- *Corresponding Author:
- Shaikh Darakhshan Afreen
Department of Pharmaceutics, Srinath College of Pharmacy, Aurangabad, Maharashtra 431136, India
E-mail: darakhshan201992@gmail.com
| Date of Received | 21 October 2022 |
| Date of Revision | 11 June 2023 |
| Date of Acceptance | 17 January 2024 |
| Indian J Pharm Sci 2023;86(1):211-221 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In ocular delivery, conventional drug administration systems have significant pitfalls, such as low bioavailability, low residence time and rapid precorneal drainage. An in situ gel is a novel drug delivery system with prolonged pharmacological action and simpler production techniques. This investigation aims to formulate and evaluate pH-responsive ophthalmic in situ gel of Ciprofloxacin hydrochloride and Olopatadine hydrochloride. The nine batches of ophthalmic in situ gel were prepared using carbapol-980 as a gelling agent and hydroxypropyl cellulose as a viscosity modifier. The designed formulations were examined for visual appearance, clarity, pH, gelling time, viscosity, drug content, in vitro drug release and stability study. The study's outcome explored that in situ gelling systems were translucent and clear, with a pH ranging from 4±0.26 to 7± 0.27, suitable for ocular application. In gelling time study, F1, F2, and F3 batches required 34-39 min for gel formation and showed approximately a 4-5 times increase in the viscosity after gelation compared to all batches. In drug content analysis F3 batch exhibited the highest amount of drug content, 99.90±0.25 % for Ciprofloxacin hydrochloride and 101.45±0.93 % for Olopatadine hydrochloride; hence this batch was subjected to a drug release study. The kinetic study explored the resulting data best fitted to Korsmeyer-Peppas than zero order, first order, Higuchi models. A stability investigation exhibited no alteration in the formulation at 40°±2° with a relative humidity of 75 %-5 % RH after 3 mo. A compressive finding explored that ophthalmic pH-responsive in situ gel can be a potential approach for treating conjunctivitis.
Keywords
pH-responsive in situ gel, ciprofloxacin hydrochloride, olopatadine hydrochloride, conjunctivitis
The term "conjunctivitis" refers to a broad spectrum of disorders characterized by conjunctival inflammation[1,2]. It is the most frequent consequence of red eyes, typically associated with viral or bacterial infection[3]. Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus sp., Pseudomonas aeruginosa, and Chlamydia trachomatis are the most typical pathogens that aggravate bacterial conjunctivitis[4,5]. Ciprofloxacin Hydrochloride (CFH) is a second-generation fluoroquinolone, a broad-spectrum antibiotic utilized to treat eye disorders[6]. It is chemically 1-cyclopropyl-6-Fluro-1, 4-dihydro-4-oxo-7- (piperazine-1-yl)-3-quinoline carboxylic acid (fig. 1A)[7]. Olopatadine Hydrochloride (OLH) is an antihistaminic used to treat allergic conjunctivitis by inhibiting the discharge of histamine from mast cells[8]. It is chemically 11-[(Z)-3-(Dimethylamino) propylidene]-6, 11-dihydrodibenzo [b, e] oxepin- 2-acetic acid hydrochloride (fig. 1B). It is commercially available in the form of eye drops [e.g. Ciplox D (Cipla), Olohyd (Oculent) etc.] and ointment [e.g. Ciplox D (Cipla), Zoxan (Apollo) etc.]. In the event of a severe infection, topical administration of 0.3 % CFH and 0.1 %, 0.2 %, 0.7 % OLH solutions are recommended for the patient[9]. The topical administration of eye drops in the lower cul-de-sac is the major systematic approach for managing ocular disorders[10].
Moreover, one of the primary complaints with eye drops is the quick and widespread removal caused by tear turnover, blinking and formulation drainage, resulting in a low precorneal residence period and ocular bioavailability[11,12]. Over the last decade, In situ Gel (ISG)-based Drug Delivery System (DDS) have received much attention[13]. Before administration, ISG is in a sol state and proficient in forming a gel in response to various extracellular stimuli such as temperature, pH and ion incidence[14,15]. These delivery systems are comprised of polymers that undergo a sol-to-gel phase transition in the eye due to advances in particular physiological circumstances[16,17]. Three kinds of systems are distinguished based on the approach used to establish a phase transition on the ocular surface pH-activated systems encompass hydrogen phthalate, cellulose acetate and carbopol, temperature-dependent systems involve pluronic and tetronics and ion-activated systems comprise gellan gum and sodium alginate[18,19]. Compared to traditional DDS, the sol-gel transition of this stimuli-responsive system provides a feasible method of drug delivery, sustained and prolonged drug distribution, high stability and biocompatibility[20,21]. When the tear fluid pH is raised to 7.4, pH-triggered systems undergo a solgel transformation; most pH-sensitive polymers are acidic polyanions with a low density[17-22]. Furthermore, when water is present, the polymer becomes ionised and surges as the pH rises to that of the body[16]. Existing studies developed several ISG formulations using single CFH and OLH for various eye disorders. In literature, different polymers are implemented to establish formulations, such as sodium alginate, chitosan, Hydroxypropyl Methylcellulose (HPMC), Carbapol-980 (CBL-980), and poloxamers[23-25]. Single drugs with a combination of polymers were previously developed, but neither CFH nor OLH has been formulated yet in conjunction using two polymer combinations[21-26]. Additionally, the combination of two drugs is more effective than an individual drug; hence in this present investigation, efforts have been taken to develop an ISG formulation incorporating a pH-dependant polymer such as CBL-980 and viscosity grade Hydroxypropylcellulose (HPC) for the effective administration of the CFH and OLH into the eye for prolonged drug release and increased ocular drug bioavailability. The detailed materials and methods employed in the investigation are explored in a subsequent section.
Materials and Methods
Materials:
CFH and OLH were received as gift samples from Aadhaar Life Sciences Pvt. Ltd, Solapur, India and Indoco Remedies Pvt. Ltd, Mumbai, India, respectively. Carbopol 980 procured from Lubrizol India Pvt. Ltd, Navi Mumbai, India. Viscositygrade HPC was obtained as a gift sample from Ashland India Pvt. Ltd. Mumbai, India. Sodium chloride procured from Thomas Baker, Pvt. Ltd, Mumbai, India. Benzalkonium chloride was purchased from Loba Chemie Pvt. Ltd, Mumbai, India. The other chemicals and solvents utilised in the research were of analytical grade.
Methods:
Pre-formulation studies: Melting points of both drugs were determined using a glass capillary technique. CFH and OLH powders were packed separately into glass capillaries immersed in liquid paraffin containing Thiele's tube and heated with a silicon oil heater at a heating rate of 1°/min. The melting points were monitored by analysing the temperature at which drugs began to melt[27].
Solubility measurement was performed following the consensus recommendations for the vigorous shaking method with slight modifications in the methodology reported by Bharate et al.[28]. The solubility of CFH was examined in water, ethanol methanol and OLH in water, formic acid, and dehydrated alcohol. 5 ml of each tube was laden with excess CFH and OLH. Afterwards, the test tubes were covered with aluminium foil and the amount of drug solubilized was observed[29].
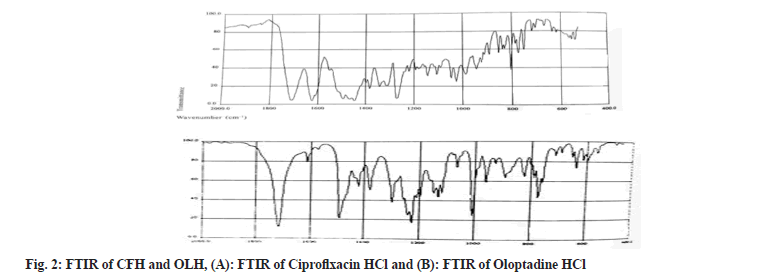
The functional groups existing in both drugs were identified by Fourier Transformed Infrared Spectroscopy (FTIR) spectroscopy. The CFH and OLH (3 mg each) were precisely weighed, combined with IR-grade potassium bromide, compressed into discs by applying hydraulic pressure, and examined on a (FTIR- 8400S, Shimadzu, Japan). The scanning was done at a resolution of 0.48-1.93 cm-1 in 2000-400 cm-1 wavelength[30].
The drug excipient compatibility study determined the interaction between the drug and excipients utilized in the ISG. The physical combinations of drugs and excipients were developed by the same concentrations in five batches and stored for 30 d at 40°±2° and 75 %±5 % relative humidity. Afterwards, the percentage assays of all batches were determined using Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) [31].
Formulation and development of pH-dependant ophthalmic ISG: The nine pH-dependent CFH and OLH ISG formulations were designed by employing differing concentrations of viscosity grade HPC, CBL-980, as shown in Table 1. To get a transparent dispersion, the HPC was gently added to the 70 ml of purified water at 30° with continuous stirring on a hot plate (Stuart, UK). The CBL-980 was then evenly dispersed over the HPC solution while being heated to approximately 50° and constantly stirred on the magnetic stirrer. Afterwards, a clear drug solution was obtained by dissolving the 0.35 % and 0.55 % CFH and OLH in 20 ml of purified water. This solution was combined with the polymer's dispersion, and the final volume was made using 100 ml of water. The formulation was filtered through a sterile membrane filter with a 0.22 μm pore size into final containers that had already been sanitised and sealed to exclude microorganisms. 0.50 % Sodium Chloride (NaCl) and 0.02 % BKC were employed as buffering agent and preservatives to prevent irritation of the ocular membrane and the growth of microorganisms[32,33].
| S no. | Ingredients (%) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CBL-980 | 0.2 | 0.25 | 0.3 | 0.3 | 0.5 | 0.7 | 0.5 | 0.75 | 1 |
| 2 | HPC | 0.3 | 0.25 | 0.2 | 0.7 | 0.5 | 0.3 | 1 | 0.75 | 0.5 |
Note: (*) concentrations of both drugs were constant in all batches
Table 1: Formula for pH-dependent ISG
Evaluations of ISG: The developed formulations of the ISG were evaluated by determining visual appearance clarity, pH, gelling time, viscosity, drug content evaluation, drug release studies, sterilization effect, and stability studies.
Visual appearance, clarity and pH: The clarity study was performed under visual inspection in adequate illumination to determine turbidity or the presence of any foreign particles. All established formulations were observed in contrast to a black and white background, with the components swirling in motion. ISG formulation’s pH was evaluated employing a digital pH meter (Mettler Toledo, Greifensee, Switzerland)[34].
Gelling time: The gelation experiment was conducted in cylindrical tubes with 5 ml of synthetic, Simulated Lacrimal Fluid (SLF) phosphate buffer pH 7.4, which simulated the cation content. A 50 μl of each formulation was incorporated using a standard dropper into a tube comprising SLF and was then visually ascertained for the gelation time and gel dissolved time[35].
Viscosity: The viscosity of the ISG was examined by (Brookfield Viscometer LVT model) at a shear rate of 10-200 N/m. The viscosity measurements were conducted before the dilution of the sol and after diluting the formulation with SLF to quantify the gelation of the formulation after installation in the eye. The viscosity of the sample solution was examined using spindle number 62 over a speed range of 0.3 to 30 rpm. It was calculated using the average of the two dial readings[36].
Drug content estimation: The drug content of F1, F2 and F3 batches were determined by diluting 100 μl of CFH and OLH ISG to 25 ml with distilled water in the decontaminated volumetric flask. The RP-HPLC method was employed to determine the content of drugs in the ISG by employing Acetonitrile:0.1 % trifluoroacetic acid in water (40:60 v/v) as the mobile phase. The samples were collected and analysed by RP-HPLC method using an Ultra Violet (UV) detector[37].
In vitro Release Test (IVRT) studies: The IVRT experimentation was performed on an optimized single batch utilising Franz diffusion cells (orifice diameter 0.9 cm; Perme Gear Inc. Hellertown, PA, USA). The Polytetrafluoroethylene (PTFE) membrane was attached to one end of a primarily built glass cylinder (open at both ends) with an inner diameter of 3.4 cm2. Before assembling in Franz-diffusion cells, the dehydrated membrane was rehydrated by immersing it in (20 ml) of pH 7.4 Phosphate Buffer (PB). A drug release diffusion study was conducted on ISG formulation. To ensure sink conditions, the receptor chamber of the cell comprised (5 ml) of SLF pH 7.4 that was agitated at 500 rpm and at 32°±1°. 3 g of the ISG was applied on membrane surfaces and wrapped with parafilm in the donor compartment to prevent evaporation. 0.5 ml sample was pipette out from release media at appropriate intervals of 30, 60, 90, 120, 180 and up to 240 min and replaced with an equivalent quantity of fresh SLF. CFH and OLH concentrations were assessed by the RP-HPLC method using a UV detector[38,39].
Drug release mechanism and kinetic modelling: The drug release mechanism was investigated using the goodness-of-fit procedure for IVRT release data. Different kinetic models such as zeroorder, first-order, Higuchi, and Korsmeyer-Peppas were used to establish the kinetic modelling of drug release from an optimized single batch. The following equations were utilized for computing the release kinetics[22].
Zero-order kinetics: dQ/dt=K0 (1)
First-order kinetics: dQ/dt=K1Q (2)
Higuchi release model: Q=KHt1/2 (3)
Korsmeyer-Peppas: Mt/M∞=KKPtn (4)
Where Q is extent of drug release; K0 is zero-order release rate constant; t is release time; K1 is firstorder release rate constant; t1/2 is half-life of the drug; Mt/M∞ is fraction of drug release and KKP is release rate constant for Korsmeyer-Peppas.
Effect of sterilization test: The sterility of F1, F2, and F3 optimised formulation batches were tested to determine the impact of aerobic, anaerobic bacteria and fungi before and after total sterilisation in an autoclave. For complete sterilization, the procedure was divided into four parts. In the first part of the sterilization process, both drugs and the aforementioned preservatives were mixed thoroughly in a separate beaker (A) and filtered through a 0.2-micron filter. Following that, CBL- 980 and HPC were thoroughly mixed in a beaker (B). Before sterilisation, both mixtures (C) were combined and evaluated for viscosity. Afterwards, mixture (C) was subjected to the autoclave for 1 h, and viscosity was measured[35].
Accelerated stability study: To evaluate the drug and formulation stability, an accelerated stability study was performed according to ICH Q1A (R2) standards at 4 different time points ((T0-zero time point-d 1), (T1-1 mo from the initiation date), (T2-2 mo from the date of initiation), (T3-3 mo from the date of initiation)). Optimized F1, F2 and F3 formulations were packed in 5 ml white HDPE plastic dropper bottles separately and kept in a stability chamber at definite temperature and humidity (40o±2°/75 %±5 % RH) for 3 mo. The physical stability of the three formulations were evaluated by determining pH, osmolality, surface tension, the viscosity of sol and gel and chemical stability by evaluating the percentage assay of the formulation[40].
Results and Discussion
The melting points of CFH and OLH were successfully ascertained by the glass capillary method. The melting points of both drugs were found to be 256°±2° and 238°±2°, which were in the range of their standard values (255°-257° CFH) and (>240° OLH). Sterilization is an essential aspect of ocular formulation. The obtained higher melting points showed no degradation of drugs during the sterilization process.
The solubility study was performed for both drugs, and it was observed that CFH was soluble in water, very soluble in ethanol, methanol and OLH was very soluble in formic acid, sparingly soluble in water, and very slightly soluble in dehydrated alcohol. Water is a potent solvent for ocular drug delivery instead of organic solvents. The solubility study explored that both drugs were soluble in water; hence it was used as a solvent for ISG formulation.
FTIR spectrum of CFH marked the presence of quinolones at 1650-1600 cm-1. In contrast, the bands at 1750-1700 cm-1 ascribed to carbonyl C=O stretching. The peaks revealed the bending vibrations of the O-H group at 1300-1250 cm-1. The existence of carboxylic acid was explored between the FT-IR ranges 1450-1400 cm-1. Furthermore, the C-F group exposed an absorption peak between 1050 and 1000 cm-1. The FTIR spectrum of OLH showed that the band at 1750 cm-1 corresponds to the C=O bond, whereas the peak at 1600 cm-1 and 1000 cm-1 exhibit the stretching vibrations of C=H and C-H, respectively. All observed functional groups in both drug’s spectrums were found as per standard ranges; hence it's confirmed that both drugs were in pure form and represented in fig. 2.
In the drug excipient compatibility study, the percentage assay for CFH was found between 97 %-101 % and for OLH 98 %-100 %. The excipient compatibility study explored that all batches (EC- 1 to EC-5) did not showed incompatibility; hence both drugs, in conjunction with excipients, were utilized in ISG formulation. The summary of the percentage assay excipient compatibility study is shown in the Table 2 and fig. 3 represents the study chromatograms. The nine batches of pH-dependant ISG formulation were developed successfully by employing CBL-980 and viscosity grade HPC and subjected to the following evaluation parameters.
| Batches | % Assay for CFH | % Assay for OLH |
|---|---|---|
| EC-1 | 97.39±0.59 | 99.61±0.17 |
| EC-2 | 98.21±0.25 | 98.07±0.42 |
| EC-3 | 99.93±0.35 | 99.27±0.69 |
| EC-4 | 100.49±0.27 | 99.63±0.48 |
| EC-5 | 101.32±0.18 | 100.56±0.16 |
Table 2: Summary of excipient compatibility study
Visual inspection and clarity study of the developed pH-dependant ISG formulations revealed that all nine batches were translucent and clear in appearance, ensuring patient compliance. Additionally, it’s indicated that the formulation did not contain any particulate matter, which may harm the ocular area. The pH of the F1-F3 batches were found to be 6.35±0.12 to 7±0.27, which correlated to the lacrimal fluid pH, whereas the pH of the F4- F6 batches were found to be 5±0.22 to 6±0.37, and F7-F9 batch showed 4±0.26 to 5.1±0.27 pH which was not suitable for ocular administration.
The gelling time was studied by adding 50 μl of each formulation to the cylindrical fluid containing 5 ml of SLF (pH 7.4), and the time required to form the gel was noted in min. After adding a drop of SLF, the gel was formed in seconds and lasted for several min. The study outcomes showed that batches F1, F2 and F3 required 34-39 min to assemble the gel. Batch F4, F5 and F6 required 43-49 min to form the gel. Batch F7, F8 and F9 consist 38-53 min to form the gel. In all batches, F1, F2 and F3 showed a consistent gelling time suitable for ocular administration because a higher gelling time reduces formulation efficacy.
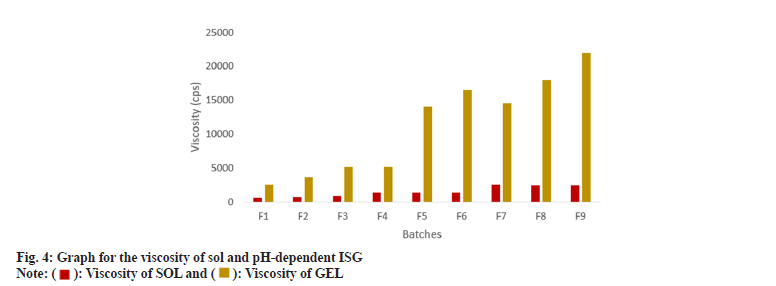
The viscosity of formulations was determined for sol and gel. Batch F1, F2 and F3 showed approximately a 4-5 times increase in the viscosity after gelation. In contrast, other batches showed an approximately 10 times increase which consisted of 0.5 %-1 % CBL-980, but the viscosity was significantly higher in comparison to what is required for ophthalmic preparation; hence 0.3 % concentration of CBL-980 and HPC were suitable for the ISG formulation as mentioned in fig. 4. The F1, F2 and F3 batches did not exhibit stiff form ISG hence the obtained viscosity was feasible for ocular DDS. The summary of overall evaluations is indicated in Table 3.
| Parameters | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Clarity | Clear | Clear | Clear | Clear | Clear | Clear | Clear | Clear | Clear |
| Viscosity of sol (cps) | 631±2.2 | 783±1.7 | 861±5.4 | 1389±3.54 | 1362±6.9 | 1331±9.25 | 2475±11.57 | 2460±21.08 | 2438±19.84 |
| Viscosity of gel (cps) | 2500±17 | 3700±23 | 5100±27 | 5100±1.5 | 14000±131 | 16500±100 | 14500±126 | 18000±119 | 22000±185 |
| Gelling time (min) | 39 | 36 | 34 | 55 | 43 | 49 | 38 | 48 | 53 |
| pH of sol | 6.35 | 6.75 | 6.47 | 5.74 | 5.88 | 5.66 | 4.75 | 4.33 | 4.76 |
Table 3: Summary of pH-dependent In Situ gel evaluations
The three batches were selected for drug content estimation based on the aforementioned evaluations. In the visual appearance, clarity, pH, gelling time and viscosity study, it was observed that F1, F2, and F3 batches showed relevant results for ocular drug delivery; hence these batches were considered optimized batches subjected to drug content estimation. The drug content of all three batches for CFH was found in ranges of 98.89±0.49 to 100.88±0.29, respectively, and for OLH was found to be 98.37±0.54 to 101.45±0.93, respectively, as shown in fig. 5 and Table 4. F3 showed maximum drug content; hence this batch was subjected to an IVRT study. The minimal deviation in drug content implies the uniform distribution of CFH and OLH in the developed formulation.
| Batches | % Assay for CFH | % Assay for OLH |
|---|---|---|
| F1 | 99.90±0.25 | 101.45±0.93 |
| F2 | 98.89±0.49 | 98.37±0.54 |
| F3 | 100.88±0.29 | 99.35±0.62 |
Table 4: Summary of drug content
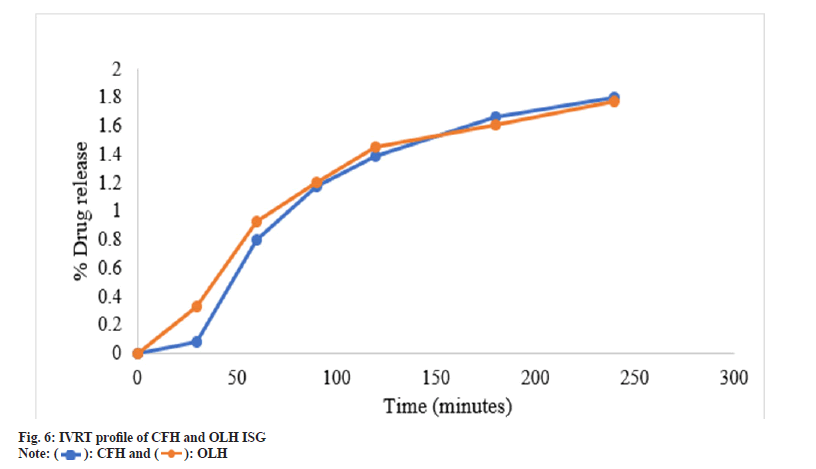
The efficacy of the drug release from developed gel employing synthetic membrane was compared by adopting an in vitro Franz cell system. When developing ocular drug delivery formulation, IVRT is used as a screening method to evaluate the efficiency characteristics of multiple prototype formulations. In drug content estimation, it was observed that F3 showed the highest amount of drug content from three selected batches, 99.56±0.42 for CFH and 99.89±0.51 for OLH; hence this batch was selected for the IVRT study. The percentage of CFH and OLH from the F3 batch released through the PTFE synthetic membrane was evaluated for up to 240 min and plotted as a function of time, as shown in Table 5 and fig. 6. The resulting data explored that pH-dependant formulation showed 1.80 % log drug release of CFH and 1.77 % log drug release for OLH within 240 min, which was satisfactory for ocular application and sustained drug delivery.
| Time (min) | Log of time | CFH | OLH | ||||
|---|---|---|---|---|---|---|---|
| The square root of time | % drug release | Log % drug release | square root of time | % drug release | Log % drug release | ||
| 30 | 1.48 | 5.48 | 1.21±0.012 | 0.08 | 5.48 | 2.15±0.015 | 0.33 |
| 60 | 1.78 | 7.75 | 6.25±0.023 | 0.8 | 7.75 | 8.45±0.013 | 0.93 |
| 90 | 1.95 | 9.49 | 14.95±0.17 | 1.17 | 9.49 | 15.93±0.17 | 1.2 |
| 120 | 2.08 | 10.95 | 24.78±0.15 | 1.39 | 10.95 | 28.34±0.23 | 1.45 |
| 180 | 2.26 | 13.42 | 45.24±0.26 | 1.66 | 13.42 | 40.33±0.31 | 1.61 |
| 240 | 2.38 | 15.5 | 62.45±0.23 | 1.8 | 15.49 | 58.64±0.12 | 1.77 |
Table 5: IVRT data of CFH and OLH For pH-dependent formulation
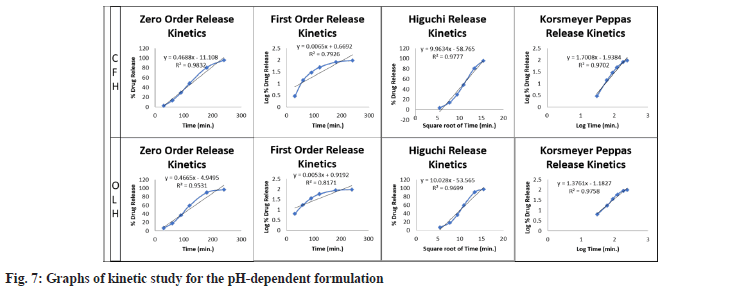
The resulting data were fitted into the preceding mathematical models to establish the optimised F3 batch release pattern and mechanism. The ISG drug release data was plotted utilizing several kinetic models, including zero order, first order, Higuchi and Korsmeyer-Peppas. Table 6 summarises the R2 values of the drug-loaded ISG formulation. The best fit model of the release pattern was estimated with zero and first-order maximum regression values (R2). Higuchi model regression values showed diffusion functions were comparable to specifying the release mechanism. The slope value ‘n’ of the Korsmeyer-Peppas model further demonstrates the release mechanism shown in fig. 7.
| Kinetic models | CFH (R2) | OLH (R2) |
|---|---|---|
| Zero-order | 0.9947 | 0.9937 |
| First order | 0.8261 | 0.8302 |
| Higuchi release kinetics | 0.959 | 0.9699 |
| Korsmeyer-Peppas model | 0.9827 | 0.9826 |
Table 6: Kinetic study data for pH-dependent ISG
The effect of sterilization was studied on F1, F2 and F3 batches before and after putting the formulation in an autoclave for total sterilization. The viscosity of formulations was measured and reported in Table 7. It observed no changes in viscosity after pre-sterilization and post-sterilization. Therefore, indicating that the product can be sterilized before filling during manufacturing.
| Batches | Viscosity | |
|---|---|---|
| Pre-sterilization | Post-sterilization | |
| F1 | 2609±5.8 | 2578±17.2 |
| F2 | 3803±4.6 | 3817±28.4 |
| F3 | 5091±16.5 | 5231±47.3 |
Table 7: Summary of sterilization effect study
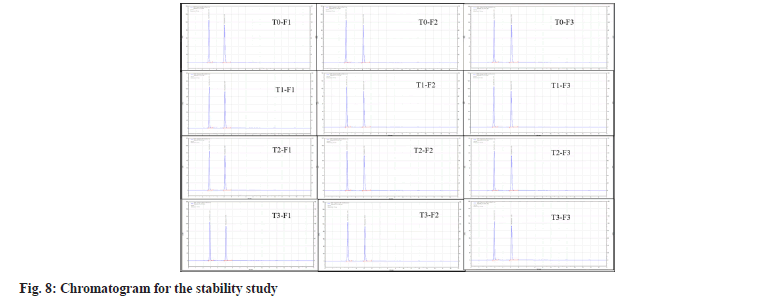
The stability of the F1, F2 and F3 batches were evaluated at 40°±2° with a relative humidity of 75 %±5 % RH for 6 mo, as displayed in Table 8 and fig. 8. The sample exhibited outstanding physical characteristics under the circumstances specified each time. Besides, the results showed no remarkable changes in all investigated parameters, pH, viscosity, and percentage assay. In addition, the gelling properties and appearance remained unchanged, stating that the formulation was stable and effective for 3 mo.
| Time | Parameters | 0 mo (T0) | 1 mo (T1) | 2 mo (T2) | 3 mo (T3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F1 | F2 | F3 | F1 | F2 | F3 | F1 | F2 | F3 | ||
| pH | 6.35 | 6.75 | 6.47 | 6.49 | 6.88 | 6.91 | 7.34 | 7.01 | 7.31 | 7.21 | 7 | 7.14 | |
| Viscosity (cps) | Sol | 631±2.5 | 783±5.4 | 861±7.3 | 644±3.3 | 749±2.1 | 852±5.4 | 59 ±0.14 | 784±0.29 | 8177±0.58 | 601±0.11 | 817±0.17 | 7915±0.25 |
| Gel | 2500±12.5 | 3700±28.3 | 5100±17.6 | 2600±17 | 3780±15 | 5000±23 | 2704±0.32 | 3715±0.22 | 5102 ±0.57 | 2617±2.6 | 3745±2.4 | 5101±3.6 | |
| % assay | CFH | 99.90±0.25 | 98.89±0.49 | 100.88±0.29 | 97.31±0.52 | 98.11±0.67 | 99.44±0.51 | 97.87±0.67 | 99.01±0.51 | 99.41±0.52 | 97.90±0.11 | 99.15±0.25 | 99.00±0.14 |
| OLH | 101.45±0.93 | 98.37±0.54 | 99.35±0.62 | 98.16±0.84 | 99.76±0.75 | 98.17±0.34 | 98.41±0.57 | 99.81±0.65 | 98.01±0.71 | 98.56±0.34 | 99.89±0.25 | 98.18±0.31 | |
Table 8: Stability study data for pH-dependent ISG formulations
Acknowledgements:
The authors are thankful to the Aadhaar Life Sciences Pvt. Ltd, Solapur, India and Indoco Remedies Pvt.Ltd, Mumbai, India, Lubrizol India Pvt. Ltd, Navi Mumbai, India. Ashland India Pvt. Ltd. Mumbai, India, Thomas Baker, Pvt. Ltd, Mumbai, India and Loba Chemie Pvt. Ltd, Mumbai, India for providing CFH, OLH, Carbopol 980, Viscosity-grade HPC, Sodium Chloride and Benzalkonium Chloride as gift samples respectively.
Conflict of interest:
The authors declared no conflict of interests.
References
- Biswas P, Batra S, Gurha N, Maksane N. Emerging antimicrobial resistance and need for antimicrobial stewardship for ocular infections in India: A narrative review. Indian J Ophthalmol 2022;70(5):1513.
[Crossref] [Google Scholar] [PubMed]
- Zi Y, Deng Y, Ji M, Qin Y, Nong L, Liu Z, et al. The effectiveness of olopatadine hydrochloride eye drops for allergic conjunctivitis: Protocol for a systematic review. Medicine 2020;99(7):e18618.
[Crossref] [Google Scholar] [PubMed]
- Azari AA, Arabi A. Conjunctivitis: A systematic review. J Ophthalmic Vision Res 2020;15(3):372.
[Crossref] [Google Scholar] [PubMed]
- Sheikh A, Hurwitz B. Topical antibiotics for acute bacterial conjunctivitis: Cochrane systematic review and meta-analysis update. Br J Gen Pract 2005;55(521):962-4.
[Google Scholar] [PubMed]
- Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am 2008;28(1):43-58.
- Davis R, Anthony M, Julia A. Ciprofloxacin. Drugs 1996;51:1019-74.
- Bartzatt R. Assay of ciprofloxacin by ultraviolet-visible spectroscopy. 2019.
- McGill JI. A review of the use of olopatadine in allergic conjunctivitis. Int Ophthalmol 2004;25(3):171-9.
[Crossref] [Google Scholar] [PubMed]
- Dubald M, Bourgeois S, Andrieu V, Fessi H. Ophthalmic drug delivery systems for antibiotherapy—A review. Pharmaceutics 2018;10(1):10.
[Crossref] [Google Scholar] [PubMed]
- SALMINEN L. Systemic absorption of topically applied ocular drugs in humans. J Ocul Pharmacol Ther 1990;6(3):243-9.
[Crossref] [Google Scholar] [PubMed]
- Zhu H, Chauhan A. Effect of viscosity on tear drainage and ocular residence time. Optom Vis Sci 2008;85(8):e715-25.
[Crossref] [Google Scholar] [PubMed]
- Achouri D, Alhanout K, Piccerelle P, Andrieu V. Recent advances in ocular drug delivery. Drug Dev Indu Pharm 2013;39(11):1599-617.
[Crossref] [Google Scholar] [PubMed]
- Wadhwa K, Sharma C, Goswami M, Thakur N. Formulation and evaluation of pH triggered in-situ ocular gel of ofloxacin. Int J Pharm Sci 2019;10:4507-12.
- Morrison PW, Khutoryanskiy VV. Advances in ophthalmic drug delivery. Ther Deliv 2014;5(12):1297-315.
[Crossref] [Google Scholar] [PubMed]
- Padmasri BU, Nagaraju RA, Prasanth DA. A comprehensive review on in situ gels. Int J Appl Pharm 2020;12(6):24-33.
- Davaran S, Lotfipour F, Sedghipour N, Sedghipour MR, Alimohammadi S, Salehi R. Preparation and in vivo evaluation of in situ gel system as dual thermo-/pH-responsive nanocarriers for sustained ocular drug delivery. J Microencapsul 2015;32(5):511-9.
[Google Scholar] [PubMed]
- Kurniawansyah IS, Sopyan I, Wathoni N, Fillah DL, Praditya RU. Application and characterization of in situ gel. Int J App Pharm 2018;10(6):34-7.
- Madan M, Bajaj A, Lewis S, Udupa N, Baig JA. In situ forming polymeric drug delivery systems. Indian J Pharm Sci 2009;71(3):242.
[Crossref] [Google Scholar] [PubMed]
- Chen H. Recent developments in ocular drug delivery. J Drug Target 2015;23:597-604.
- Allam A, Elsabahy M, El Badry M, Eleraky NE. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int J Pharm 2021;598:120380.
[Crossref] [Google Scholar] [PubMed]
- Pandey M, Choudhury H, Binti Abd Aziz A, Bhattamisra SK, Gorain B, Su JS, et al. Potential of stimuli-responsive in situ gel system for sustained ocular drug delivery: Recent progress and contemporary research. Polymers 2021;13(8):1340.
[Crossref] [Google Scholar] [PubMed]
- Rahmi F, Sopyan I. pH Triggered in situ gelling ophthalmic drug delivery system. Int J Drug Deliv Technol 2018;8(1);1-5.
- Eram F. In vivo Evaluation and characterization of novel in situ gelling system as controlled delivery system containing ciprofloxacin for ocular drug delivery. J Drug Deliv Ther 2020 Oct 15;10(5-s):32-9.
- Makwana SB, Patel VA, Parmar SJ. Development and characterization of in situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharm Sci 2016;6:1-6.
[Crossref] [Google Scholar] [PubMed]
- Kadam AT, Jadhav RI, Salunke PB, Kadam SS. Design and evaluation of modified chitosan based in situ gel for ocular drug delivery. Int J Pharm Pharm Sci 2017;9:87-91.
- Guven UM, Berkman MS, Şenel B, Yazan Y. Development and in vitro/in vivo evaluation of thermo-sensitive in situ gelling systems for ocular allergy. Braz J Pharm Sci 2019;23:55.
- Kardos F. Objective method for determining melting point. Anal Chem 1950;22(12):1569-70.
- Bharate SS, Vishwakarma RA. Thermodynamic equilibrium solubility measurements in simulated fluids by 96-well plate method in early drug discovery. Bioorgan Med Chem Lett 2015;25(7):1561-7.
[Crossref] [Google Scholar] [PubMed]
- Silverstein RM, Bassler GC. Spectrometric identification of organic compounds. J Chem Edu 1962;39(11):546.
- Skoog DA, James Holler F, Stanley R. Crouch. Principles of instrumental analysis. Cengage learning; 2017.
- Munday Dale L. Design, Development and evaluation of encapsulated oral controlled release theophylline mini-tablets. Diss Rhodes University; 1990.
- Makwana SB, Patel VA, Parmar SJ. Development and characterization of in situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharm Sci 2016;6:1-6.
[Crossref] [Google Scholar] [PubMed]
- Patel N, Thakkar V, Metalia V, Baldaniya L, Gandhi T, Gohel M. Formulation and development of ophthalmic in situ gel for the treatment ocular inflammation and infection using application of quality by design concept. Drug Dev Ind Pharm 2016;42(9):1406-23.
[Crossref] [Google Scholar] [PubMed]
- Kesarla R, Tank T, Vora PA, Shah T, Parmar S, Omri A. Preparation and evaluation of nanoparticles loaded ophthalmic in situ gel. Drug Deliv 2016;23(7):2363-70.
[Crossref] [Google Scholar] [PubMed]
- Ranch KM, Maulvi FA, Naik MJ, Koli AR, Parikh RK, Shah DO. Optimization of a novel in situ gel for sustained ocular drug delivery using Box-Behnken design: In vitro, ex vivo, in vivo and human studies. Int J Pharm 2019;554:264-75.
[Crossref] [Google Scholar] [PubMed]
- Aminabhavi TM, Agnihotri SA, Naidu BV. Rheological properties and drug release characteristics of pH‐responsive hydrogels. J Appl Polym Sci 2004;94(5):2057-64.
- Dasankoppa FS, Solankiy P, Sholapur HN, Jamakandi VG, Sajjanar VM, Walveka PM. Design, formulation, and evaluation of in situ gelling ophthalmic drug delivery system comprising anionic and nonionic polymers. Indian J Health Sci Biomed Res 2017;10(3):323-30.
- Allam A, Elsabahy M, El Badry M, Eleraky NE. Betaxolol‐loaded niosomes integrated within pH‐sensitive in situ forming gel for management of glaucoma. Int J Pharm 2021;598:120380.
[Crossref] [Google Scholar] [PubMed]
- Bisht R, Nirmal S, Agrawal R, Jain GK, Nirmal J. Injectable in situ gel depot system for targeted delivery of biologics to the retina. J Drug Target 2021;29(1):46-59.
[Crossref] [Google Scholar] [PubMed]
- Muthu MS, Feng SS. Pharmaceutical stability aspects of nanomedicines. Nanomedicine 2009;4(8):857-60.
[Crossref] [Google Scholar] [PubMed]
 ): Viscosity of SOL and (
): Viscosity of SOL and ( ): Viscosity of GEL
): Viscosity of GEL 
 ): CFH and (
): CFH and (  ): OLH
): OLH