.012) | 0.98 (0.02) | |
| D | P=8 | D-I | D-II | D-III | D-IV | D-V |
| D=2 | 0.06 (0.001) | 0.10 (0.001) | 0.172 (0.016) | 0.24 (0.012) | 0.328 (0.004) | |
D= Drug solution (3 mg/ml), P= Polymer solution (20%), I to V indicates number of coatings given to fibres
Table 1: Amount of Loaded Drug In Fibres Coated By Solution Technique (Ref: Method A)
B. Using concentrated solution of drug
In this technique, concentrated solutions of drug were prepared by dissolving 50 mg and 100 mg drug in 5.0 ml of ethanol, resulting in concentrations of 10 and 20 mg/ml respectively. Rest of the procedure was same as discussed in section A. The amount of drug in coated fibres with different number of coatings is presented in Table 2.
| Set | Coating | Average content of drug in fibre (mg) n=3 (±S.D) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | composition (g) | |||||||||
| E | P=6 | E-II | E-III | E-IV | E-V | |||||
| D=4 | 0.54 | (0.012) | 0.66 | (0.044) | 0.91 | (0.001) | 1.40 | (0.040) | ||
| F | P=5 | F-II | F-III | F-IV | F-V | |||||
| D=5 | 0.63 | (0.014) | 0.82 | (0.016) | 1.16 | (0.0014) | 2.09 | (0.069) | ||
| G | P=4 | G-II | G-III | G-IV | G-V | |||||
| D=6 | 1.1 | (0.081) | 1.47 | (0.00) | 1.99 | (0.024) | 2.84 | (0.044) | ||
| H | P=8 | H-II | H-III | H-IV | H-V | |||||
| D=2 | 1.04 | (0.016) | 1.6 | (0.016) | 2.15 | (0.069) | 2.80 | (0.081) | ||
Set E and F were prepared by taking 50 mg drug in coating solution (total drug content in 5 g of drug solution and in 4 g of drug solution respectively). Set G and H were prepared by taking 100 mg drug in coating solution (total drug content in 5 g of drug solution and in 4 g of drug solution respectively). II to V indicate the number of coatings given to fibres.
Table 2: Amount of Loaded Drug In Fibres Coated By Concentrated Drug Solution Technique (Method B)
In vitro release of drug from coated fibres [9]
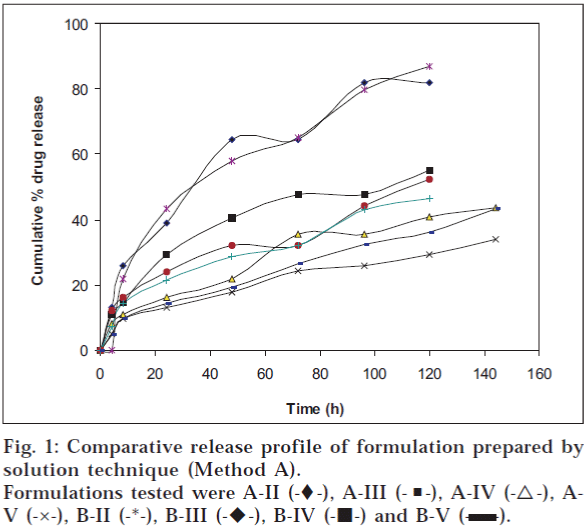
Release of amoxycillin from the fibre was determined by immersing the sample in a container filled with 50 ml of isotonic phosphate buffer having pH 6.6 and containing 2.25% glycoproteins. The container was sealed and then incubated at 37° in a shaking-water bath (Veego, Mumbai). Three millilitres of sample was withdrawn, filtered and analysed for drug content at 229 nm. Same volume of fresh dissolution media was replaced. The amount of drug released was determined at different time intervals. The results are shown in figs. 1 and 2 and Tables 3 and 4.
| Formulation code | Maximum amount of drug released n=3 (±S.D) | Time for maximum drug release (hrs) | |
|---|---|---|---|
| A-II | 81.8 (0.808) | 120 | |
| A-III | 55.14 (0.930) | 120 | |
| A-IV | 43.62 (1.071) | 144 | |
| A-V | 34.09 (1.071) | 144 | |
| B-II | 86.9 (1.189) | 120 | |
| B-III | 52.25 (0.707) | 120 | |
| B-IV | 46042 (0.08) | 120 | |
| B-V | 43.26 (1.428) | 144 | |
Table 3: Comparative In Vitro Release of Drug From The Fibres Prepared By Solution Technique (Method A)
| Formulation | Maximum amount of | Time for maximum |
|---|---|---|
| code | drug released N=3 (±S.D) | drug release (h) |
| E-II | 87.96 (6.094) | 144 |
| E-III | 83.3 (2.93) | 216 |
| E-IV | 5201 (3.336) | 216 |
| E-V | 39.2 (2.619) | 216 |
| F-II | 83.33 (3.763) | 168 |
| F-III | 45.7 (1.421) | 216 |
| F-IV | 49.5 (1.673) | 216 |
| F-V | 39.4 (0.989) | 216 |
| G-II | 84.0 (1.4) | 264 |
| G-III | 51.02 (1.424) | 240 |
| G-IV | 45.20 (0.457) | 264 |
| G-V | 35.21 (1.747) | 264 |
| H-II | 67.3 (0.823) | 264 |
| H-III | 53.12 (2.860) | 264 |
| H-IV | 36.04 (0.130) | 288 |
| H-V | 30.35 (0.694) | 240 |
Table 4: Comparative In Vitro Release Of Drug From The Fibres Prepared By Concentrated Solution Technique (Method B)
Analysis of dissolution data
Dissolution data was analysed using the equation proposed by Ritger and Peppas to describe the drug release of the drug from the matrix10 — Mt/M∞ = Ktn, where Mt corresponds to the amount of drug released in time t, M∞ is the total amount of drug released after infinite time, K denotes a constant and n is the release exponent indicating the type of drug release mechanism. Different kinetic equations (zero-order, first-order and Higuchi’s equation) were applied to interpret the release rates of drug from the fibre at pH 6.6.
Optimization of the formulation
The formulation was optimized on the basis of percent drug release over a period of time. Formulation A-II (‘A’ indicates the coating composition containing polymer solution and drug solution, i.e., 6 g and 4 g respectively; and II indicates two coatings given to the fibres), prepared by solution technique (Method A), showed maximum drug release of 81.8% over a period of 120 h; whereas formulation G-II [‘G’ indicates coating composition containing 5 g of drug solution (content of drug 100 mg) and 5 g of polymer solution and II indicates two coatings given to the fibres], prepared by method B, released 84% drug over a period of 264 h (11 d). On the basis of percent drug release over a period of time, it was concluded that G-II was the optimized formulation. The optimized fibre of amoxycillin trihydrate (G-II) was evaluated for general appearance. The colour of the formulation was white with a very slight bitter taste. The fibre was slightly flexible in texture with a diameter of 0.6 mm and length of 10 cm.
In situ release studies on the optimized fibre [11,12]
Continuous-flow-through apparatus was designed. It simulated the in vivo conditions of periodontal pocket for release rate studies. The flow-through cell was made up of glass and had a length of 10.5 cm and diameter of 2.1 cm, closed at one end and open on the other end. A small inlet tube of diameter 0.5 cm was attached to one end of the cell and another outlet tube of the same diameter was attached to the opposite end. In the centre of the lower chamber, there was a cavity of 8 mm length and 3 mm width for placement of fibre. The study was conducted using mucosal membrane of the bovine cheek pouch. The animal was sacrificed in the slaughter house and the cheek pouch was excised. It was washed thoroughly with distilled water and was then dipped in ammonia solution (0.2 M). This treatment leads to separation of buccal mucosa from the underlying tissues. The mucosal membrane so separated was cut into strips of 3 × 3 mm. A strip of mucosal membrane was washed with isotonic phosphate buffer of pH 6.6 and kept in the central cavity. It was stabilized with isotonic phosphate buffer pH 6.6 in order to remove soluble components. After stabilization, the fibre was placed on the mucosal membrane. One end of the flow-through cell was attached to a reservoir containing isotonic phosphate buffer of pH 6.6. Isotonic phosphate buffer of pH 6.6 simulating the salivary pH was continuously pumped at a flow rate of 0.65 ml/min using flow regulators. The flow rate chosen corresponded with the mean salivary flow rate [13]. The whole assembly was maintained at 37°. The samples were collected from the cavity at different time intervals and analysed spectrophotometrically at 229 nm for drug content.
Microbiological evaluation of in situ release samples [14,15]
The nutrient agar media and blood agar media were prepared by standard procedures. Sterilized Petri dishes were taken and 25 ml of media was poured into Petri dishes aseptically and allowed to solidify. Before solidification, it was inoculated with about 0.2 ml culture of microbes (0.5 MacFarland standard) implicated in such infections. Nutrient agar was inoculated with E. coli and S. aureus, whereas blood agar media was inoculated with S. mutans and B. cereus.
The samples obtained from in situ release studies were filtered through sterilized Millipore membrane filters (0.2 mm) and added in cups bored in inoculated solidified media. These were incubated at 37° for a period of 48 h in an incubator. The diameter of the zone of inhibition was noted. The same procedure was adopted for placebo formulation.
Permeation studies on the optimized fibre across buccal membrane [16,17]
A modified version of Franz diffusion cell was used to study the permeation of drug from bovine mucosal membrane; to achieve this, isotonic phosphate buffer pH bovine buccal mucosa at the base and the fibre was placed on top of it. The lower chamber was in the form of a closed cylinder containing a sampling port and had Teflon-coated magnetic needle at the base. The junction between the two chambers was tightly secured by placing buccal mucosa in between the two chambers. Fifteen millilitres of isotonic phosphate buffer of pH 7.4 was added to the lower chamber containing Teflon-coated needle. Upper chamber contained 10 ml of isotonic phosphate buffer of pH 6.6. The cell was placed on a magnetic stirrer. The whole setup was kept at 37° in an oven. Three millilitres of sample was withdrawn at different time intervals over a period of 11 d from the lower chamber. The samples were filtered, diluted suitably and were analysed spectrophotometrically at 229 nm for the amount of drug permeated.7.4 (representing the physiological pH of plasma) was utilized in the receiver compartment. The assembly consisted of two chambers. The upper cylindrical chamber, which was open from above, harboured the bovine buccal mucosa at the base and the fibre was placed on top of it. The lower chamber was in the form of a closed cylinder containing a sampling port and had Teflon-coated magnetic needle at the base. The junction between the two chambers was tightly secured by placing buccal mucosa in between the two chambers. Fifteen millilitres of isotonic phosphate buffer of pH 7.4 was added to the lower chamber containing Teflon-coated needle. Upper chamber contained 10 ml of isotonic phosphate buffer of pH 6.6. The cell was placed on a magnetic stirrer. The whole setup was kept at 37° in an oven. Three millilitres of sample was withdrawn at different time intervals over a period of 11 d from the lower chamber. The samples were filtered, diluted suitably and were analysed spectrophotometrically at 229 nm for the amount of drug permeated.
Results and Discussion
The optimized coating composition prepared by solution technique was found to be one having 6 g polymer solution and 4 g drug solution (Set A), while for method B (concentrated solution technique) it was combination of 5 g polymer solution along with 5 g drug solution (Set G). As the amount of drug in the solution increased, amount of drug incorporated in the coated fibre increased. Hence maximum amount of drug could be incorporated into the fibres prepared by using method B (concentrated solution technique). The optimized fibre was cut into pieces and the content of drug was estimated on each section. It was found that drug content was uniform throughout the length of the fibre. The optimized fibre was flexible since the folding endurance was found to be more than 300. It had nylon as core material. The fibre can be easily placed inside the periodontal pocket by first filling the deepest portion of the pocket and by wrapping the fibre around the teeth; a thin layer of cyanoacrylate adhesive is applied to keep the delivery system in place. Dentists carry out this procedure in the hospital.
Formulation A-II, prepared by solution technique (Method A), released 81.8% drug over a period of 120 h; whereas G-II, prepared by concentrated solution technique (Method B), released 84% drug over a period of 264 h. Presence of glycoproteins did not alter the λ max of the drug analysed by the UV method. It was revealed that as the number of coatings increased, the percentage of drug released decreased because increase in the number of polymer coats decreased diffusivity of the drug.
Different kinetic equations were applied to interpret the release rate from the optimized fibre G-II at pH 6.6. The best fit with the highest correlation was achieved with the zero-order equation. When n approximates to 0.5, a Fickian/diffusion-controlled release is implied; when 0.5<n<1.0 a non-Fickian transport is implied; and when n = 1, zero-order release is indicated. When values of n approach 1.0, one can conclude that release is approaching zero order. For the optimized formulation, value of n is calculated to be 0.9317.
The concentration of drug in samples obtained from in situ release studies remained well above the minimum inhibitory concentration (≅1.5 μg/ml) value of the drug over a period of 264 h. In situ release study samples when tested against S. aureus, S. mutans, B. cereus and E. coli showed that samples inhibited the growth of all the above-mentioned microorganisms except E. coli. The microbiological study at one end revealed that the drug released at various time intervals was able to inhibit the growth of microbes, whereas at the other end, it also revealed that the drug was stable to inhibit the growth of microbes even after 264 h. Amoxycillin was not able to inhibit the growth of E. coli as it is not effective against Gram-ve. Fibre without the drug was also tested against the above-mentioned microorganisms and it was found that the fibre without the drug was not effective against the microorganisms.
Permeation studies across bovine cheek pouch membrane using modified Franz diffusion cell showed that only 12.3% drug permeated in 264 h, indicating that low amount of drug is expected to go into systemic circulation. The study revealed that the drug in this formulation is released locally; hence it has a high benefit-to-risk ratio. Analysis of permeation rate data showed that permeation followed zero-order kinetics rather than first-order because coefficient of variation was lower for zero-order (14.9) as compared to first-order (91.9).
The present study was an attempt to develop local targeted drug delivery system in the form of a fibre. It was developed to a satisfactory level in terms of drug content, drug release, mechanical properties, in vitro release and in situ release of drug. Fibres were found to be a suitable drug delivery system, an alternative to conventional administration of systemic antibiotics. The fibres were flexible. The placement and removal of the fibre from periodontal pocket is easy. Since the drug release occurs locally, local administration is more desirable because of high benefit-to-risk ratio as compared to systemic administration, which is unacceptable due to low benefit-to-risk ratio. Hence low-dose site-specific fibre is a better alternative.
References
- William, R.C., N. Engl. J. Med., 1990, 322, 373.
- Rahman, S., Ahuja, A., Ali, J. and Khar, R.K., Indian J. Pharm.Sci., 2003, 65, 106
- Goodson, J.M., Offenbacher, S., Farr. D.H. and Hogan, P.E., J.Periodontal.,1985, 56, 265.
- Schwach-abdellaoui, K., Vivien-Casioni, N, and Gumy, R., Eur. J.Pharm. Biopharm., 2000, 50, 83.
- Kornman, K.S., J. Periodontal., 1993, 64, 782.
- Deasy, P.B., Collins, A.E.M., MacCarthy, D.J. and Russell, R.J., J.Pharm. Pharmacol.,1989, 41, 694.
- Abu Fanas, S.H., Drucker, D.B. and Hull, P.S., J. Dent., 1991, 19, 92.
- Goodson, J.M., Haffajee, A. and Socransky, S.S., J. Clin. Periodontal.,1979, 6, 83.
- Goodson, J.M., Holbrow, D., Dunn, R.L., Hogan, P. and Dunham, S., J. Periodontal., 1983, 54, 575.
- Ritger, P.L. and Peppas, N.A., J. Control. Release, 1987, 5, 37.
- Mumtaz, A.M. and Chng, H.S., Int. J. Pharm., 1995,121, 129.
- Ali, J., Khar , R.K. and Ahuja, A., Pharmazie, 1998, 53, 329.
- Schneyer, L. H. and Levin, L.K., J. Appl. Physiol., 1995, 7, 609.
- Rahman, S., Ahuja, A., Ali, J. and Chaudhry, R., Pharmazie, 2003,58, 716.
- Ahuja, A., Ali, J. and Rahman, S., Pharmazie, 2005. In Press
- Ali, J., Khar, R.K., Ahuja, A and Khurana, R., Int. J. Pharm., 2002,283, 93.
- Ahuja, A, Ali, J., R. Sarkar, Shareef, A. and Khar, R.K., Int. J.Pharm., 2003, 259, 47.
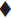 -), A-III (-?-), A-IV (-?-), A-GV (-×-), B-II (-*-), B-III (-
-), A-III (-?-), A-IV (-?-), A-GV (-×-), B-II (-*-), B-III (- -), B-IV (-?-) and B-V (-?-).
-), B-IV (-?-) and B-V (-?-).